Abstract
Metallic glasses (MGs) or amorphous alloys are an important engineering material that has a history of research of about 80–90 years. While different fast cooling methods were developed for multi-component MGs between 1960s and 1980s, 1990s witnessed a surge of research interest in the development of bulk metallic glasses (BGMs). Since then, one central theme of research in the metallic-glass community has been compositional design that aims to search for MGs with a better glass forming ability, a larger size and/or more interesting properties, which can hence meet the demands from more important applications. In this review article, we focus on the recent development of chemically complex MGs, such as high entropy MGs, with new tools that were not available or mature yet until recently, such as the state-of-the-art additive manufacturing technologies, high throughput materials design techniques and the methods for big data analyses (e.g. machine learning and artificial intelligence). We also discuss the recent use of MGs in a variety of novel and important applications, from personal healthcare, electric energy transfer to nuclear energy that plays a pivotal role in the battle against global warming.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Metals and alloys have been one of the key materials in shaping human society since ancient times. Over the past centuries, human civilization has relied on the discovery and development of new metallic materials which can spur innovation and drive the technologic developments in almost all industrial sectors, from civil and commercial products, high-tech manufacturing to national defense. In light of this, the discovery of the first amorphous alloy by Kramer [1] through electrodeposition in the 1930s marked a new epoch for the research and application of metallic materials. Following Kramer [1], Brenner et al [2] produced amorphous Ni–P thin films by electrodeposition in the 1950s. In the 1960s, Duwez et al [3] successfully obtained Au–Si glassy ribbons with a thickness of ∼10 μm through vacuum melt spinning. With the development of rapid cooling techniques, thicker and larger amorphous metals or metallic glasses (MGs) with more complicated chemical compositions were developed [4–6]. For instance, Chen fabricated Pd-based MGs in a cylindrical shape with a diameter of 3 mm by water quenching in fused quartz capillary in 1974 [5] and after that, Turnbull et al obtained a fully glassy ingot with a size larger than 10 mm using the 'boron oxide fluxing' method in 1984 [6]. The 1990s witnessed a surge of research interest in the development of bulk metallic glasses (BMGs), as inspired by the seminar works from Inoue's group in 1990 [7, 8] and from Johnson's group in 1993 [9], both of which successfully obtained a series of BMGs with a diameter ranging from 1 to 15 mm [7–9] through compositional design. These early efforts were even 'translated' into several empirical rules [10–12] in order to guide the development of BMGs with a good glass-forming ability (GFA). Aside from transition metal-based BMGs, Wang's group has developed a series of rare earth metal-based BMGs [13] since 2003, such as La- [14], Ce- [15], and Pr-based [16] BMGs. Today, after almost six decades of intense research, over 800 BMG compositions have been developed and reported in the literature [17], out of which the most studied base elements are Fe, Zr, Ni, Al, Co, Mg, Cu, La, Pd, etc [17], and their percentages are shown in figure 1. To accelerate the compositional search of BMGs, people also utilized novel high throughput methods, such as multi-target physical vapor deposition (MT-PVD) [18, 19] and machine learning (ML) reinforced compositional design [20], to pinpoint the chemical composition with a good GFA. The central theme of research for these efforts has been to make larger and more affordable BMGs with better properties (such as GFA), which however still remains a challenge today.
Figure 1. Comparison of different classes of BMGs in terms of their percentage. Note that the comparison is based on a collection of ∼1000 BMGs with measured GFAs. The inset highlights the elements with counts indicating the total number of times an individual element being found in the reported MG compositions. Reproduced from [17]. CC BY 4.0.
