Abstract
The ability to establish successful and efficient extracellular electron transfer (EET) between bacteria and electrode surfaces is critical for the development of mediated microbial electrochemical technologies. Here, we describe a phenazine-based mediator system to facilitate electron transfer from the model bacterium Escherichia coli during glucose metabolism. Phenazine redox mediators were experimentally evaluated, demonstrating distinct mediated currents, dependent on mediator structure. Our results show that the choice of a mediator with the appropriate redox potential is not the single aspect to consider when rationally designing future mediator-based EET systems.
Export citation and abstract BibTeX RIS
The expansion of the global population has led to a continuous increase in energy consumption. Consequently, there is a growing demand for the development and consistent improvement of renewable energy sources to meet global energy demands. 1,2 Bioelectrochemical systems have rapidly emerged as promising, sustainable technologies for energy-efficient wastewater treatment, more sustainable bioremediation, and other potential applications. 2–4 Microbial electrochemical technologies utilize the electrochemical interaction between microorganisms as biocatalysts and conductive electrode surfaces. 5–7 This interaction, most commonly studied on the anode, is driven by the metabolism of abundant organic substances (e.g., sugars and low molecular weight organic acids) via complex oxidation-reduction (redox) reactions during microbial respiration. 8–13 The released electrons from these microbial redox processes are transferred from the microbes to the anode through various mechanisms of extracellular electron transfer (EET), reaching the cathode via an external electric circuit. 14 In this context, establishing an efficient EET between the microorganism and the anode surface plays a fundamental role in improving the power generation and performance of microbial-based bioelectrochemical systems. 15–18 Thus, the development of advanced microbial electrochemical systems requires a more thorough understanding of possible EET mechanisms.
Efficient EET is one of the dominant challenges in the field of bioelectrocatalysis. 15 The EET mechanisms of microorganisms are divided into two main types: (1) direct electron transfer (DET) from membrane-bound redox proteins and (2) indirect, mediated electron transfer (MET) via exogenous or endogenous electron redox shuttles (mediators). In DET, anodes are in physical contact with functional motifs (redox-active proteins) on cellular surfaces, facilitating EET. 14,15,17 The comprehensive mechanisms of protein-protein interactions, the involvement of bacterial nanowires in electron transport, and microbe-electrode communication remain highly discussed topics. 15,16,18 However, with the exception of a few microorganisms, most bacteria do not have redox-active surface proteins to achieve DET. Thus, the majority of microorganisms require the use of redox-active mediator systems to facilitate and/or increase electron transfer. 17,19 Particularly, non-electrogenic microbes (e.g., those that do not readily carry out EET) can be encouraged to chemically interact with an electrode via the addition of soluble, diffusive electron shuttles, known as mediators. 12,17,20,21 A suitable electron shuttle for bacterial-based bioelectrochemical systems should be (1) soluble, (2) environmentally friendly, (3) biologically compatible, (4) stable and reusable, and (5) should have a suitable redox potential. (3) Redox mediators, such as quinones, 20 flavins, 22 and phenazines, 23,24 have a significant role in bioenergy metabolism and are recognized for their ability to enhance electron transfer to anode surfaces. 17
