Abstract
Material genetic engineering can significantly accelerate the development of new materials. As an important topic in material science and condensed matter physics, the development of metallic glasses (MGs) with specific properties has largely been the result of trial and error since their discovery in 1960. Yet, property design based on the physical parameters of constituent elements of MGs remains a huge challenge owing to the lack of an understanding of the property inheritance from constitute elements to the resultant alloys. In this work, we report the inherent relationships of the yield strength σy, Young's modulus E, and shear Modulus G with the valence electron density. More importantly, we reveal that the electronic density of states (EDOSs) at the Fermi surface (EF) is an inheritance factor for the physical properties of MGs. The physical properties of MGs are inherited from the specific element with the largest coefficient of electronic specific heat (γi), which dominates the value of the EDOS at EF. This work not only contributes to the understanding of property inheritances but also guides the design of novel MGs with specific properties based on material genetic engineering.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Future perspectives
As a new class of metastable materials with unique structures and excellent properties, metallic glasses have been one of the hot topics in the field of materials science. Establishing the relationship between components and their properties is the basis for the performance design of metallic glasses and the future direction in this field. Similar to the genetics of living things, some properties of metallic glasses are also inherited from the genes in some of their components. Revealing the inheritance factor on the physical properties in metallic glasses may provide the possibility for the performance optimization and design of novel metallic glasses in the future. This work may also provide novel ideas and methods for the design of intermetallic compounds based on materials genetic engineering.
1. Introduction
As one of the important systems in condensed matter physics and material science, metallic glasses (MGs) have attracted intense attention over the past decades owing to their distinctive properties, e.g. high strength, good corrosion resistance, and excellent soft magnetic properties [1]. Since their discovery in 1960 [2], thousands of MG compositions have been developed based on a wide range of elements [3]. In parallel with experimental research, many theoretical works have sought to establish the relationships between the structure and physical properties of MGs [4–8]. However, theoretical predictions of properties remain challenging due to their disordered structures and metastable states. Consequently, the design of alloys with specific properties has largely been the result of trial-and-error efforts [9]. Material genetic engineering can greatly improve and accelerate the efficiency of the development of new materials [10, 11]. Therefore, revealing the key inheritance factor(s) that determine the properties of MGs is an urgent need for directing the development of new MGs.
Recently, Ma and Wang et al [12–14] demonstrated that a variety of MGs inherit their elastic modulus from the base components. Furthermore, in subsequent studies, other physical properties of MGs have been revealed to be related to the corresponding properties of the principal elements. Examples of this phenomenon include Gd-, Ho-, Dy-, Er-, and La-based MGs with promising magnetocaloric properties [15–17], Nd-, Pr-, and Sm-based MGs showing hard magnetic behavior [18], Ce-based MGs that display heavy fermionic behavior [19]; and superconductive MG systems exploiting the superconductive properties of La, Fe, Zr, Hf, and Cu [20–23]. While, for other MGs [24], it is not always the case. Many Cu-based MGs show elastic moduli that are markedly different from those of their base components (see table S1). For example, the bulk modulus of the Cu60Zr20Hf10Ti10 glass is quite different from that of its base element Cu and is closer to the bulk modulus of Zr [13]. This underlines that the key inheritance factors determining MG properties are still unclear. The above results raise the following questions: what is the key factor that determines property inheritance in MGs? Revealing the physical parameters of the components that determine these properties is highly important for understanding the inheritance phenomenon and for accelerating the design of MGs with tailored properties.
In this work, we reveal the inheritance factor of physical properties from the perspective of electronic structure by analyzing a total of 90 MG compositions. We have found a universal scaling law that can be rationalized with the fundamental thermodynamics' principles [25, 26] and Fermi sphere-Brillouin zone interactions [27, 28]. Based on this scaling law, we provide a close correlation between the mechanical properties and a certain component that dominates the electronic density of states (EDOSs) at the Fermi surface (EF) in MGs. These findings guide the design of novel MGs with desirable properties during material genetic engineering.