Download figure:
Standard image High-resolution imageApart from the development of BMGs through compositional design, various additive manufacturing (AM) techniques were explored to fabricate bulk structures out of MGs even with poor GFA, which include spark plasma sintering (SPS) [21, 22], selected laser melting (SLM) enabled three-dimensional (3D) printing [23, 24], friction welding [25, 26], electron-beam welding [27, 28], laser welding [29, 30], ultrasound welding, etc [31–34]. The earliest attempt to fabricate bulk sized MGs with AM might be traced back to 1988 when Kawamura et al [35] prepared BMGs from ribbons by high temperature sintering. Later in 1994, Kawamura et al further adopted warm extrusion to produce Al- [36] and Zr-based [37] amorphous compacts nearly without porosity. In 1999, Inoue's group started to fabricate Fe-based soft magnetic BMGs with SPS [21, 22]. Considering that SPS possesses a very short processing time with better temperature control compared to other powder consolidation methods, people also fabricated Zr- [38], Ti- [39], and Ni-based [40] BMGs successfully through SPS in the first decade of this century. Meanwhile, Sun et al [41] attempted to print Zr-based BMGs through laser engineered net shaping in 2008. However, this early effort failed to produce fully amorphous structures because of significant crystallization in heat affected zones. In 2013, Pauly et al successfully printed out Fe-based BMGs with a fully amorphous structure and complex shape via SLM [23]. According to reference [24], the cooling rate in the typical heat affected zone can reach as high as 106 K s−1 in SLM. Notably, many good MG-formers can afford this cooling rate without crystallization, which unlocks the door for SLM enabled 3D printing of BMGs. On the other hand, various welding or joining techniques were developed and studied for the fabrication of BMGs. Since 2001, Kawamura et al have studied a number of welding techniques for the fabrication of BMGs, such as electron-beam welding [27], laser welding [29], and explosive welding [31]. In 2014, Jan Schroers et al [33] demonstrated the joining of Zr-based BMGs through thermoplastic compression in air. Regardless of the physical mechanisms underpinning these welding methods, they all require that the temperature in the welding area be above the glass transition temperature or melting temperature [33]. More interestingly, Ma et al [34] developed a cold joining technique to join MG ribbons or BMGs even at temperatures close to room temperature in 2019. These signs of progress are promising, which suggests that, with these new AM techniques, we may be very close to overcoming the size limit imposed on MGs by the high cooling rate.
Future perspectives
Metallic glasses are well known for their potential engineering applications; however, their widespread use is yet to emerge due to several limitations they are facing, such as the issue of poor glass forming ability and limited size. In this focused review, we discuss the research efforts in tackling this important issue with the tools that were available only recently, such as combinatorial alloy design, big data analysis, machine learning and additive manufacturing. With these novel tools, the design for good performing glass-forming alloys has been greatly accelerated, and bulk structures can be even made of poor glass-forming alloys through additive manufacturing, thereby overcoming the longstanding issue of limited size for metallic glasses. In addition, despite all the limitations facing metallic glasses as a structural material, we also highlight some of their recent applications in biomedical, nuclear and electronic engineering. These progresses deliver a strong message that metallic glasses are still a very attractive material with a bright future ahead.
To date, there are still several major roadblocks against the widespread structural applications of BMGs, such as medium temperature embrittlement [42], high materials cost, and sustainability [43]; however, their functional applications are attractive and burgeoning. For example, Fe-based amorphous ribbons have already been widely used in China to replace crystalline Fe–Si (silicon steel) in making the next generation electric transformers for their low cost and excellent soft magnetic properties, such as the very low coercivity and high electrical resistance, which can reduce the energy core loss by 70%∼80% [44]. Also, MGs are a good candidate material for the next generation of micro- and nano-devices because of their excellent thermo-plastic formability [39]. Meanwhile, Ti-, Mg- and Zr-based BMGs could be good aerospace and biomedical materials for their high strength, low moduli, and excellent corrosion resistance [45–47]. More importantly, some BMGs were proven to be good catalytic materials, which can be utilized in various applications related to chemical catalysis, such as wastewater degradation [48], water splitting [49], and CO2 reduction [50]. In this article, we would like to provide a focused overview of the recent advancement in the research of MGs in three fast-growing areas: (a) accelerated compositional design, (b) AM, and (c) new applications.