The majority of microbial MET-based bioelectrochemical systems have focused on studying and utilizing well-characterized electrogenic microorganisms, such as Shewanella oneidensis and Pseudomonas aeruginosa, which produce endogenous electron shuttles. While S. oneidensis naturally secretes yellow-pigmented flavin molecules, 22 P. aeruginosa self-produces nitrogen-containing heterocyclic phenazine metabolites, 10,23 which can facilitate electron transfer across cell membranes. 25 Redox mediators can also be added exogenously to enhance the performance of microbial bioelectrochemical systems. 24 For instance, Rabaey et al. have reported the utilization of phenazine-producing P. aeruginosa and exogenous addition of phenazines to improve electron transfer rates in microbial fuel cells. 24 However, the complex regulatory network of the P. aeruginosa phenazine biosynthesis and highly pathogenic nature of P. aeruginosa strains limit the practical application of phenazine mediated microbial bioelectrochemical systems with this microorganism as the host. 26,27 On the other hand, a large number of microorganisms are considered electrochemically inactive as they do not have a fully functional EET system. Such bacterial strains have not been shown to secrete endogenous electron carriers. 5,28 Non-electroactive microorganism strains, including Escherichia coli, Actinobacillus succinogenes, 29 and Clostridium 30 can utilize electron shuttles to achieve electron transfer to anodes. The Gram-negative and versatile model microorganism E. coli has been used in microbial bioelectrochemical systems. 15,31–34 Recognized for its expansive synthetic biology toolkit for bioelectrochemical purposes, E. coli offers various advantages, such as fast growth rates, easy access and culturing methods, as well as the ability to metabolize several different substrates (e.g., glucose, lactose, sucrose, xylose). 35 Additionally, as a non-pathogenic microbe, E. coli has emerged as a versatile host for bioelectrochemical applications. 15 The respiratory metabolism and activity of E. coli can be used in bioelectrochemical applications by supplying exogenous redox mediators to the medium, which also provides insight into the electron transfer mechanisms from E. coli. Previous studies have shown the use of thionine, 2-hydroxy-1,4-naphthoquinone (HNQ), and neutral red as exogenous electron mediators for E. coli-based bioelectrochemical systems. 17,19,36–38 However, detailed information on EET in E. coli with electrode surfaces and its interaction with redox mediators remains unclear. Thus, understanding the properties of mediator systems that facilitate efficient EET from E. coli to anodes provides a means for a unique cell design in genetically engineering E. coli for (1) improving the performance of microbial-based bioelectrochemical systems, (2) metabolizing new substrates for bioremediation, and (3) the synthesis of value-added products.
Herein, we report an experimental electrochemical evaluation of different, soluble phenazines as exogenous redox mediators that control EET in the model microorganism E. coli, providing pivotal understanding for rationally designing mediated microbial electrochemical systems with enhanced performances. This study investigates the utilization of phenazine-based soluble electron mediators to facilitate electron transfer between E. coli cells and carbon-based electrodes during respiration metabolism, with glucose as the substrate. Experimental data for the generation of mediated current were obtained for nine commercially-available phenazines mediators (Scheme 1, SI): (1) neutral red (NR), (2) pyocyanin (PYO), (3) phenazine-1-carboxamide (PCX), (4) phenazine (PHZ), (5) 1-hydroxyphenazine (OHPHZ), (6) phenazine methosulfate (PMS), (7) phenazine ethosulfate (PES), (8) 1-methoxy-5-methylphenazinium methyl sulfate (MPMS), and (9) benzo(A)phenazine-7,12-dioxide (BAPD). Additionally, as part of the phenazine biosynthetic pathway, P. aeruginosa produces redox-active phenazine metabolites, which can have toxic effects on other competing microbes. 39 Therefore, we examined the mediator cytotoxicity by monitoring the E. coli bacterial growth rates in the presence of phenazine mediators at different concentrations.
Experimental
General
All reagents and chemicals were used as received. D-glucose (glucose), sodium bicarbonate (NaHCO3), magnesium chloride (MgCl2), and lysogeny broth (LB) LB agar were purchased from Fisher Scientific Inc. Sodium phosphate monobasic (NaH2PO4), sodium chloride (NaCl), bovine serum albumin (BSA), Tris hydrochloride (Tris-HCl), imidazole, lysozyme from egg white (≥40,000 units mg−1), yeast extract, and 3-(N-morpholino)propane sulfonic acid (MOPS) were obtained from Sigma-Aldrich Co. Pyocyanin, 1-hydroxyphenazine, 1-methoxy-5-methylphenazinium methyl sulfate, and benzo(A)phenazine-7,12-dioxide were acquired from Cayman Chemical Company. Neutral red, phenazine, phenazine methosulfate, phenazine ethosulfate, and phenazine-1-carboxamide were purchased from Sigma-Aldrich Co.