2. Materials and methods
Cu64Zr36 and Ni45Ti20Zr25Al10 (at. %) MGs with nominal compositions were prepared by arc-melting of a mixture of pure Cu (99.99%), Zr (99.99%), Ni (99.99%), Ti (99.99%) and Al (99.99%) in a highly purified argon atmosphere. Glassy ribbons were produced from these master alloys by rapid quenching onto a single copper wheel at a speed of 45 m s−1. Fe80P13C7 and Fe74Mo6P13C7 (at. %) MGs were prepared by torch-melting a mixture of pure Fe (99.9 mass %), Mo (99.9 mass %), C (99.95 mass %), and Fe3P (99.5 mass %) in a clear fused-silica tube under a high-purity argon atmosphere by a torch. The as-prepared master alloy ingots were fluxed in a fluxing agent composed of B2O3 and CaO with a mass ratio of 3:1 at an elevated temperature for 4 h under a vacuum with a pressure of ∼10 Pa. After fluxing, each ingot was re-melted and cast into a copper mold with an inner diameter of 1.0 mm under a high-purity argon atmosphere.
The glassy nature of the obtained samples was ascertained by x-ray diffraction (D8 Advance, Bruker, Germany) using Cu Kα radiation and by differential scanning calorimetry (DSC-404, NETZSCH, Germany) with a heating rate of 0.67 K s−1. The bonding states were evaluated by x-ray photoelectron spectroscopy (XPS) using a Kratos AXIS ULTRADLD instrument with a monochromic Al Kα x-ray source (hν= 1486.6 eV). The power was 120 W and the x-ray spot size was set to 700 × 300 μm. The pass energy of the XPS analyzer was set at 20 eV. The base pressure of the analysis chamber was greater than 5 × 10−9 Torr. All of the spectra were calibrated using the binding energy of C 1 s (284.8 eV) as the reference, and the etching conditions of beam energy of 2 kV, extractor current of 100 μA, and raster size of 4 mm. Oxygen-free surfaces of glassy samples were obtained by Ar-ion sputtering. Young's modulus and shear modulus were determined using a Quasar RUSpec resonant ultrasound spectrometer. The low-temperature specific heats of the studied amorphous alloys were evaluated using a physical property measurement system (Model-9, Quantum Design Systems, USA). The specific heat measurements were performed in triplicate and the final data were obtained by averaging the three results. The relative error for the specific heat measurements is less than 2%.
Molecular dynamics calculations were performed using the Vienna ab initio simulation package [29]. All simulations were carried out in the canonical ensemble with the temperature controlled by the Nose thermostat [30]. Cubic supercells containing 100 atoms were used, and the dimensions of the cells were estimated according to the alloy densities at room temperature. The cells were first melted and equilibrated at 2000 K, and then gradually cooled first to 1000 K and then to 300 K at a cooling rate of 1.67 × 1014 K s−1 with a time step of 3 fs. A 5 × 5 × 5 Monkhorst-Pack mesh was used for k-point sampling, and the EDOSs were calculated considering spin polarization.
