Abstract
We report on the extensive structural and optical studies of Re-doped molybdenum disulfide (MoS2) grown by the chemical vapor transport (CVT) method using Br2 as a transport agent. To evaluate the influence of Re on the structural properties of crystals, we have conducted X-ray diffraction (XRD) and transmission electron microscopy (TEM) experiments. For optical characterization, we carried out piezoreflectance (PzR) and electrolyte electroreflectance (EER) measurements. The Re dopant clearly caused a structural change in MoS2 from a two-layer hexagonal (2H) structure to a three-layer rhombohedral (3R) structure, which has been clearly verified and identified herein.
Export citation and abstract BibTeX RIS
1. Introduction
Molybdenum disulphide belongs to the family of transition-metal dichalcogenides,1) MX2 where M = Mo or W and X = S or Se. In particular, the as-crystallized two-dimensional layered-type structure can impart substantial anisotropy to most of the physical properties of these compounds and has attracted investigators aiming to acquire a better insight into the fundamental physics of these compounds.1–3) The materials have also been extensively investigated because of their possible practical applications such as efficient electrodes in photoelectrochemical solar cells,4–6) catalysts in industrial applications and in secondary batteries,7–9) and solid-state lubricants.10–12) The successful application of this semiconductor compound originates largely from its sandwich interlayer structure, loosely bound by the weak van der Waals forces, as evidenced by easy cleavage perpendicular to the c-direction along which the S–Mo–S layers are stacked to form a crystal. There are two known polytypes of MoS2;1,13) two-layer hexagonal and three-layer rhombohedral termed 2H and 3R, respectively. Naturally occurring 3R-MoS2 has been found to be consistently rich in certain minor elements such as Re and Nb.14) The incorporation of the impurity elements will essentially influence the structural symmetry of MoS2, that is, the adoption of the polytype 3R-MoS2. Zelikman et al. reported that the 3R modification of MoS2 is a result of substitution of some part of the Mo atoms by Re in the MoS2 lattice, which made the three layer 3R packing more stable than the two-layer 2H polytype.15) In the previous work, we investigated the electrical properties of Re-doped MoS2 by temperature-dependent resistivity and Hall coefficient measurements; the optical absorption measurements indicated that Re-doped MoS2 is an indirect semiconductor and its energy gap shows a red-shift with increasing dopant concentration.16) Herein, we have also undertaken a series of experiments to analyze the structural and optical properties of undoped and Re-doped MoS2.
In this study, both MoS2 samples with and without the Re dopant were prepared by the chemical vapor transport (CVT) method. To determine the structural and optical properties of both samples, X-ray diffraction (XRD), scanning transmission electron microscopy (STEM), piezoreflectance (PzR), and electrolyte electroreflectance (EER) measurements were carried out. A series of experimental results confirmed the Re dopant effect on the fundamental material properties, which could provide guidance for further electronics and optoelectronics production of MoS2.
2. Experimental methods
Single crystals of the Re-doped MoS2 were grown by the CVT method with Br2 as a transport agent. The total charge used in each growth experiment was about 10 g. A stoichiometrically determined weight of the doping material was added in the hope that it would be transported at a rate similar to that of Mo. Before the crystal growth, the powdered compounds were prepared from the elements by reaction at 1,000 °C for 10 days in an evacuated quartz ampoule. Prior to the crystal growth, a quartz ampoule (22 mm OD, 17 mm ID, 20 cm length) containing Br2 (∼5 mg/cm3) and the elements (purity: Mo, 99.99%; Re, 99.99%; S, 99.999%) was evacuated to 10−6 Torr and sealed. It was shaken well for uniform mixing of the powder. The ampoule was placed in a three-zone furnace and the charge prereacted for 24 h at 800 °C with the growth zone at 950 °C, preventing the transport of the product. The temperature of the furnace was increased slowly. The slow heating was necessary to avoid any possibility of explosion due to exothermic reaction between the elements. The furnace was then equilibrated to give a constant temperature across the reaction tube and programmed over 24 h to produce the temperature gradient at which single crystal growth took place. Optimal results were obtained with the temperature gradient of approximately 960 → 930 °C. After 240 h, the furnace was allowed to cool slowly (40 °C/h) to about 200 °C. The ampoule was then removed and wet tissues were applied rapidly to the end away from the crystals to condense the Br2 vapor. When the ampoule reached room temperature, it was opened and the crystals were removed. The crystals were then rinsed with acetone and deionized water. Single crystalline platelets up to 10 × 10 mm2 in surface area and 2 mm in thickness were obtained.
