Abstract
Interesting phenomena were observed during an investigation on the accelerating effects of 3-mercapto-1-propane sulfonic acid (MPSA), i.e., different aging times of MPSA result in different filling profiles. When MPSA was added to the electrolyte immediately before electrodeposition, subconformal deposits appeared, whereas MPSA aged over 12 h enabled superfilling. From UV-visible analysis, over 99% MPSA was converted to bis(3-sulfopropyl)disulfide (SPS) within 12 h through the reaction with  which means that SPS was, in terms of "visible" superfilling, the real accelerator. This arose from the fact that SPS experienced adsorption first, while MPSA underwent
which means that SPS was, in terms of "visible" superfilling, the real accelerator. This arose from the fact that SPS experienced adsorption first, while MPSA underwent  reduction first at the trench entrance. © 2004 The Electrochemical Society. All rights reserved.
reduction first at the trench entrance. © 2004 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Extensive research on Cu superfilling has been carried out using thiol/disulfide-based compounds as accelerators in three-additives systems.1
2
3
4
5
6
7
8
9
10
11 Typical compounds of these substances are 3-mercapto-1-propane sulfonic acid, sodium salt (MPSA,  and bis(3-sulfopropyl)disulfide (SPS,
and bis(3-sulfopropyl)disulfide (SPS,  . So far, studies on MPSA have been focused on the modeling studies4
7
8
9
12 or on verifying the catalytic effects of MPSA.11
13
14
15 MPSA in an electrolyte is not stable and undergoes aging with
. So far, studies on MPSA have been focused on the modeling studies4
7
8
9
12 or on verifying the catalytic effects of MPSA.11
13
14
15 MPSA in an electrolyte is not stable and undergoes aging with  ions to form SPS or its derivatives. Farandon et al.13 proposed reactions among MPSA, SPS, and copper ions. In the authors' previous research,11 oxidative dimerization of MPSA to SPS by
ions to form SPS or its derivatives. Farandon et al.13 proposed reactions among MPSA, SPS, and copper ions. In the authors' previous research,11 oxidative dimerization of MPSA to SPS by  ions has been demonstrated through Raman analysis. At around that time, Frank and Bard14 mentioned the formation of a Cu(I):SPS complex from MPSA during their research on the decomposition of SPS, using extensive analytical techniques. Moffat and his co-workers15 also investigated the similarity in slow sweep voltammetry curves between an overnight-aged MPSA electrolyte and an SPS electrolyte indicating the oxidation of thiol to form disulfide.
ions has been demonstrated through Raman analysis. At around that time, Frank and Bard14 mentioned the formation of a Cu(I):SPS complex from MPSA during their research on the decomposition of SPS, using extensive analytical techniques. Moffat and his co-workers15 also investigated the similarity in slow sweep voltammetry curves between an overnight-aged MPSA electrolyte and an SPS electrolyte indicating the oxidation of thiol to form disulfide.
Despite the many studies on MPSA, a superfilling profile with distinct bumps (although the formation of bumps is not always desirable), which is indicative of bottom-up acceleration, has been hardly observed with MPSA, whereas it has been with SPS. Likewise, from our investigations that are being introduced here, MPSA is incompetent for superfilling, which conflicts with existing studies which dealt with the accelerating role of MPSA. This is not surprising, though, if it is considered that major researches regarding MPSA have focused on modeling or demonstrating its catalytic effect by electrochemical analysis techniques. In addition, the interval between the time of mixing of additives with electrolyte (i.e., mixing of MPSA with  ions) and the time of applying them to electrodeposition has been treated as unimportant. However, the deposition profile may be dependent upon the actual compound of the accelerator, which is the reaction product of MPSA and
ions) and the time of applying them to electrodeposition has been treated as unimportant. However, the deposition profile may be dependent upon the actual compound of the accelerator, which is the reaction product of MPSA and  at a given aging time. Consequently, there is an ample chance that MPSA which is used in existing researches and has achieved a "visible" superfilling may actually be aged MPSA during the custody, that is, SPS.
at a given aging time. Consequently, there is an ample chance that MPSA which is used in existing researches and has achieved a "visible" superfilling may actually be aged MPSA during the custody, that is, SPS.
Of particular interest here, therefore, are the time-dependent aging phenomena of MPSA and corresponding gap-filling profiles when it is used for electrodeposition at different aging times, investigations on which are rare. Differences in reaction mechanisms compared to SPS are also included in the subject on considerations.