3. Results and discussion
Figures 1(a)–(d) show the comparisons of Young's moduli (E) and shear moduli (G) between the Cu64Zr36, Ni45Ti20Zr25Al10, Fe80P13C7 and Fe74Mo6P13C7 glassy samples (the glassy nature of samples was shown in figure S1) and their components, respectively. In figures 1(a) and (b), the values of elastic moduli are close to those of a single element, but this element is not the major component. For instance, for the Ni45Ti20Zr25Al10 glass, E is 114 GPa, which is quite close to that of pure polycrystalline Ti (116 GPa) but is different from that of Ni (200 GPa) and Zr (98 GPa). This is unusual because the minor component rather than the major component appears to be responsible for the properties of MGs. An examination of figures 1(c) and (d) show that the elastic moduli are close to the corresponding values of their major component Fe, but minor changes in the Mo component (e.g. through microalloying) affect the values of the elastic moduli. E of Fe80P13C7 glass can be dramatically enhanced from 137 to 174 GPa, and G increases from 49 to 65 GPa when 6 at. % Mo was added. This phenomenon seems contradictory but is curious. It is well known that the interatomic bonding controls the elastic moduli of MGs [31] and the characteristics of interatomic bonds are mostly determined by the valence electrons (d, p, and/or s) [32]. To reveal the mechanism of inheritance in MGs, the XPS valence-band spectra, and spin-polarized total and partial DOS of the valence electrons in the corresponding MGs were obtained as shown in figures 1(a1)∼(d1), respectively. The EDOS at EF of Cu64Zr36 and Ni45Ti20Zr25Al10 MGs are dominated by the d electrons of Zr and Ti, respectively, as shown in figures 1(a1) and (b1), in good agreement with previous results [33]. As shown in figures 1(c1) and (d1), Fe contributes to most of the EDOS in the Fe-based MGs at EF, while the Mo d electrons also make a significant contribution to the EDOS at EF in Fe74Mo6P13C7 (figure S2). It is observed that the modulus inheritance and electronic structure are correlated, namely, the elastic moduli of MGs are mainly inherited from the elements that dominate the EDOS at the EF in MGs.
Figure 1. Comparisons of Young's moduli (E) and shear moduli (G) for the (a) Cu64Zr36, (b) Ni45Ti20Zr25Al10, (c) Fe80P13C7 and (d) Fe74Mo6P13C7 glassy samples and their components. (a1)∼(d1) XPS spectra and electronic densities of states for the Cu64Zr36, Ni45Ti20Zr25Al10, Fe80P13C7, and Fe74Mo6P13C7 glassy samples, respectively.
Download figure:
Standard image High-resolution imageTo further explore the underlying physics of inheritance, the relationship between the elastic modulus and structural parameters was investigated. In previous studies, the elastic modulus of MGs was found to have a clear relationship to the glass transition temperature Tg and molar volume V [7, 34]. The inherent relationship of E with Tg and V of MGs in a universal scaling equation is given by [25, 26]:
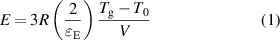
where R is the gas constant, T0 is room temperature,  E is the room temperature elastic limit of bulk MGs (BMGs) that is approximately 2% [35]. Since E = σy/
E is the room temperature elastic limit of bulk MGs (BMGs) that is approximately 2% [35]. Since E = σy/ E and E= 2.61 G [24], we can thus obtain a universal scaling relationship between σy, G, and (Tg–T0)/V.
E and E= 2.61 G [24], we can thus obtain a universal scaling relationship between σy, G, and (Tg–T0)/V.
Recently, fractal concepts have been introduced to describe atomic structure [36, 37]. These models have been invoked to explain the widely observed noncubic power laws (D) correlating the positions of the first sharp diffraction peak, q1, with V [37], that is:

From equations (1) and (2), a strong dependence of the mechanical properties on the structural vector q1 can be obtained as follows:
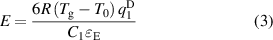
where C1 is a constant. The above relationship is generally applicable to different types of MGs, independent of their chemical compositions and bonding types. Thus, different types of MGs should follow a similar underlying mechanism of deformation and fracture. Generally, an increase in Tg and q1 will result in a stronger MG and vice versa. In figure 2, we plotted the correlations between the mechanical properties σy (a), E (b), and G (c) and q1 for 57 MGs (the data are summarized in table S2) as solid lines. It is observed that equation (3) fits the experimental results well, verifying the existence of an intrinsic correlation. The value of the exponent D extracted from the fitting (2.45) is consistent with D= 2.3 [37] and 2.5 [38] that have been measured for MGs in previous work. This indicates that although the fractal atomic packing in MGs has not been proven [39], the conclusions obtained by using this fractal model are acceptable and are in good agreement with the experimental results.
Figure 2. Relationship between yield strength σy (a), Young's modulus E (b), shear modulus G (c), Tg, and structural vector q1 for 57 MGs [40].