XRD patterns of single crystals were obtained using a Rigaku RTP300RC X-ray with Ni-filtered Cu Kα radiation (λ = 1.5418 Å), and a silicon standard was used to calibrate the diffractometer. The morphologies of the samples were investigated by STEM at a high magnification. A JEOL 2100F STEM equipped with a delta corrector and a cold field emission gun was employed at 60 kV in these experiments. Single-layer and few-layer Re-doped MoS2 samples were mechanically exfoliated from the CVT synthesized crystals using scotch tape and transferred onto a silicon substrate with 300 nm thermal oxide. The surfaces of Re-doped MoS2/SiO2/Si samples were spin coated with 1.5-µm-thick polycarbonate (1 wt % dissolved in chloroform). The single-layered Re-doped MoS2 flakes were transferred from the SiO2 surface to the TEM microgrid (quantifoil) using 2-propanol and cleaned using chloroform for 12 h before the TEM observation. Then, the specimens were cleaned by baking in air at 200 °C for 10 min, after which they were placed in the TEM chamber. The JEOL double tilt holder was precleaned using ion-plasma cleaner (JEOL, operated at 360 V for 10 min) after ethanol treatment. The vacuum level in the TEM chamber was ∼1.7 × 10−5 Pa.
The experimental setup for the PzR measurements has been described elsewhere.17,18) Our systems were prepared by gluing the thin single crystal specimen onto a 0.15-cm-thick lead–zirconate–titanate (PZT) piezoelectric transducer driven by a 200 Vrms sinusoidal wave at 200 Hz. The alternating expansion and contraction of the transducer subjects the sample to an alternating strain with a typical rms Δl/l of ∼10−5. A 150 W tungsten–halogen lamp filtered using a McPherson 0.35 m monochromator provided the monochromatic light. The reflected light was detected by an EG&G HUV-2000B silicon photodiode. The DC output of the silicon photodiode was maintained constant by the servo mechanism of a variable neutral density filter. A dual-phase lock-in amplifier was used to measure the detected signal. Modulated spectra were normalized to the reflectance to obtain ΔR/R. An RMC 22 closed-cycle cryogenic refrigerator equipped with a model 4075 digital thermometer controller was used to control the measurement temperature between 25 and 300 K with a temperature stability of 0.5 K or better.
For the EER experiment, maximum-size crystals of 1% Re-doped MoS2 were selected. The EER measurements were taken on a fully computerized setup for modulation spectroscopy described elsewhere.17,19,20) Detailed investigations of the polarization dependence of the prominent features, A and B excitons in the energy range of 1.75–2.25 eV, were undertaken. For the Re-doped MoS2, the spectra were recorded for the perpendicular (E ⊥ c) and parallel (E ∥ c) polarizations together with an unpolarized spectrum for the k ⊥ c (edge plane) configuration, whereas only the unpolarized spectrum was recorded for the k ∥ c (van der Waals plane) configuration. For the undoped MoS2, only the k ∥ c and unpolarized spectrum was taken. The detector response to the DC component of the reflected light is kept constant by either an electronic servo mechanism or a neutral density filter so that the AC reflectance corresponds to ΔR/R, the differential reflectance. Scans of ΔR/R versus wavelength were obtained using a 0.35 m McPherson grating monochromator together with an Oriel 150 W xenon arc lamp as a monochromatic light source. Phase-sensitive detection was carried out to measure the differential reflectance. The electrolyte was a 1 N H2SO4 aqueous solution, and the counter-electrode was a 5 cm2 platinum plate. A 200 Hz 100 mV peak-to-peak square wave with VDC = 0 V versus a platinum electrode was used to modulate the electric field in the space charge region of the MoS2 electrode.