Experimental
Two kinds of electrolytes composed of base electrolyte and different additive compositions were used in the experiments, where the base electrolyte consisted of 1 M  0.25 M
0.25 M  and deionized (DI) water. The first electrolyte is made up of a base electrolyte, 100 μM MPSA, 88 μM poly(ethylene glycol) (PEG, Mw 3400), and 1 mM NaCl (for details of the electrolyte composition, see Ref. 2, 5, 6, and 10). This electrolyte was aged over various durations at room temperature with gentle stirring prior to being applied to electrodeposition. 50 μM SPS was added in the second electrolyte in place of MPSA. This electrolyte was used without the aging step. Electrodeposition was performed on a trench-type single damascene (with a linewidth of 500 nm and an aspect ratio of 2.5) Si wafer with a structure of physical vapor deposition (PVD) Cu (70 nm, seed layer)/chemical vapor deposition (CVD) TiN (10 nm, diffusion barrier)/PVD Ti (15 nm, glue layer)/Si. The equipment used in applying the constant potential of
and deionized (DI) water. The first electrolyte is made up of a base electrolyte, 100 μM MPSA, 88 μM poly(ethylene glycol) (PEG, Mw 3400), and 1 mM NaCl (for details of the electrolyte composition, see Ref. 2, 5, 6, and 10). This electrolyte was aged over various durations at room temperature with gentle stirring prior to being applied to electrodeposition. 50 μM SPS was added in the second electrolyte in place of MPSA. This electrolyte was used without the aging step. Electrodeposition was performed on a trench-type single damascene (with a linewidth of 500 nm and an aspect ratio of 2.5) Si wafer with a structure of physical vapor deposition (PVD) Cu (70 nm, seed layer)/chemical vapor deposition (CVD) TiN (10 nm, diffusion barrier)/PVD Ti (15 nm, glue layer)/Si. The equipment used in applying the constant potential of  vs. a saturated calomel electrode (SCE) was a PAR 263 potentiostat (EG&G Princeton Applied Research Corporation), and a Cu bar was used as an anode. After electrodeposition, all samples were rinsed with DI water and dried in an
vs. a saturated calomel electrode (SCE) was a PAR 263 potentiostat (EG&G Princeton Applied Research Corporation), and a Cu bar was used as an anode. After electrodeposition, all samples were rinsed with DI water and dried in an  stream.
stream.
The aging of MPSA in the electrolyte was monitored by UV-visible spectroscopy (UV-160A, Shimadzu, Japan) in the wavelength range of 200-800 nm. A base electrolyte composed of  and
and  was used as a reference solvent to eliminate the peak interferences from sulfate and
was used as a reference solvent to eliminate the peak interferences from sulfate and  ions. Samples were mixtures of a base electrolyte with 10 mM MPSA, and mixtures of a base electrolyte with 5 mM SPS. The scale-up of the additive concentration compared to the actual concentration used in the gap filling was due to the relatively small molar absorptivities of both additives at their intensity maxima
ions. Samples were mixtures of a base electrolyte with 10 mM MPSA, and mixtures of a base electrolyte with 5 mM SPS. The scale-up of the additive concentration compared to the actual concentration used in the gap filling was due to the relatively small molar absorptivities of both additives at their intensity maxima  which were measured from their as-made electrolytes to be 268.6 and
which were measured from their as-made electrolytes to be 268.6 and  respectively. These values correspond to forbidden transition,16 and signal amplification can be achieved with an increase in the concentrations.
respectively. These values correspond to forbidden transition,16 and signal amplification can be achieved with an increase in the concentrations.