Download figure:
Standard image High-resolution imageFurthermore, many studies [27, 28, 41] have shown that MGs are stabilized when the assumed spherical Fermi surface coincides with the diffused pseudo-Brillouin zone boundary, which can be expressed as q1 = 2kF, where kF is the Fermi wave vector. This is also known as Fermi surface-Brillouin zone interaction or the Hume-Rothery stabilization mechanism [42]. All binary and some ternary MGs compositions have been precisely verified experimentally to satisfy the stability criterion [43]. Then, a universal correlation between E and kF can be obtained as:
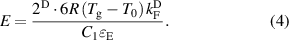
On the one hand, kF can be given by  [28], where ρ is the valence electron density. The correlation between E and ρ can then be described by:
[28], where ρ is the valence electron density. The correlation between E and ρ can then be described by:
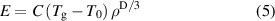
where C is a constant. It is noted that equation (5) is based on the assumption that q1 represents the effect of both atomic spacing V and valence electron density ρ. Fortunately, MGs can be considered metallic materials with isotropic electron clouds. Despite the lack of long-range periodicity in their atomic structures, MGs can be considered virtually isotropic (i.e. a close-to-spherical symmetry) even down to the atomic level [40]. Thus, the 2kF = q1 relationship is valid, as can be further confirmed in figure 3. As shown in figure 3(a), equation (5) fits the experimental data well (the data are also summarized in table S3), demonstrating that σy of MGs is intrinsically linked with ρ, which is also consistent with previous works [44]. A remarkable agreement was also observed between the calculated and measured E and G values of various MGs, as shown in figures 3(b) and (c), respectively. Thus, equation (5) demonstrates that in MGs, Young's modulus is directly related to the valence electron density.
Figure 3. Relationship between yield strength σy (a), Young's modulus E (b), shear modulus G (c), glass transition temperature Tg, and valence electron density ρ for a variety of MGs [14, 24, 40].
Download figure:
Standard image High-resolution imageOn the other hand, EDOS at the Fermi level n(EF) and kF is also related to each other by  [45]. Then, the relationship between E and n(EF) can be expressed as:
[45]. Then, the relationship between E and n(EF) can be expressed as:

Equation (6) indicates that the mechanical properties of MGs depended on the EDOS at EF. A straightforward way to obtain experimental information regarding the EDOS at EF is to measure the low-temperature specific heat Cp . The EDOS at EF is directly related to the linear term temperature coefficient of the electronic specific heat [46], γ = (1/3)π2 kB 2(1 + λep) n(EF), where kB is the Boltzmann constant and λep accounts for the enhancement of γ due to the electron-phonon interaction. Since the electron-phonon enhancement turns out to be of minor importance [47], the composition dependence of γ already reflects the band structure at EF well. The relationship between E and γ can be approximately expressed as:
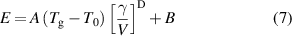
where A and B are constants. This indicates that the mechanical properties should be related to γ and n(EF) values of MGs. To confirm this correlation, the Cp values of Cu64Zr36, Ni45Ti20Zr25Al10, Fe80P13C7, and Fe74Mo6P13C7 BMGs are presented in figure 4(a). Cp can be separated into the linear electronic contribution (γT) and the cubic Debye's contribution (δT3) below 20 K [48], δ= 12π4 R/(5θD 3), where θD is the Debye temperature. The relationship between Cp and T can be fitted using Cp /T= γ+ δT2 with different γ and δ. The results of this fit are shown in figure 4(b) and demonstrate the good linear relationships between Cp /T and T2 for these MGs. The θD values of these MGs derived from the δ coefficients and those of their components are compared in figure 4(c). Similar to EDOS and moduli inheritance, the θD of these MGs are also inherited from one of their components. The γ values of MGs, together with their σy, E, G, V, and Tg are summarized in table 1.
Figure 4. Specific heat Cp of the MGs in the temperature range from 3 to 33 K. (a) Specific heat plotted versus the temperature. (b) Specific heat, shown as Cp /T vs T2, where the solid lines are the results of the fitting of the specific heat data to Cp /T= γ+ δT2. (c) Histograms of θD for MGs and one of their dominant components.