3. Results and discussion
Figure 1 reveals the XRD patterns of doped and undoped MoS2 single crystals. The relative intensity and resolution of observed peaks change for the Re-doped sample. For Re-doped crystals, the observed peaks correspond to the rhombohedral structure (3R) crystal with the cell dimension of a = 3.164 Å and c = 18.371 Å, whereas for the undoped crystal, the patterns correspond to the hexagonal structure (2H). The lines were identified with a 3R in which, by referring to the 2H, the a parameter of the unit cell is similar to that of the 3R (a = 3.160 Å) but the c parameter was about 1.5 times larger than that of that 2H (c = 12.295 Å). We observed that the a parameter remains unchanged for the undoped and Re-doped samples, whereas the c parameter shows an appreciable increase, which is in agreement with the increase in the d-spacing.
Fig. 1. XRD patterns of the undoped and Re-doped MoS2 single crystals.
Download figure:
Standard image High-resolution imageIn Figs. 2 and 3, the STEM images of Re-doped MoS2 crystals are presented. It is clearly seen that Re atoms tend to occupy or substitute Mo atoms in the host MoS2 lattice. The Re dopants are well dispersed in MoS2 layers and show no formation of clusters in the host material.21) The observed doping concentration of ∼1% Re is in agreement with the synthesis condition. About 7% of the Re dopants were found as adatoms. Those Re adatom dopants on MoS2 can become mobile by receiving kinetic energy (Ek) from the focused incident electron beam. The energy transferred from the 60 kV electron beam to Re atoms is Ek = 0.75 eV.22–24) In this case, substitution to the Mo site has the lowest formation energy by a large margin. Among the adatom sites, the position on the top of Mo is favored. This is in good agreement with the observations that nearly all Re atoms are located at Mo sites, and rarely on the adatom sites on top of Mo and S. The way the Re atoms moved has been discussed in more detail in our previous work.24)
Fig. 2. Low magnification annular dark-field STEM image of Re-doped few-layer stacked MoS2 flakes, where the atomic element arrangements and the number of MoS2 layers can be clearly distinguished by the Z-contrast. The numbers 0, 1, 2, 3, and 4 correspond to the number of layers.
Download figure:
Standard image High-resolution imageFig. 3. ADF-STEM image of Re atoms tending to occupy Mo atoms.
Download figure:
Standard image High-resolution imageThe PzR spectra near the direct band edge over the range of 1.7–2.3 eV for the undoped and Re-doped MoS2 single crystals are respectively shown in Figs. 4(a) and 4(b). The spectra are characterized by observing two prominent excitonic transitions, A and B. In the case of undoped MoS2, a higher-series A exciton, denoted as A2, is also detected. The dashed lines in Fig. 4 are the experimental PzR spectra and the solid curves are the least-square fits to a derivative Lorentzian functional form appropriate for the interband transitions expressed as17,19)
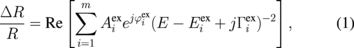
where  and
and  are the amplitude and phase of the line shape, and
are the amplitude and phase of the line shape, and  and
and  are the energy and broadening parameters of the interband excitonic transitions, respectively. For the first derivative functional form, the exponential term 2.0 is appropriate for the bound states, such as excitons or impurity transitions.19) The fits yield the parameters Ai, Ei, and Γi. The obtained values of Ei are indicated as arrows and denoted as A1, A2, and B. The nomenclature was commonly used previously by Wilson and Yoffe,1) Beal et al.,25) and Fortin and Raga.3) The fitted values of Ei are listed in Table I together with some of the values from previous works.3,25–28) The energies of prominent A and B excitons of 3R are observed to be lower than those of the 2H undoped MoS2. The energy separations between A and B excitons (ΔBA = EB − EA) are 152 ± 5 meV for Re-doped MoS2 and 212 ± 8 meV for undoped MoS2. The Re incorporation can reduce the energy separation between A and B excitons. The linewidth of the exciton B is much broader than that of exciton A. The A exciton originates from the valence-band top whereas the B exciton comes from the valence-band splitting. In general, the Rydberg series of the A and B excitons of undoped MoS2 can be described by three-dimensional Mott–Wannier excitons,25,26,29) as En = E∞ − Rn−2, where n = 1, 2, 3, ..., and E∞ and R are the critical and binding energies, respectively. For Re-doped MoS2, only n = 1 Rydberg series for both excitonic transitions are observed. It