Mediator preparation
Nine phenazine-based redox mediators were used in this study with different concentrations ranging from 25 to 200 μM. Specifically, neutral red (NR), pyocyanin (PYO), phenazine-1-carboxamide (PCX), phenazine (PHZ), 1-hydroxyphenazine (OHPHZ), phenazine methosulfate (PMS), phenazine ethosulfate (PES), 1-methoxy-5-methylphenazinium methyl sulfate (MPMS), and benzo(A)phenazine-7,12-dioxide (BAPD) were utilized to examine the generation of mediated currents. Stock solutions for each mediator with a concentration of 2 mM were prepared in the MOPS buffer (20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 100 mM glucose). For NR, PYO, OHPHZ, PMS, PES, MPMS, and BAPD, 20% ethanol was added to the 2 mM stock solution to solubilize the compounds, giving a final ethanol concentration below 0.1% in the electrolyte solution. For PHZ and PCX, 20% sulfuric acid was added to the 2 mM stock solution to solubilize the compounds, giving a final ethanol concentration below 0.1% in the electrolyte solution. The 2 mM stock solution for each mediator was then diluted to make a 500 μM stock solution in the MOPS buffer. From the 500 μM stock solution, respective mediator solutions were prepared to have the following concentrations: 25, 50, 75, 100, 150, and 200 μM, in the MOPS buffer.
Cell culture and bacteria growth
Escherichia coli strain BL21(DE3) was obtained from New England BioLabs, Ipswich, MA. E. coli bacterial cells were cultured from freezer stock on LB agar plates. Using E. coli initially grown on LB agar plates, overnight liquid cultures were prepared by inoculating 250 mL cell culture flasks filled to a volume of 150 mL with liquid broth growth medium of the following composition: 10 g L−1 glucose, 5 g L−1 yeast extract, 8.5 g L−1 NaH2PO4 and 10 g L−1 NaHCO3 in 1 L of Milli-Q water. Glucose was added to the growth medium after sterilization at 125 °C for 25 min. Bacterial cells were grown in a 37 °C incubator for 18 h with a measured optical density at 600 nm (OD600) of 2. After this 18-h bacterial culture growth, the E. coli cells were collected by centrifugation at 5000× g (Allegra X-15R Benchtop Centrifuge, Beckman Coulter) for 20 min. In the following washing step, the bacterial cells were resuspended in 1 mL of 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 100 mM glucose and further concentrated by centrifugation at 15,000 rpm (Eppendorf Centrifuge 5424 R) for 10 min. In the final step, a solution with a bacterial cell concentration of 1 g mL−1 of E. coli was prepared using 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 100 mM glucose. Only for the amperometric i-t experiments to monitor the glucose-oxidizing activity of E. coli with phenazine mediators, the E. coli cells were resuspended as outlined in steps above in a 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 0 mM glucose solution.
Preparation of heat-killed and lysed E. coli
Control amperometric i-t experiments to monitor the glucose-oxidizing activity with phenazine mediators were performed using AvCarb carbon paper electrodes with immobilized heat-killed E. coli and lysed E. coli cells. For the experiments with heat-killed E. coli, 18-h liquid bacterial cultures were autoclaved at 121 °C and 15 psi. For the experiments with lysed E. coli cells, cell lysis was performed using a general bacterial lysate protocol. Specifically, E. coli cells were harvested from a bacterial culture grown at 37 °C for 18 h by centrifugation at 6,000 rpm for 10 min and the resulting bacterial cell pellet was stored at –80 °C. The pellet was then resuspended in 2 mL of Millipore water in a clean tube and lysozyme was added. The mixture was incubated on ice for 30 min, followed by 30 min at 30 °C. The resulting viscous mixture was then sonicated using three 10-second fragments at high intensity, allowing the mixture to cool down for 30 s on ice between each burst. Using a lysis buffer of 100 mM NaCl + 25 mM Tris-HCl + 10 mM imidazole (pH 8), the lysate was diluted to ∼50 mL and centrifuged at 35,000 rpm for 30 min at 4 °C to pellet the cellular debris in the lysate. Both the heat-killed cells and the lysed bacteria were collected via the series of centrifugation and washing steps described above in order to prepare a solution of 1 g mL−1 concentration with either heat-killed or lysed E. coli cells in 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 0 mM glucose.