Results and Discussion
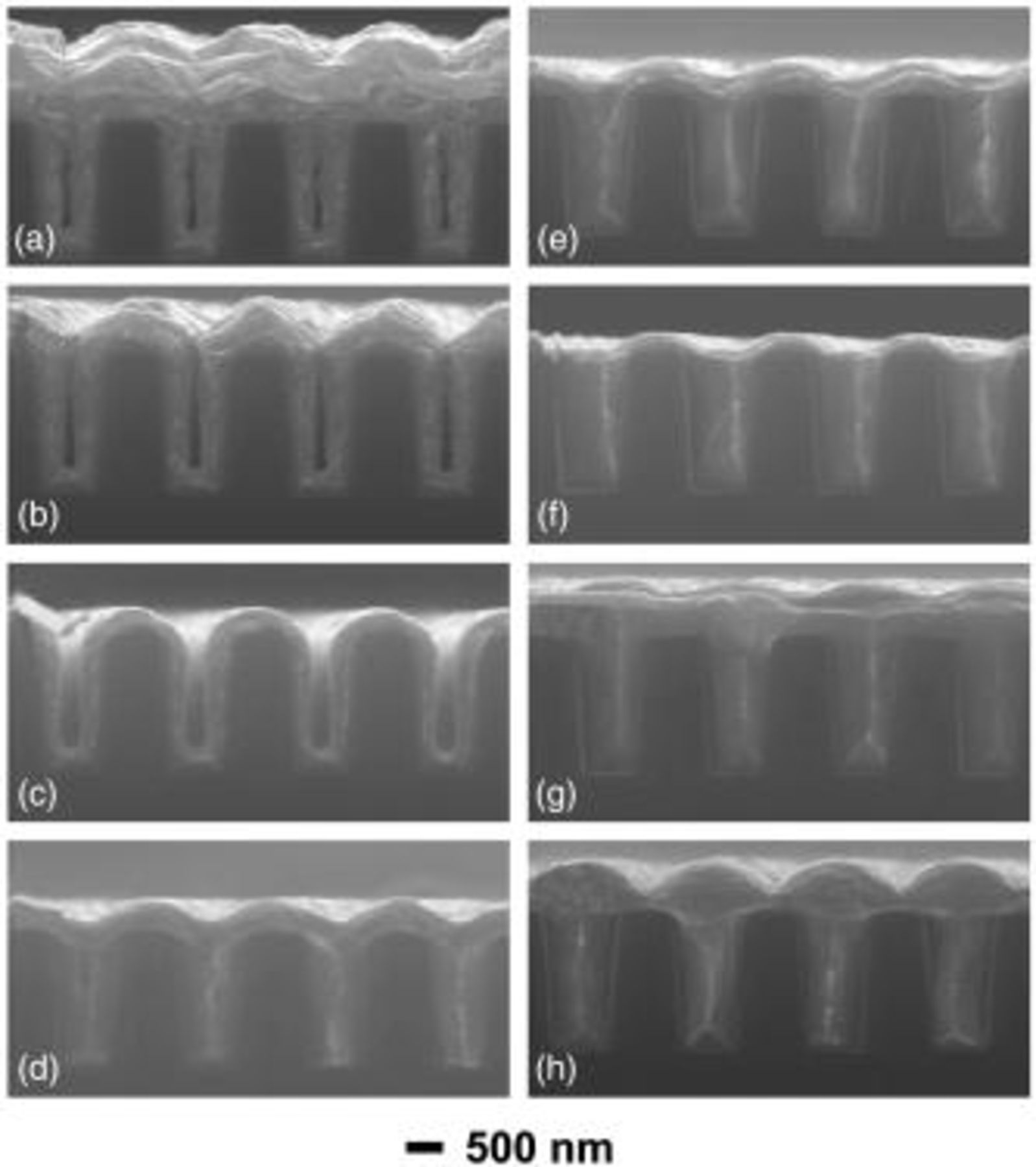
Filling profiles according to the aging time of MPSA were investigated and corresponding field-emission scanning electron microscopy (FESEM) images are presented in Fig. 1. The profiles shown in a-h represent electrodeposits from the electrolyte with three additives aged for 0 s (immediately after mixing), 5 s, 30 min, 3 h, 12 h, 24 h, and 24 h with only an increase in the deposition time, and with three additives including SPS instead of MPSA, respectively. For the as-mixed MPSA shown in Fig. 1a, the electrodeposits exhibited a highly subconformal profile with center voids. The deposits were concentrated on the prominent part (the trench opening) rather than on the recessed area (the trench bottom). Even after a very short aging time, 5 s (Fig. 1b), the deposits showed a transition from being subconformal to somewhat conformal at the sidewall of the trench, still the deposit thickness of the prominence was thicker than that of the recess. This tendency became conspicuous with the increase in the aging time and the deposit with the MPSA aged for 30 min showed a highly conformal contour (Fig. 1c). The transition to a superconformal filling was observed for 3 h aged MPSA (Fig. 1d) and the consummation of the superfilling appeared with the aged MPSA at more than 12 h (Fig. 1e and f). For the deposits with MPSA aged for 24 h, the increase in the deposition time from 300 to 400 s resulted in the formation of bumps, which was indicative of bottom-up acceleration (Fig. 1g). Contrary to MPSA, SPS showed no dependency on the aging time in the filling profile with it and gave a typical contour of superfilling that was similar to that in Fig. 1g, as shown in Fig. 1h. Comparing Fig. 1a, g, and h, deposits with MPSA aged within sufficient time assumed an aspect close to that of SPS rather than to pure (as mixed) MPSA. Collectively, MPSA in the electrolyte underwent a spontaneous reaction to make SPS or its derivatives within several hours. Likewise, the real accelerator that enables Cu superfilling in a damascene structure is aged MPSA (presumably SPS) rather than pure MPSA.