has been shown that excitons for the 3R MoS2 are more appropriately described by the two-dimensional Mott–Wannier excitons.25,26,29) The absence of higher-order series is an inherent nature of the two-dimensional Mott–Wannier excitons.26) From more recent theoretical and experimental studies,30,31) the A and B excitons are attributed to the smallest direct transitions at the K point of the Brillouin zone split by interlayer interaction and spin–orbit splitting. The A exciton belongs to K4 to K5 optical transition whereas the B exciton corresponds to K1 to K5 optical transition. The K states have been shown to be predominantly determined by the metal d states with a small contribution from the non-metal p states.30,31) The energies and linewidths of A and B excitons can be determined accurately by using the fits of Eq. (1). When the temperature increased, both excitonic transitions showed energy reduction which is a general semiconductor behavior. The linewidths also become broadened in the process. The temperature-dependent variations of the energies of A and B excitons for the undoped and Re-doped MoS2 are shown in Fig. 5. The dashed curves in Fig. 5(a) are the least-squares fits to the Varshni-type equation:32)
are the energy and broadening parameters of the interband excitonic transitions, respectively. For the first derivative functional form, the exponential term 2.0 is appropriate for the bound states, such as excitons or impurity transitions.19) The fits yield the parameters Ai, Ei, and Γi. The obtained values of Ei are indicated as arrows and denoted as A1, A2, and B. The nomenclature was commonly used previously by Wilson and Yoffe,1) Beal et al.,25) and Fortin and Raga.3) The fitted values of Ei are listed in Table I together with some of the values from previous works.3,25–28) The energies of prominent A and B excitons of 3R are observed to be lower than those of the 2H undoped MoS2. The energy separations between A and B excitons (ΔBA = EB − EA) are 152 ± 5 meV for Re-doped MoS2 and 212 ± 8 meV for undoped MoS2. The Re incorporation can reduce the energy separation between A and B excitons. The linewidth of the exciton B is much broader than that of exciton A. The A exciton originates from the valence-band top whereas the B exciton comes from the valence-band splitting. In general, the Rydberg series of the A and B excitons of undoped MoS2 can be described by three-dimensional Mott–Wannier excitons,25,26,29) as En = E∞ − Rn−2, where n = 1, 2, 3, ..., and E∞ and R are the critical and binding energies, respectively. For Re-doped MoS2, only n = 1 Rydberg series for both excitonic transitions are observed. It has been shown that excitons for the 3R MoS2 are more appropriately described by the two-dimensional Mott–Wannier excitons.25,26,29) The absence of higher-order series is an inherent nature of the two-dimensional Mott–Wannier excitons.26) From more recent theoretical and experimental studies,30,31) the A and B excitons are attributed to the smallest direct transitions at the K point of the Brillouin zone split by interlayer interaction and spin–orbit splitting. The A exciton belongs to K4 to K5 optical transition whereas the B exciton corresponds to K1 to K5 optical transition. The K states have been shown to be predominantly determined by the metal d states with a small contribution from the non-metal p states.30,31) The energies and linewidths of A and B excitons can be determined accurately by using the fits of Eq. (1). When the temperature increased, both excitonic transitions showed energy reduction which is a general semiconductor behavior. The linewidths also become broadened in the process. The temperature-dependent variations of the energies of A and B excitons for the undoped and Re-doped MoS2 are shown in Fig. 5. The dashed curves in Fig. 5(a) are the least-squares fits to the Varshni-type equation:32)
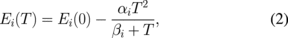
where i = A or B, Ei(0) is the transition energy at 0 K, and αi and βi are the Varshni coefficients. The constant αi is related to the electron (exciton)–phonon interaction and βi is closely related to the Debye temperature. For the undoped sample, the fitted values for exciton A are E(0) = 1.935 ± 0.005 eV, α = 0.47 ± 0.05 meV K−1, and β = 148 ± 45 K; and for exciton B, they are E(0) = 2.151 ± 0.005 eV, α = 0.46 ± 0.05 meV K−1 and β = 188 ± 45 K, whereas for the Re-doped MoS2, the fitted values for exciton A are E(0) = 1.917 ± 0.005 eV, α = 0.43 ± 0.05 meV K−1, and β = 170 ± 45 K; and for exciton B they are E(0) = 2.071 ± 0.005 eV, α = 0.43 ± 0.05 meV K−1, and β = 185 ± 45 K.