Electrode preparation and cell immobilization
The general cell immobilization on the working electrodes was performed using steps previously established and reported by our research group. 20,40,41 The working electrodes used in this study were obtained by cutting AvCarb carbon paper electrodes (AvCarb MGL 190, Fuel Cell Store) to have an area of 1 cm2. The electrodes were sterilized via exposure to UV-light. Using the prepared 1 g mL−1 bacterial solution, 30 μL bacterial cells were deposited on each electrode surface, resulting in a cell density of 30 mg cm−2. The solution on the electrodes was allowed to dry under N2 gas atmosphere for 1 h. The dry AvCarb carbon paper electrodes with immobilized E. coli were stored under N2 gas until use in electrochemical experiments. Control amperometric i–t tests were performed to monitor the glucose-oxidizing activity with phenazine mediators on AvCarb carbon paper electrodes with bovine serum albumin (BSA), immobilized heat-killed E. coli, and immobilized lysed E. coli. For BSA carbon electrodes, 30 μL of 10 mg mL−1 BSA in sodium phosphate buffer (pH 7) solution were deposited on carbon electrodes and then dried under N2 for 2 h. For the control experiments with immobilized heat-killed E. coli and lysed E. coli, 30 μL of the above-described 1 g mL−1 solution with either heat-killed cells or lysed cells, were added to sterile carbon paper electrodes. The prepared electrodes were allowed to dry under N2 for 1 h before use in electrochemical control tests.
Electrochemical setup and measurements
All electrochemical measurements were performed using a three-electrode cell system at 20 ± 1 °C. The current generation in E. coli with different phenazine-based soluble mediators was examined by cyclic voltammetry (CV) (CH660 potentiostat, CH Instruments) and amperometric i–t tests (Bio-Logic VSP, BioLogic Sciences Instruments). AvCarb carbon paper electrodes with immobilized E. coli cells on the electrode surface were used as the working electrodes. For control experiments, sterile AvCarb carbon paper electrodes without E. coli cells were used as the working electrodes. A Pt mesh electrode and saturated calomel electrode (SCE) were used as the counter electrode and as the reference electrode, respectively. All the potentials discussed in this paper refer to the SCE reference electrode. The 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 100 mM glucose was used as the electrolyte solution for all electrochemical experiments, glucose being the sole carbon source. The electrolyte solution was used with differing concentrations of all phenazine redox mediators. Only for the amperometric i–t curves to examine the glucose-oxidizing activity of E. coli using phenazine mediators, a 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 0 mM glucose was used as the electrolyte solution and 0.5 M glucose solution was added at various time points during those amperometric experiments. CV tests were performed at a scan rate of 5 mV s−1 in different potential windows depending on the mediator used. Specifically, for PYO, PES, PMS, PCX, PHZ, OHPHZ, and MPMS, the CV potential window used was from 0.1 to –0.6 V vs SCE; CV potential windows from –0.3 to –1.0 V vs SCE and from –0.2 to –0.8 V vs SCE were used for NR and BAPD, respectively. The redox potential for the oxidation of each mediator was determined from the CVs. Amperometric i–t measurements were performed for 900 s at a constant potential by applying an anodic overpotential of 50 mV (η) for each phenazine redox mediator. The current was evaluated and calculated at the end of the 900 s amperometric i–t tests. For the amperometric detection of glucose-oxidizing activity of E. coli with phenazine mediators (here, 100 μM NR), i–t tests were performed for 3000 s and 0.5 M glucose was added at 500, 750, 1000, and 1500 s while stirring the solution. All solutions were deaerated with N2 gas.
Calculation of mediated current densities
The mediated current densities were calculated by subtracting the current obtained with E. coli cells immobilized on the AvCarb carbon paper electrode from the current obtained with sterile AvCarb carbon paper electrode (without immobilized bacterial cells as a control experiment) at the end of the amperometric i–t test (900 s) (Fig. S3 available online at stacks.iop.org/JES/168/025503/mmedia of the Supplementary Information, SI). All of the amperometric experiments, with and without immobilized bacterial cells, were performed a total of five times. The average value of mediated current was calculated, and the standard deviation was used to calculate the error.
Cytotoxicity studies using 96-well plate optical density measurements
The cytotoxicity assays were performed in a 96-well plate reader (BioTek) using polystyrene 96-well plates (Thermo Fisher Scientific). The plates were used with a working volume of 200 μL and run for 20 h while holding a temperature of 37 °C with constant slow orbital shaking. Optical density measurements at 600 nm (OD600) were obtained by taking absorbance readings every 10 min. Each phenazine mediator was added at 25, 100, or 200 μM final concentration from a 4 mM working stock solution. Each bacteria-containing well was inoculated using 20 μL (10% inoculation) of an E. coli growth at OD600. Each plate contained a negative control containing only media to ensure sterile conditions throughout the experiments as well as controls including only the phenazine mediators at the corresponding concentrations with no bacterial cells to ensure these compounds were not contributing to the absorbances at 600 nm throughout the experiments. The data were plotted and analyzed using Excel and OriginLab Graphing and Analysis software.