Download figure:
Standard image High-resolution imageTable 1. Summary of the glass transition temperature Tg, calculated molar volume V, yield strength σy, Young's modulus E, shear modulus G, and linear term low-temperature coefficient of the electronic specific heat γ of 15 MGs [14, 24]. The values of molar volumes V of the MGs are calculated according to the rule of mixtures [49].
| Label | Composition | Tg (K) | V (cm3 mol−1) | σy (GPa) | E (GPa) | G (GPa) | γ (mJ mol−1 K2) | References. |
|---|---|---|---|---|---|---|---|---|
| A | Cu64Zr36 | 744 | 9.646 | 1.94 | 92 | 34 | 2.64 | This work |
| B | Ni45Ti20Zr25Al10 | 733 | 9.110 | 2.37 | 114 | 44 | 8.01 | This work |
| C | Fe74Mo6P13C7 | 715 | 6.999 | 3.30 | 173 | 65 | 6.00 | This work |
| D | Fe80P13C7 | 669 | 6.740 | 3.14 | 137 | 49 | 6.70 | This work |
| E | Cu50Zr50 | 733 | 10.451 | 1.92 | 85 | 32 | 4.30 | [50] |
| F | (Fe0.8Co0.2)72B20Si4Nb4 | 830 | 6.218 | 4.17 | 205 | 78 | 6.94 | [51] |
| G | Zr46.75Ti8.25Cu7.5Ni10Be27.5 | 623 | 9.930 | 1.83 | 100 | 37.2 | 2.82 | [52] |
| H | Zr41Ti14Cu12.5Ni10Be22.5 | 620 | 9.788 | 1.8 | 101 | 37.4 | 3.03 | [53] |
| I | Co56Ta9B35 | 945 | 5.923 | 5.80 | 240 | 91.5 | 7.10 | [54] |
| J | Pd40Ni10Cu30P20 | 564 | 7.959 | 1.52 | 98 | 35.1 | 0.67 | [52] |
| K | Cu60Zr20Hf10Ti10 | 734 | 9.501 | 1.95 | 101 | 36.9 | 2.73 | [55] |
| L | Pd77.5Cu6Si16.5 | 630 | 8.742 | 1.55 | 96 | 34.8 | 0.40 | [56] |
| M | Cu45Zr45Al7Gd3 | 670 | 10.645 | 1.81 | 90.6 | 32.9 | 4.90 | [57] |
| N | Pd40Ni40P20 | 630 | 7.6805 | 1.84 | 91.9 | 32.7 | 2.19 | [50] |
| O | Zr52.5Ti5Cu17.9Ni14.6Al10 | 672 | 10.768 | 1.77 | 88.6 | 32.3 | 3.44 | [58] |
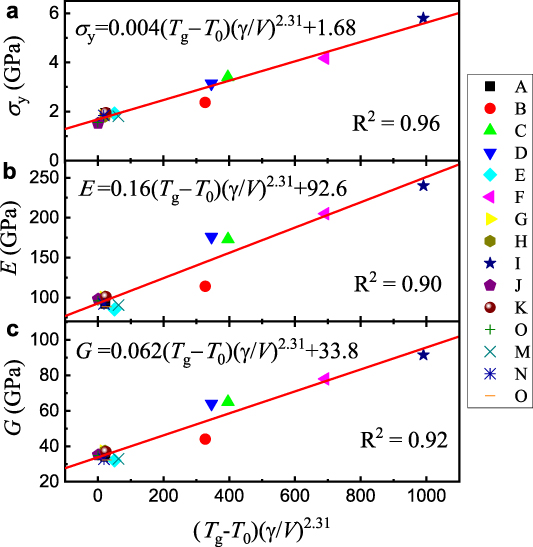
The relationships between σy, E, and G vs γ/V are shown by the solid lines plotted in figure 5. It is observed that equation (7) fits the experimental results very well, verifying the existence of a correlation between the mechanical properties and γ values. Generally, two characteristics of the electron-density distribution, namely bond directionality and charge polarizability, determine the strength and modulus [31]. In principle, the bonding characteristics mainly depend on the EDOS at EF. The γ values are determined by all the electrons contained in the components and their hybridization [59, 60]. If the effects of covalent bonds and electron hybridization are not considered, the γ of a MG shows a correlation with a weighted average of the coefficient of the electronic specific heat γi for the constituent element, γ= Σγi × fi , where fi denotes the atomic percentage of the constituent element. It indicates that the component with the largest γi × fi that dominates the EDOS at EF has a decisive influence on the physical properties of MGs. The minor changes in atom components (e.g. through microalloying) may significantly change the EDOS of an MG and accordingly influence the physical properties. As shown in figure 6, γCu = 0.7 mJ mol−1 K2 is much smaller than γZr= 2.8 mJ mol−1 K2, which means that the EDOS at EF of Cu–Zr glassy systems is mainly dominated by Zr rather than by Cu. Moreover, γCe = 1620 mJ mol−1 K2 is much larger than γLa = 10 mJ mol−1 K2. In (Cex La100−x )-based MGs (x = 10 ∼ 80) [61], even though the Ce content is very low, the EDOS value at EF of (Ce10La90)68Al10Cu20Co2 glass is mainly determined by Ce [62]. Correspondingly, the elastic moduli of (Ce10La90)68Al10Cu20Co2 MG are closer to those of Ce rather than to those of La (the data are also summarized in table S4). These results further confirm that EDOS at EF or the γi value is an inheritance factor of physical properties in MGs, i.e. the basic physical properties of MGs are inherited from a certain component with the largest γi × fi . The properties of the MGs can be judged preliminarily through the γi values in figure 6 and their atomic percentage fi .