Download figure:
Standard image High-resolution imageFig. 4. Piezoreflectance spectra of (a) undoped and (b) Re-doped MoS2 at several temperatures between 25 and 300 K. The dashed curves are the experimental results, and the solid curves are the least-squares fits of Eq. (1).
Download figure:
Standard image High-resolution imageTable I. Energies of excitons A and B for the undoped and Re-doped MoS2. The corresponding values in previous reports are included.
| Material | 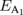 (eV) (eV) |
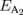 (eV) (eV) |
EB (eV) | Temperature (K) |
|---|---|---|---|---|
| Undoped MoS2a) | 1.932 ± 0.005 | 1.970 ± 0.005 | 2.148 ± 0.008 | 25 |
| 1.927 ± 0.005 | 1.966 ± 0.005 | 2.144 ± 0.008 | 77 | |
| 1.853 ± 0.008 | 1.903 ± 0.008 | 2.070 ± 0.008 | 300 | |
| Re-doped MoS2a) | 1.910 ± 0.005 | 2.068 ± 0.008 | 25 | |
| 1.905 ± 0.005 | 2.062 ± 0.008 | 77 | ||
| 1.846 ± 0.008 | 2.004 ± 0.008 | 300 | ||
| Re-doped WS2b) | 2.039 ± 0.002 | 2.454 ± 0.003 | 15 | |
| 1.958 ± 0.005 | 2.340 ± 0.008 | 300 | ||
| Undoped MoS2c) | 1.929 ± 0.005 | 2.136 ± 0.008 | 25 | |
| 1.845 ± 0.008 | 2.053 ± 0.010 | 300 | ||
| MoS2d) | 1.88 | 2.06 | 300 | |
| MoS2e) | 1.9255 | 2.137 | 4.2 | |
| MoS2f) | 1.92 | 2.124 | 4.2 | |
| 2H-MoS2g) | 1.910 | 2.112 | 5 | |
| 3R-MoS2g) | 1.908 | 2.057 | 5 |
a) Present work. b) Ref. 28 (PzR). c) Ref. 27 (PzR).
d) Ref. 26 (reflectance). e) Ref. 3 (WMR). f) Ref. 3 (photoconductivity).
g) Ref. 25 (transmission).
Fig. 5. Temperature-dependent variations of the energies of the A–B excitonic pair for undoped and Re-doped MoS2. Representative error bars are shown. The dashed and solid curves are least-squares fits to Eqs. (2) and (3), respectively.