Results and Discussion
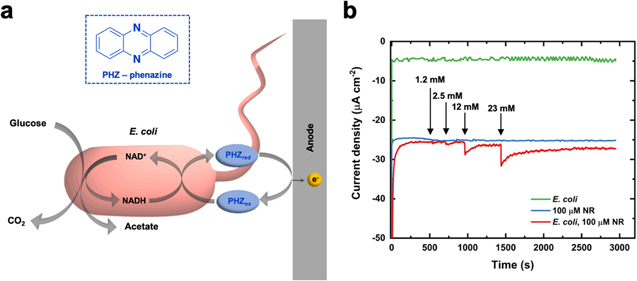
To achieve efficient extracellular electron transfer, specific features of electron transport chains of E. coli as the microbial catalytic host need to be considered. 42 Glucose oxidation in E. coli (Fig. 1a) is achieved through glycolysis and cofactor recycling reactions, resulting in the generation of NADH. 43 For an electron transfer from an E. coli microbial electron carrier to an electrode to occur, an exogenous redox mediator is required. To achieve the conversion of metabolic reducing power into electricity, an electron mediator should (1) have a high negative redox potential of a significant magnitude (E0' for NADH –0.32 V vs SHE) to link to the NADH metabolism, (2) be a reversible redox couple, stable in both the oxidized and reduced forms, on the anode surface, and (3) should not decompose during long-term redox cycling. Finally, the mediator should be soluble in aqueous media (pH 7) and have the ability to pass through the microbial cytoplasmic membrane. For monitoring of the glucose-oxidizing activity of E. coli, the cells were grown in minimal glucose media and were then resuspended in a buffer without glucose before immobilization on electrodes. The redox potential for the oxidation reaction was determined via cyclic voltammetry for NR (Fig. S1, SI) and a 50 mV overpotential was applied for the amperometric i–t tests. The current was measured at a constant potential of –0.5 V vs SCE using immobilized E. coli on AvCarb carbon paper electrodes immersed in a buffer solution of 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 0 mM glucose, with 100 μM NR (Fig. 1b). During these amperometric i–t tests, varying volumes of 0.5 M glucose solution were added to the buffer at 500, 750, 1000, and 1500 s, to give final glucose concentrations of 1.2 mM, 2.5 mM, 12 mM, and 23 mM, respectively. Using a carbon paper electrode with immobilized E. coli cells and 100 μM NR solution, the current was monitored over time. The current-time response in Fig. 1b (red curve) shows that the addition of glucose results in anodic currents. Control experiments were performed using (1) sterile carbon paper electrodes (without E. coli cells immobilized) and a 100 μM NR solution (blue curve) and (2) carbon paper electrodes with immobilized E. coli and a mediator-less buffer (green curve). The control experiments did not result in the generation of anodic currents with the addition of glucose at 500, 750, 1000, and 1500 s. These results indicate that NR (and other phenazines) can act as an electron acceptor in the bacterial oxidation of glucose and as a redox mediator between E. coli and carbon electrodes. Amperometric detection measurements of substrate-oxidizing activity in Fig. 1b were also performed using (1) BSA, (2) heat-killed E. coli, and (3) lysed E. coli cells immobilized on AvCarb carbon paper electrodes (Fig. S2, SI) as controls. The resulting amperometric i–t curves in Fig. S2 did not result in the generation of anodic currents with the addition of glucose at 500, 750, 1000, and 1500 s, which indicate that viable E. coli cells perform the glucose oxidation.
Figure 1. (a) Schematic of E. coli glucose metabolism with phenazine (PHZ) as an electron mediator. (b) Amperometric detection of substrate-oxidizing activity of E. coli using neutral red (NR) as the electron mediator. The amperometric current was measured as a function of time with immobilized E. coli on carbon paper electrodes immersed in a buffer solution of 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 0 mM glucose, with 100 μM NR. 0.5 M glucose was added to the buffer at 500, 750, 1000, and 1500 s. The amperometric i-t curve shows that the addition of glucose to the buffer results in anodic currents (red). Control experiments were performed using sterile carbon paper electrodes (without E. coli) and 100 μM NR (blue) and carbon paper electrodes with immobilized E. coli and a buffer without a mediator (green). No anodic currents were observed with the addition of glucose at 500, 750, 1000, and 1500 s. To verify that viable E. coli cells perform the glucose oxidation, additional control experiments were performed by recording amperometric i-t current responses using carbon paper electrodes with bovine serum albumin (BSA), immobilized heat-killed E. coli cells, and immobilized lysed E. coli cells (Fig. S2, SI).