Figure 1. FESEM images of electrodeposited Cu films on damascene structure. Electrolyte for (a-g) was composed of 88 μM PEG, 1 mM NaCl, and 100 μM MPSA and the electrolyte went through an aging step prior to electrodeposition for (a) 0 s, (b) 5 s, (c) 30 min, (d) 3 h, (e) 12 h, (f) 24 h, and (g) 24 h (increase in deposition time). 50 μM SPS was used in (h) instead of 100 μM MPSA. Deposition was carried out at  (vs. SCE) for 400 s for (g) and 300 s for the rest.
(vs. SCE) for 400 s for (g) and 300 s for the rest.
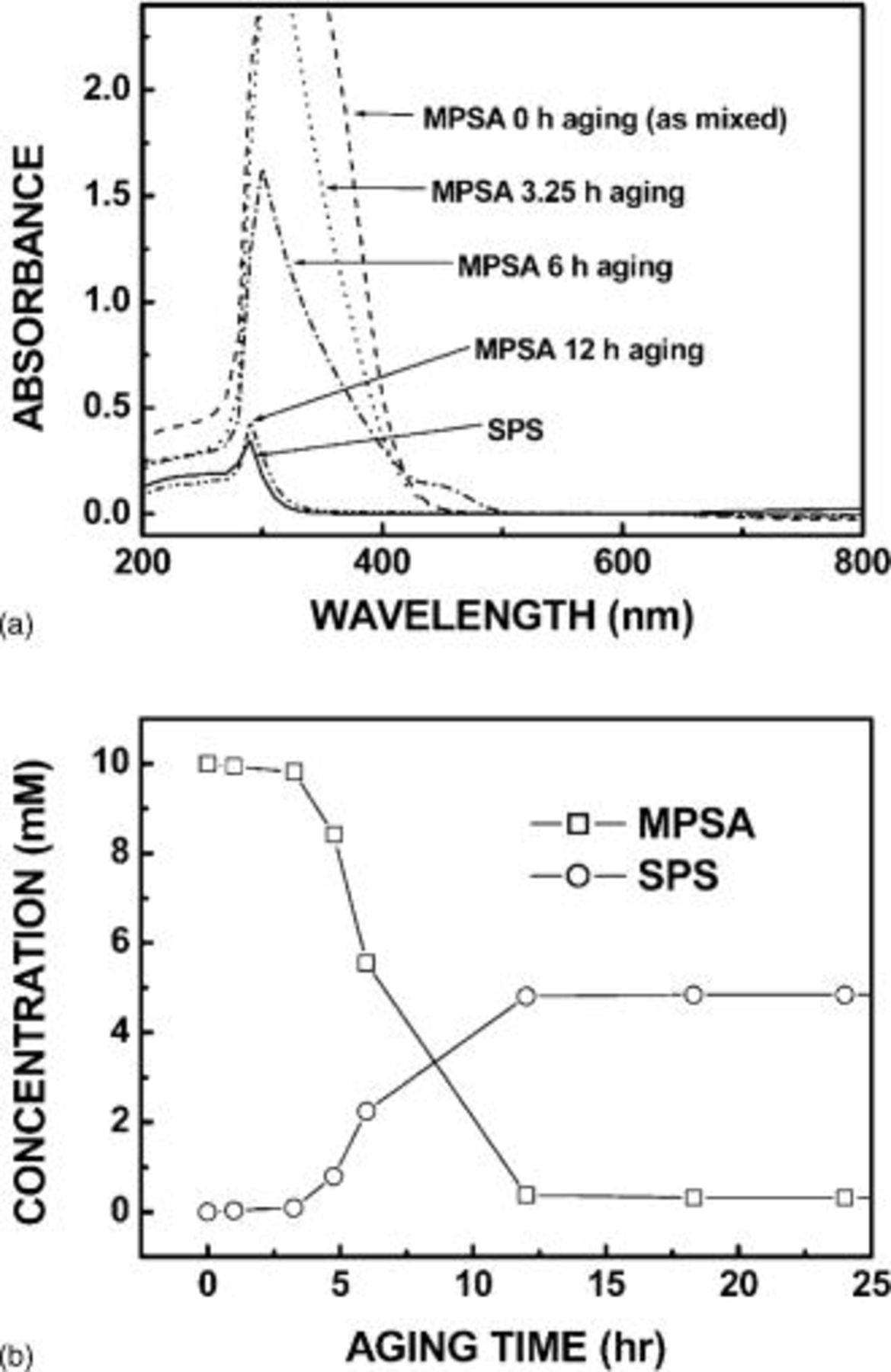
For a precise understanding of the time-dependent aging of MPSA and its conversion to SPS, UV-vis spectroscopy was performed and the results are depicted in Fig. 2. Because the σ→σ* transition does not occur at a wavelength of over 185 nm,17 and both additives have nonbonding electrons at the S atom, peaks of MPSA and SPS shown in Fig. 2a correspond to the  transition. With an increase in the aging time of MPSA, the peak of MPSA converged to that of SPS. After aging for 12 h, the MPSA peak coincided with the SPS peak, which strongly implied the dimerization of MPSA to SPS. Furthermore, from the absorbance of as-mixed 10 mM MPSA and 5 mM SPS, the molar absorptivities (ɛ) of each additive can be calculated. Moreover, by solving the following equations, we can monitor the time-dependent concentration change of initially added MPSA and produced SPS
transition. With an increase in the aging time of MPSA, the peak of MPSA converged to that of SPS. After aging for 12 h, the MPSA peak coincided with the SPS peak, which strongly implied the dimerization of MPSA to SPS. Furthermore, from the absorbance of as-mixed 10 mM MPSA and 5 mM SPS, the molar absorptivities (ɛ) of each additive can be calculated. Moreover, by solving the following equations, we can monitor the time-dependent concentration change of initially added MPSA and produced SPS
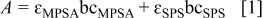
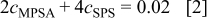
Where  is the absorbance,
is the absorbance,  is the path length (1 cm), and