Download figure:
Standard image High-resolution imageThe temperature dependence of excitonic transition energies EA(T) and EB(T) of the undoped and Re-doped MoS2 can also be analyzed (solid curves in Fig. 5) by using another expression containing the Bose–Einstein occupation factor for phonons33,34)
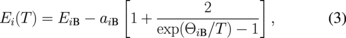
where i = A or B, aiB represents the strength of the electron (exciton)–phonon interaction, and ΘiB corresponds to the average phonon temperature. For the undoped sample, the fitted values for exciton A are EAB = 1.932 ± 0.01 eV, aAB = 40 ± 15 meV, and ΘAB = 180 ± 65 K; and for exciton B, they are EBB = 2.148 ± 0.01 eV, aBB = 41 ± 15 meV, and ΘBB = 190 ± 65 K, whereas for the Re-doped samples, the fitted values for exciton A are EAB = 1.905 ± 0.01 eV, aAB = 42 ± 15 meV, and ΘAB = 220 ± 65 K; and for exciton B EBB = 2.07 ± 0.01 eV, aBB = 40 ± 15 meV, and ΘBB = 205 ± 65 K. The obtained values are typical of the layered-type transition metal dichalcogenides.27,35–37) The line-width broadening parameter of the A and B excitons can be analyzed by33,34)
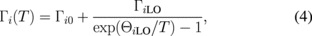
where i = A or B. The first term represents the broadening induced by temperature-independent mechanisms, such as impurity, dislocation, electron interaction, and Auger processes, whereas the second term is caused by the Fröhlich interaction. The quantity ΓiLO represents the strength of the electron (exciton)–LO phonon coupling, whereas ΘiLO is the LO phonon temperature. The solid curves in Fig. 6 represent least-squares fits to Eq. (4), which determine the values of Γi0, ΓiLO, and ΘiLO for the excitonic transitions. For the undoped MoS2, the fitted values for exciton A are Γ0 = 18.3 ± 1.0 meV, ΓLO = 77 ± 20 meV K−1, and ΘLO = 562 ± 50 K; and for exciton B they are Γ0 = 37.6 ± 2.0 meV, ΓLO = 76 ± 30 meV K−1, and ΘLO = 562 ± 50 K, whereas for the Re-doped MoS2, the fitted values for exciton A are Γ0 = 16.2 ± 1.0 meV, ΓLO = 83 ± 20 meV K−1, and ΘLO = 515 ± 50 K; and for exciton B they are Γ0 = 38.5 ± 2.0 meV, ΓLO = 88 ± 30 meV K−1, and ΘLO = 522 ± 50 K. These values are typical and similar to all of the layered structure transition metal dichalcogenides.27,35–37)
Fig. 6. Temperature-dependent variations of the broadening parameters of A and B excitonic transitions for undoped and Re-doped MoS2. Representative error bars are shown. The solid curves are the least-squares fits to Eq. (4).
Download figure:
Standard image High-resolution imageFigure 7 shows the polarized and unpolarized EER spectra of undoped and Re-doped MoS2 measured from the van der Waals plane or edge plane in the energy range of 1.75 to 2.25 eV. The EER spectrum of the undoped MoS2 is displayed on the bottom of Fig. 7. Two bands, EA1 = 1.881 eV and EA2 = 1.925 eV, were detected for exciton A. The direct band gap Eg = 1.94 eV and the exciton binding energy R = 58.7 meV can be estimated from the A exciton series.25,26) These values are similar to those in Ref. 25. The features of ARA = 1.986 eV and ARB = 2.15 eV are identified to be those of the antiresonance structures.25) For exciton B, the EER transition feature is much broadened and the energy is approximately 2.07 eV.
Fig. 7. Polarization-dependent EER spectra of undoped and Re-doped MoS2 over the range of 1.75 to 2.25 eV. The dashed curves are the experimental results, and the solid curves are the least-squares fits of Eq. (1).