Figure 5. Relationship between yield strength σy (a), Young's modulus E (b), shear modulus G (c), glass transition temperature Tg, and low-temperature coefficient of the electronic specific heat γ for 15 MGs.
Download figure:
Standard image High-resolution imageFigure 6. Experimental values [63] of the linear term temperature coefficients γi of electronic specific heat for a great number of elements.
Download figure:
Standard image High-resolution imageAlthough we have mainly focused on the inheritance factor of elastic moduli in MGs, the other physical properties such as magnetocaloric effects, Debye temperature, and superconducting temperature also follow the inheritance, as examples in table 2. The physical properties of the MGs are dominated by the major contribution composition to the γ (the EDOS at EF). Thus, the γi values in figure 6 can be used to design tailored properties of MGs by high-throughput computational simulation and/or machine learning.
Table 2. The physical properties of some typical metallic glasses inherit from their component.
| Physical properties | MGs | Values of MGs | Dominate components | Values of components | References |
|---|---|---|---|---|---|
| Magnetocaloric effect | Gd53Al24Co20Zr3 | 9.40 (5T) | Gd | 9.8 (5T) | [16] |
| Gd33Er22Al25Co20 | 9.47 (5T) | Gd | 9.8 (5T) | [16] | |
| Gd55Ni25Al20 | 9.76 (5T) | Gd | 9.8 (5T) | [64] | |
| Debye temperature | Zr55Al10Ni10Cu15Be10 | 297 K | Zr | 291 K | [24] |
| Fe60Cr10Mo9C13B6Er2 | 471 K | Fe | 470 K | [24] | |
| Ca65Mg8.54Li9.96Zn16.5 | 221 K | Ca | 230 K | [24] | |
| Superconducting temperature | La60Cu20Ni10Al10 | 2.5 K | La | 6.0 K | [20] |
| Fe30Zr70 | 1.9 K | Fe | 2.1 K | [65] | |
| Zr54Cu46 | 0.9 K | Zr | 0.85 K | [66] | |
| Zr50Cu50 | 0.7 K | Zr | 0.85 K | [66] |
It was found that elastic properties of MGs are primarily determined by their base pure polycrystalline components [12], and usually increased by about 30% after crystallization [67]. In principle, trends similar to the physical properties inheritance and correlations observed here should also exist for intermetallic compounds; however, due to a large number of dislocations and/or defects in MGs after crystallization, the factors affecting mechanical properties are more complex, and it is difficult to determine quantitative relationships between EDOS and mechanical properties. Furthermore, due to covalent bonds and sp-d hybridization, the assignment of valence electrons is complicated in most alloys containing transition metals, metalloids, and/or rare earth [59, 60]. The data scatter observed for metalloid glasses, such as Fe-, Co-, Ni-, Pd-, and Pt-based MGs, may be considered due to the perturbations stemming from the above-mentioned effects. In particular, Fe-based MGs usually contain non-metallic elements such as B, Si, P, and C [68]. These elements will form covalent bonding with Fe and generate sp-d hybridization [59]. This results in that the electron's contribution γi to the parameter γ is no longer a weighted average. Therefore, the scattering of Fe-based MGs is far more than that of others. Fortunately, the transition metals can be treated as