is the path length (1 cm), and  is the concentration. Equation 2 is the concentration balance equation of the S atom of additives generated from initially added MPSA at a given aging time. As presented in Fig. 2b, conversion of MPSA to SPS underwent about 3 h of incubation period. And over 99% of initially added 10 mM MPSA was converted into 5 mM SPS within 12 h of aging, which is in agreement with the slow sweep voltammetry result of Moffat et al.15 For the 3 h incubation period, it is not easy to suggest a definite answer. However, if we introduce the results of Healey et al.18 and Moffat et al. ,15 it can be assumed gingerly that the entire reactions between the MPSA/SPS and
is the concentration. Equation 2 is the concentration balance equation of the S atom of additives generated from initially added MPSA at a given aging time. As presented in Fig. 2b, conversion of MPSA to SPS underwent about 3 h of incubation period. And over 99% of initially added 10 mM MPSA was converted into 5 mM SPS within 12 h of aging, which is in agreement with the slow sweep voltammetry result of Moffat et al.15 For the 3 h incubation period, it is not easy to suggest a definite answer. However, if we introduce the results of Healey et al.18 and Moffat et al. ,15 it can be assumed gingerly that the entire reactions between the MPSA/SPS and  are circulating and autocatalytic reaction systems, where the reaction products involve the initial reaction again and the reaction rate is slow initially.19
are circulating and autocatalytic reaction systems, where the reaction products involve the initial reaction again and the reaction rate is slow initially.19
Figure 2. (a) UV-vis spectroscopy analysis for SPS and aged MPSA and (b) concentration changes of initially added MPSA and generated SPS as a function of aging time of MPSA, which is calculated from the results of (a). Reference solvent for (a) is composed of 0.25 M  and 1 M
and 1 M  and samples are a mixture of reference solvent and each additive.
and samples are a mixture of reference solvent and each additive.