Download figure:
Standard image High-resolution imageThe top four curves in Fig. 7 are the EER spectra of the 1% Re-doped MoS2 sample obtained on the edge plane [(1) E ∥ c and k ⊥ c, (2) E ⊥ c and k ⊥ c, and (3) unpolarized and k ⊥ c, from top] and on the basal plane [i.e., (4) unpolarized and k ∥ c spectrum]. The higher bands (i.e., A2, ARA, and ARB observed in undoped MoS2) in all the spectra of the Re-doped MoS2 are absent. The energies of excitons A and B showed a decrease when the Re element was incorporated into the MoS2. In particular, the reduction of ∼65 meV for exciton B with the k ∥ c configuration in Re-doped and undoped MoS2 is identical to the separation of the feature labeled B* from exciton B of the WMR measurement in Ref. 3 where the same k ∥ c configuration is employed. The presence of B* is attributed to the coexistence of 3R and 2H phases3,15) in the synthetic crystals. In the E ∥ c and k ⊥ c spectrum, we have additionally detected one distinct structure of unknown origin located between excitons A and B at 1.906 eV. It is possibly closely related to the antiresonance feature such as ARA that has been detected in the undoped MoS2. In addition, the undoped and Re-doped MoS2 in Fig. 7 also show that excitons A and B are respectively lowered by 27 and 65 meV for the Re-doped MoS2. This is due to the presence of the rhenium impurity altering the original crystal symmetry of MoS2. During the crystal growth of Re–MoS2, rhenium ions can substitute Mo atoms in the MoS2 lattice.15) Consequently (by the interlayer interactions), the excitonic transitions with A–B energy separation of the spin orbit doublet can reduce from ∼210 meV for undoped MoS2 (i.e., 2H) to approximately 158 meV for the Re-doped MoS2 (i.e., 3R). As shown in Fig. 7, the unpolarized spectra of k ∥ c and k ⊥ c configurations for Re-doped MoS2 show that the measured energy difference between excitons A and B is identical (∼158 meV) but the energy shift is about 21 meV. The ∼21 meV energy shift is attributed to the crystal anisotropy.38,39) The appearance of the measured crystal anisotropy for the Re-doped MoS2 [i.e., measurements on the basal (k ∥ c) and edge (k ⊥ c) planes] is due to the fact that the incorporation of Re ions not only transforms the stacking structure from 2H to 3R but also pushes the layered sample to become thicker to form a large area of the edge (side) plane available for EER measurement. This evidence also supports the dopant effect of the Re ions in MoS2 as previously described in STEM. Besides, the top two spectra in Fig. 7 from the edge plane (i.e., k ⊥ c) of E ∥ c and E ⊥ c polarizations showed A1 = 1.838 and 1.833 eV, respectively. The broadened B exciton shows insensitivity (no energy shift) to the polarizations. A measured shift of 5 meV for A1 can be consistently obtained by careful repetition of the measurements by E ∥ c and E ⊥ c operations on the edge plane. The unpolarized spectrum measured from the edge plane (i.e., unpolarized and k ⊥ c) can be regarded as a random superposition of the two spectra, whose transition energy is determined to be A1 = 1.836 eV in Fig. 7. The intralayer bonding of MoS2 is partially ionic and partially covalent with the latter being dominant in the crystal.40,41) The presence of Re atoms in the MoS2 lattice enhances the ionicity of the metal–chalcogen bonding and also increases the lattice polarizability40) in the Re-doped MoS2. From the top-two EER spectra in Fig. 7, the E ⊥ c spectrum demonstrates a more pronounced lattice field than that of the E ∥ c spectrum to render a shift of 5 meV in exciton A. From all of the optical results of the EER measurements, the Re dopant surely substitutes the Mo atom in the Re–MoS2 lattice. By the Re incorporation, the stacking structure of MoS2 has thus been changed (2H → 3R), and the crystal polarizability and lattice iconicity have therefore been reconstructed.
4. Conclusions
We have demonstrated the synthesis of single crystals of undoped and Re-doped MoS2 by the CVT method using Br2 as a transport agent. XRD revealed the 3R structure of the Re-doped compound and 2H of the undoped one. Re atoms tend to occupy or substitute Mo atoms in the host MoS2 lattice. Optical spectra were recorded to clarify the spectral features near the direct band-edge excitonic transitions and showed a splitting of approximately 150 meV between the A and B excitons for the 3R compound. The corresponding splitting is measured to be 200 meV for the 2H compound. The temperature dependence of the broadening function has also been interpreted in terms of a Bose–Einstein equation that contains the electron (exciton)–LO phonon coupling ΓLO. The Re ions stabilize the formation of 3R-MoS2. Re doping can strongly reduce the splitting between A and B excitons and cause their redshift in relation to the undoped MoS2. The electronic states of the MoS2 crystals are modified and affect the symmetry selection rules of the excitonic transitions.
Acknowledgment
The authors would like to acknowledge the financial support by the National Science Council of Taiwan under Grant No. NSC 100-2112-M-011-001-MY3.