if they have one free electron per atom [27], and the presence of the Fermi sphere-Brillouin zone interaction has been both theoretically and experimentally confirmed in transition metal and rare earth MGs [28, 69]. The E, G, and σy are complex material parameters that may be influenced by the 'micro-structure', fracture and deformation mode, preparation, loading conditions, specimen size, and aspect ratio geometry. It is important to mention that the Tg and molar volume V depends on the thermal history, such as aging, rejuvenation, and processing conditions. The structural heterogeneity and atomic density fluctuation froze during rapid quenching are also expected to result in the fluctuation of free-volume regions, valence electron density, and low-temperature specific heat. In the free-volume regions, where mechanical coupling to the surrounding atomic environment is weak, inelastic relaxation becomes possible by local atom rearrangements, without affecting the surrounding atomic environment significantly [25, 70]. Thus, these sites are the preferred regions to initiate the scattering of data. If these effects can be ruled out, the theoretical and experimental values of MGs will likely show better agreement. Namely, considering the large phase space of possible metallic glass compositions and their multicomponent nature, their exploration as the result of trial-and-error efforts will be very time-consuming. By contrast, if it is known that the EDOS at EF is controlled by a certain element, then only calculations for the properties of that element are possible to predict the properties of MGs. Thus, this work may help to design novel MGs with specific properties.
4. Conclusions
In summary, we have successfully derived a universal scaling law based on fundamental thermodynamics' principles and Fermi sphere-Brillouin zone interactions. The linearity between mechanical properties and glass transition temperature unambiguously demonstrates that the linear elastic properties of MGs are dominated by structural vector and/or valence electron density. It was found that the EDOS at EF is an inheritance factor of physical properties in MGs, i.e. the elastic moduli of MGs are inherited from the components of the MGs that dominate the EDOS at EF. This work can be used to design novel MGs with specific properties and will have a significant impact on material genetic engineering.
Acknowledgments
We thank Professor Q Li for his assistance in sample preparations. We acknowledge Y C Wang and W B Zhang for their assistance with simulations. We also thank W Y Li for their assistance with data collection. This work was supported by the National Natural Science Foundation of China (Nos. 51871237 and 52171165). Additional support was provided through the European Research Council under the Advanced Grant 'INTELHYB—Next Generation of Complex Metallic Materials in Intelligent Hybrid Structures' (No. ERC-2013-ADG-340025).
Author contributions
W M Yang, J W Li, H S Liu, and J T Huo conceived and designed the research and analysis. H Y Li contributed to data collection. J Y Mo and M Z Li performed the MD simulations. W M Yang, H S Liu, S Lan, X-L Wang, M Z Li, J Eckert, and J T Huo performed the data analysis and wrote the paper with assistance from all authors. All authors contributed to the analysis of the results and the discussion in the manuscript.
Conflict of interest
The authors declare no competing financial interests.
Supplementary data (3.0 MB DOC)