Download figure:
Standard image High-resolution imageCyclic voltammetry (CV) measurements were used to analyze the mediated bioelectrocatalytic currents. Figure 2 shows representative CV current-potential response obtained using PYO as a soluble redox mediator and E. coli immobilized on AvCarb carbon paper electrodes (red curve). The current-potential CV response was also obtained in the presence of a phenazine mediator with sterile carbon paper electrodes, without E. coli (blue curve), which shows a distinct difference, confirming the mediated anodic current. As a control study, the probability of current generation in the absence of exogenous mediators was evaluated using CV and amperometric i–t measurements in the absence of any exogenous phenazine mediator in solution. As shown in Fig. 2, no current was observed using immobilized E. coli cells on carbon electrodes without any soluble mediator (grey curve). Additionally, CV current response was not obtained in the absence of a mediator with sterile carbon paper electrodes, without E. coli cells (black curve). Representative CVs for the other eight phenazine mediators are displayed in Fig. S1, SI. It is interesting to remark the significant current responses are observed for the reduction reaction, as seen on the characteristic voltammetric data. While the solutions were purged with N2 during the duration of the experiments, it cannot be excluded that the obtained reduction current responses are related to O2 reduction and/or a specific metabolism taking place. These compelling aspects of the CV results are a subject of investigation for future studies.
Figure 2. Representative cyclic voltammograms with 100 μM pyocyanin (PYO) as a soluble redox mediator and E. coli immobilized on carbon paper electrodes (red). Control experiments were performed using (1) sterile carbon paper electrodes (without E. coli) with PYO as a soluble mediator (blue), (2) E. coli immobilized on carbon paper electrodes in the absence of PYO (grey), and (3) sterile carbon paper electrodes (without E. coli) in the absence of PYO (black). Scan rate: 5 mV s−1. Buffer: 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 100 mM glucose.
Download figure:
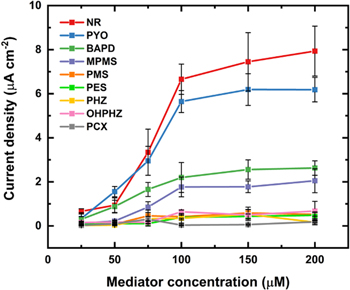
Standard image High-resolution imageThe cyclic voltammetry data was used to determine the redox potential for the oxidation reaction for all phenazine mediators under examination, and an anodic overpotential (η) of 50 mV for each phenazine mediator was applied for amperometric i–t tests. The amperometric measurements were performed with several independent replicate measurements (n ≥ 5) using both carbon paper electrodes with immobilized E. coli cells and sterile carbon paper electrodes (no E. coli as control experiments) with varying redox mediator concentrations in the 25–200 μM range. Figure 3 shows characteristic amperometric i–t tests obtained with sterile carbon paper electrodes (Fig. 3a) and carbon paper electrodes with immobilized E. coli (Fig. 3b) with six different PYO concentrations. Representative amperometric i–t curves for the other phenazine mediators are depicted in Figs. S4 and S5 (SI). The mediated currents generated for different concentrations of each mediator were calculated by subtracting the current response obtained with immobilized E. coli on carbon paper electrode from the current obtained with bare carbon paper electrode at the end of the amperometric i–t test at 900 s (Fig. S3, SI). Figure 4 shows a comparison of the calculated current densities obtained for all nine phenazines as a function of mediator concentrations. The maximum mediated current generation was achieved using 200 μM NR (7.9 ± 0.9 μA cm−2) followed by 200 μM PYO (6.2 ± 0.6 μA cm−2), 200 μM BAPD (2.6 ± 0.3 μA cm−2), 200 μM MPMS (2.0 ± 0.5 μA cm−2), 200 μM OHPHZ (0.7 ± 0.4 μA cm−2), 150 μM PMS (0.6 ± 0.3 μA cm−2), 200 μM PES (0.5 ± 0.1 μA cm−2), 150 μM PHZ (0.5 ± 0.2 μA cm−2), and 200 μM PCX (0.2 ± 0.1 μA cm−2). The correlation between mediator concentrations and mediated current densities achieved from amperometric measurements is summarized in Fig. 4 (Table SI, SI). It is important to note that small variations in the phenazine mediators and their concentrations resulted in noteworthy differences in the mediated current densities. Previous research has demonstrated that in certain cases, the mediator redox potential correlates to increased catalytic current responses. 44 However, our experimental data indicate that this explanation does not describe the catalytic currents observed in this study. As there is no apparent correlation between the redox potential for the different phenazine mediators determined via CV and the obtained mediated currents, these results show that mediated EET is far more complex than just choosing a mediator with an appropriate redox potential.