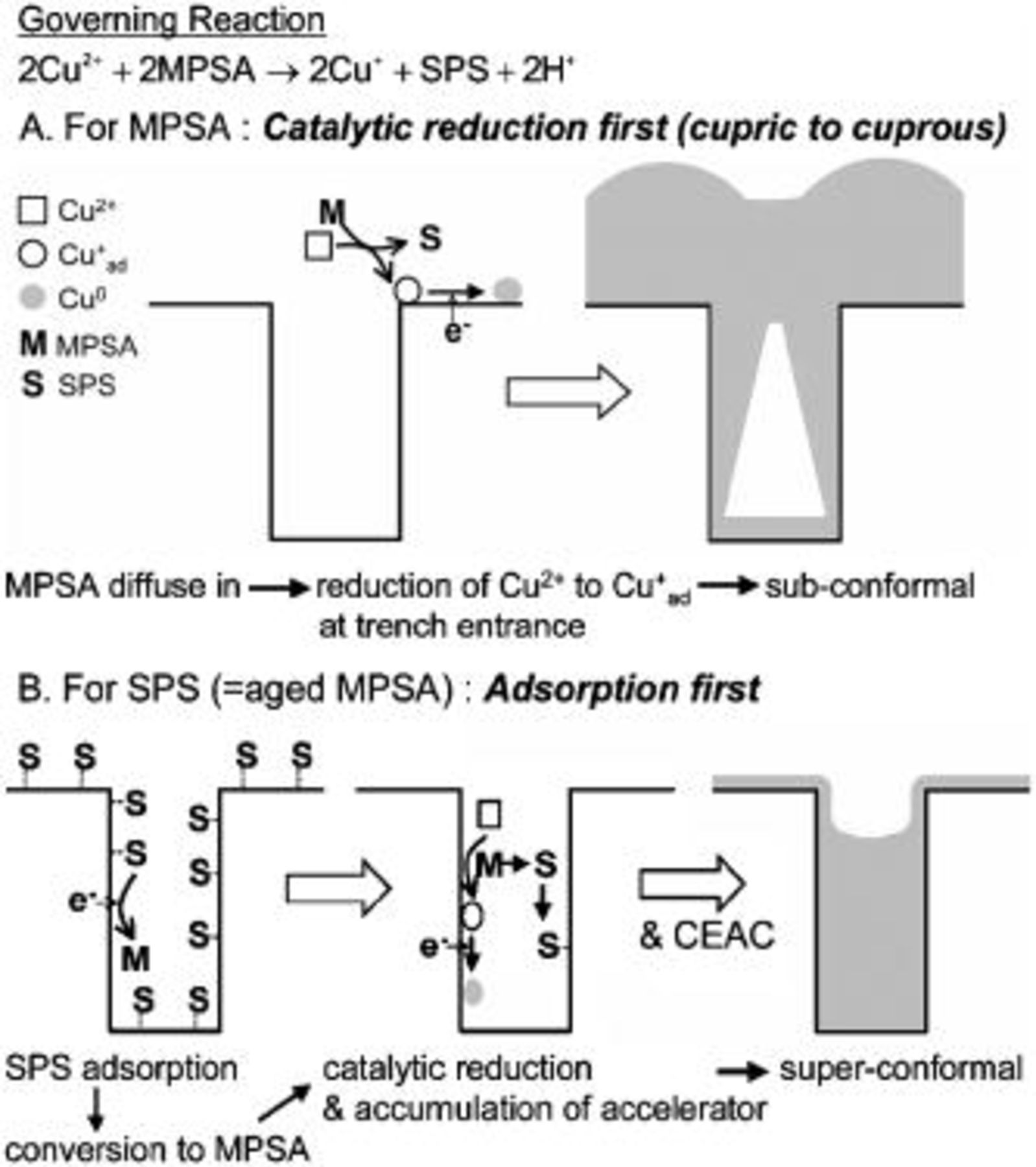
Figure 3 illustrates suggested mechanisms that can explain the different filling aspects of the two accelerators. In both cases, the two important roles of accelerators are generally accepted, i.e., catalytic reduction of the  ion by oxidation of MPSA to SPS11
13
14
15 and competitive adsorption of the accelerator with the suppressor(s), namely, the PEG-Cl complex.2
3
8
10
ion by oxidation of MPSA to SPS11
13
14
15 and competitive adsorption of the accelerator with the suppressor(s), namely, the PEG-Cl complex.2
3
8
10
Figure 3. Schematic illustration of suggested mechanisms for different filling aspects between MPSA and SPS/aged MPSA. For convenience, stoichiometry is ignored in the illustration.