Figure 3. Representative amperometric i-t tests for (a) sterile carbon paper electrodes and (b) carbon paper electrodes with immobilized E. coli cells with six different phenazine mediator concentrations of pyocyanin (PYO). Buffer: 20 mM MOPS buffer (pH 7) + 10 mM MgCl2 + 100 mM glucose. Amperometry tests were performed at an overpotential of 50 mV for all mediators over the anodic redox peak obtained from cyclic voltammetry. Representative i-t curves for the other mediators are shown in the SI.
Download figure:
Standard image High-resolution imageFigure 4. Comparison of determined mediated current densities at six different mediator concentrations (25, 50, 75, 100, 150, and 200 μM) for NR, PYO, BAPD, MPMS, PMS, PES, PHZ, OHPHZ, and PCX. The current was calculated by subtracting the current obtained with E. coli cells immobilized on carbon paper electrode from the current obtained with sterile carbon paper electrode at the end of the amperometric i-t test. Error bars represent the standard deviation (n = 5).
Download figure:
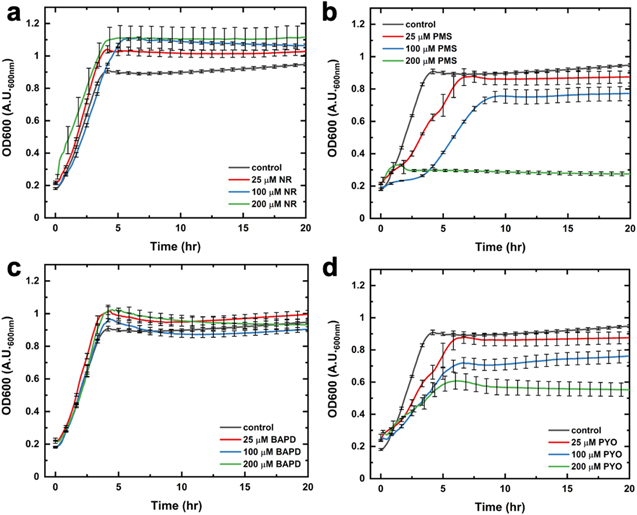
Standard image High-resolution imageThe utilization of exogenous mediators in microbial bioelectrochemical systems has limiting downsides since high mediator concentrations can have toxic effects on bacterial cells. 45,46 Thus, here, cytotoxicity studies were performed to examine the impact of phenazine mediators on the growth rates of E. coli. Namely, we obtained optical density measurements at 600 nm (OD600) for several phenazines at various concentrations (Figs. 5, S6 and S7, SI). Figure 5 shows bacterial growth curves during a 20-h period in the presence of different concentrations for NR, PYO, BAPD, and PMS. While these growth curves have a fairly sigmoidal character, they show significantly different growth rates based on the mediator type. When grown in the presence of NR, E. coli cells display enhanced growth rates at all three mediator concentrations (Fig. 5a). As such, this qualitative data is in agreement with our quantitative electrochemical results showing that the highest mediated currents are achieved with NR as an electron mediator. Furthermore, this data, showing enhanced growth rates with NR, suggests that certain phenazine mediators facilitate biofilm formation 47,48 in E. coli, which needs to be further examined in a future study. On the other hand, phenazine mediators, such as PMS, diminish the bacterial growth rates, as illustrated in Fig. 5b. The slower growth rates suggest toxic effects of PMS on E. coli with increasing mediator concentrations. This quantitative absorbance data corresponds with electrochemical results as mediated current densities are decreased at higher PMS concentrations. Figure 5c shows OD600 growth curves for E. coli in the presence of BAPD. This mediator type does not appear to impact cellular growth, indicative of non-toxic BAPD effects on E. coli cells. Interestingly, the growth measurements for E. coli with PYO (Fig. 5d) reveal that PYO exhibits toxicity effects on the cells over 20 h. These results do not agree with the electrochemical data showing PYO as the second best-performing mediator. However, it is important to note that PYO appears to enhance the E. coli growth rates only during the initial growth stages (0–2 h), as seen in Fig. 5d, which relates to our electrochemical measurements performed for 900 s. In the particular case of using PYO as a phenazine-based redox mediator, previous studies have examined its toxicity under oxic (oxygen-containing) conditions and correlated it to oxygen reactivity. 49 Namely, PYO toxicity has been mainly attributed to its ability to rapidly reduce molecular oxygen, generating superoxide species, which produce hydrogen peroxide via enzymatic or abiotic dismutation redox processes. 