For the reduction of  the
the  ion in the bulk electrolyte (the aquo complex of
ion in the bulk electrolyte (the aquo complex of  is not stable,14 which suggests that the
is not stable,14 which suggests that the  ion on a metal surface (adion,
ion on a metal surface (adion,  is thermodynamically more stable than that in the bulk electrolyte. Therefore, in the presence of a Cu seed in the electrolyte,
is thermodynamically more stable than that in the bulk electrolyte. Therefore, in the presence of a Cu seed in the electrolyte,  reduction by MPSA may prefer generation of
reduction by MPSA may prefer generation of  on a Cu surface, which implies that MPSA plays the role of a reducing agent near the Cu surface. (The conversion reaction rate measured by UV and shown in Fig. 2b cannot be applied in this case.) Eventually, when MPSA is used as an accelerator, diffused MPSA from the boundary layer reaches the trench entrance and subsequently reduces the
on a Cu surface, which implies that MPSA plays the role of a reducing agent near the Cu surface. (The conversion reaction rate measured by UV and shown in Fig. 2b cannot be applied in this case.) Eventually, when MPSA is used as an accelerator, diffused MPSA from the boundary layer reaches the trench entrance and subsequently reduces the  ion to a
ion to a  adion. Since the concentration of the added MPSA is μM scale, which is a quite small amount compared with the
adion. Since the concentration of the added MPSA is μM scale, which is a quite small amount compared with the  concentrations, this reaction is governed by mass transfer of MPSA and MPSA does not have a chance to diffuse deeply inside the trench. Therefore,
concentrations, this reaction is governed by mass transfer of MPSA and MPSA does not have a chance to diffuse deeply inside the trench. Therefore,  reduction is restricted to the trench entrance, which results in highly subconformal deposits (Fig. 1a).
reduction is restricted to the trench entrance, which results in highly subconformal deposits (Fig. 1a).
When SPS that has no catalytic activity in itself is added to the electrolyte, the first reaction it undergoes is an adsorption. It can diffuse deeply inside the trench without interference from the reaction involving it, and can adsorb uniformly on the entire seed surface. The adsorbed SPS is converted to MPSA by external electrons11 and starts its role as a catalyst. Dimerized MPSA (SPS) during the  reduction adsorbs again at the neighboring site and repeats the cycle. Therefore, SPS, once seated in the trench, does not escape from the trench and is accumulated during the progress of deposition. The well-established curvature-enhanced accelerator coverage (CEAC) mechanism4
5
7
8
9 is also available here. From these considerations, the superfilling profiles shown in Fig. 1g and h can be easily understood.
reduction adsorbs again at the neighboring site and repeats the cycle. Therefore, SPS, once seated in the trench, does not escape from the trench and is accumulated during the progress of deposition. The well-established curvature-enhanced accelerator coverage (CEAC) mechanism4
5
7
8
9 is also available here. From these considerations, the superfilling profiles shown in Fig. 1g and h can be easily understood.
Conclusions
An as-made electrolyte that contains MPSA is ineffective in the superconformal electrodeposition of Cu. This is presumably because the first reaction that the added MPSA underwent during the electrodeposition is  reduction near the trench entrance. To achieve superfilling using MPSA, aging of the electrolyte at least for 12 h prior to the electrodeposition is needed to convert over 99% MPSA into SPS. Then, SPS can diffuse inside the trench without interruption and subsequently starts the
reduction near the trench entrance. To achieve superfilling using MPSA, aging of the electrolyte at least for 12 h prior to the electrodeposition is needed to convert over 99% MPSA into SPS. Then, SPS can diffuse inside the trench without interruption and subsequently starts the  reduction. Conclusively, the first reaction that the accelerator undergoes during the initiation of electrodeposition is important.
reduction. Conclusively, the first reaction that the accelerator undergoes during the initiation of electrodeposition is important.
Acknowledgments
This work was supported by KOSEF through the Research Center for Energy Conversion and Storage (RCECS), LG Chemical Ltd., and also by Institute of Chemical Processes (ICP).
Seoul National University assisted in meeting the publication costs of this article.