50–52 It is also important to note that the E. coli growth curve assays were obtained in the presence of O2 while all solutions were deaerated with N2 gas during electrochemical measurements. Therefore, certain cytotoxicity effects to E. coli cells could be due to phenazine reactivity with molecular oxygen, explaining the observed differences between OD600 and electrochemical results. Future work will investigate these noteworthy aspects by performing experiments over extended periods in both the presence and absence of O2 to understand the impact of phenazine mediators on E. coli growth, metabolism, and biofilm formation.
Figure 5. Optical density measurements at 600 nm (OD600) for E. coli growth over 20 h in the presence of (a) neutral red (NR), (b) phenazine methosulfate (PMS), (c) benzo(A)phenazine-7,12-dioxide (BAPD), and (d) pyocyanin (PYO) at concentrations of 25 μM (red), 100 μM (blue), and 200 μM (green). Control OD600 experiments (grey) were performed with E. coli cells in the absence of phenazine mediators. Error bars correspond to the standard error of the mean (n = 3).
Download figure:
Standard image High-resolution imageIn the rational design of mediated microbial electrochemical systems with biological entities, a deeper understanding of the fundamental bioelectrochemical processes accounting for EET is important. The behavior and interaction of phenazine redox mediators with E. coli need to be further examined. Specifically, the interaction of phenazine mediators with the cell surface and/or the cell periplasm needs to be studied. While the P. aeruginosa phenazine synthetic pathway can be genetically introduced into E. coli cells, the complexity of this pathway limits the ability to access all phenazine metabolites to evaluate the mediated current densities for each mediator. The presented results provide information about which phenazine mediator results in optimal mediated current densities in E. coli glucose metabolism. As such, the data offer a means for a unique approach in genetically engineering specific proteins to optimize the self-production of endogenous phenazine redox mediators in E. coli cells.
Conclusions
In summary, we described an experimental electrochemical characterization of the attributes of nine different soluble phenazine redox mediators that support efficient EET in the model microorganism E. coli consuming glucose. This study shows that the addition of exogenous phenazine redox mediators allows for the monitoring of the E. coli glucose metabolism. Our quantitative, electrochemical results demonstrated a maximum mediated current density of 7.9 ± 0.9 μA cm−2 with NR, followed by 6.2 ± 0.6 μA cm−2 with 200 μM PYO. Cytotoxicity studies showed that the mediator structure and concentration can impact E. coli growth rates, indicating specific toxic effects. The use of NR as a mediator showed improved bacterial growth rates, which correlates with the enhanced current generation. Similarly, the cytotoxicity results for the other phenazine mediators partially agree with the electrochemical data, requiring additional investigation. Notably, our data demonstrate that the potential of the redox mediator is not the single aspect to consider for the rational design of mediator systems for EET. Therefore, a deeper understanding of the fundamental bioelectrochemical mechanisms that govern EET and careful consideration of the electrochemical behaviors of electron mediators are necessary for the development of future mediated microbial-based bioelectrochemical technologies.
Acknowledgments
The authors acknowledge the funding from the NSF grant 1561427 and the Army Research Office MURI grant W911NF1410263. O.S. gratefully acknowledges funding from the ACS Irving S. Sigal Postdoctoral Fellowship.