2. Compositional design of BMG
2.1. Traditional design approach
The traditional design of BMGs has been mainly guided by empirical rules [10, 11, 51, 52], such as the famous Inoue's rules [11], including (a) an atomic size mismatch greater than 12%, (b) highly negative heat of mixing between dislike atoms, and (c) more than three constituent elements. Up to date, the majority of BMGs were discovered and developed based on these rules [10, 11, 51, 52]. However, prior to the proposal of Inoue [11], it was proposed, as early as 1969, that the formation of MGs be correlated with the eutectic or near-eutectic composition of an alloy [51], at which the thermal stability of a metallic liquid can be enhanced, therefore leading to a better GFA. Since then, as guided by this 'eutectic point' rule, a number of BMGs have also been discovered, such as Zr48Cu45Al7 [53, 54], Zr45Cu49Al6 [54], Zr54Cu38Al8 [54], and Cu52Zr40Ti8 [55]. The 'confusion' principle is another empirical rule that is expected to apply to all kinds of BMGs [10], which is also consistent with Inoue's rules [11]. This principle is rooted in the idea that the compositional complexity of an alloy may lead to competitive crystallization of multiple species, thereby causing the 'confusion' (or interruption) of crystallization and facilitating amorphization [10]. At the fundamental level, the confusion principle rule is in line with the theory of Tanaka [52], according to which amorphization is essentially a result of interrupted crystallization because of the strong competition of multiple crystallization processes. Through transmission electron microscopy studies, Wang et al [56] found that the excellent GFA of the famous Zr-based BMG (Vitrelloy 1) could be attributed to the confusion principle or the competition between the formation of quasi-crystalline phases and conventional crystals. Over the past six decades, the traditional design approach has been proven useful and successful; nonetheless, by following this traditional design approach, it also means that one usually has to go through iterations of experimental trial and error, which is time consuming, costly and of a low efficiency, particularly when the chemical composition of an alloy becomes complicated, such as high entropy metallic glasses (HEMGs) [57–63] that contain more than five elements mixed in nearly equal molar fractions.
2.2. Combinatorial design approach
To accelerate the compositional design for BMGs, the combinatorial methods have recently attracted a great deal of research attentions [18, 19, 64–70]. These methods are usually based upon high throughput materials synthesis combined with high throughput structure and property characterization. Through the combinatorial design approach, one could prepare and characterize hundreds or even thousands of alloy compositions in a single run of experiment, which greatly improves the efficiency in compositional screening. To date, the most widely used high throughput method for materials synthesis is the MT-PVD [18, 19, 64–66]. In MT-PVD, one can control the film composition gradient by tuning the angles and intensity ratio among energy fluxes of different targets. Following MT-PVD, rapid x-ray diffraction mapping could be applied to characterize the structure of the metallic thin films as a function of their chemical composition [18, 19, 66], which can quickly lead to the construction of a phase diagram. Aside from x-ray diffraction mapping, people devised other high throughput characterization techniques, such as density mapping [64], electric conductivity mapping [19], and solidification temperature mapping [65]. For example, Li et al systematically studied the GFA of the Cu–Zr films through density mapping [64]; Li et al searched the high GFA compositions in Ir–Ni–Ta films through electric resistance mapping [19]; and Ding et al [65] investigated the GFA of the Au–Cu–Si films [65] through an optical method that enabled the detection of melting and solidification of the metallic films, as seen in figure 2. Ding et al also studied BMG-forming compositional space through parallel blow forming, which characterized the thermoplastic formability of the Mg–Cu–Y films and casted quantitative insights into their GFAs [18]. Besides, Tsai et al demonstrated that the differential interference contrast optical micrographs could be used to detect amorphous phase through surface topography [67, 68].
Figure 2. Combinatorial methods for MG synthesis and characterization. The variation of contrast in the heating and cooling cycle of Au–Cu–Si library section. Reprinted from [65], with the permission of AIP Publishing.
Download figure:
Standard image High-resolution imageAlthough the MT-PVD method is popular as a high throughput method for materials synthesis, it does not involve explicitly the effect of cooling rate on the formation of amorphous structures. By comparison, the conventional wedge casting may be the first high throughput method that enables the study of various cooling rates, usually in the range of 101∼103 K s−1, within one experimental run for a targeted composition [69, 70]. Direct laser AM provides another high-throughput technique to tune the heating/cooling rate, in the range from 102 to 104 K s−1, through the adjustment of laser power [72], which could be coupled with compositional mapping to search for the alloy composition with a good GFA [67, 68].
2.3. Data driven design approach
In contrast to the traditional design approach, data driven design approach, such as those based on ML [20, 66, 71, 73–78], provides an alternative to accelerate the compositional design of BMGs. Built upon high-fidelity data, ML has been proven useful, highly efficient in compositional screening for the synthesis of complex materials [20, 79–81]. For the design of next generation BMGs with ML, the sources of high fidelity data are the pre-requisite (see figure 3(a)). To date, there are a number of data sources available in the open literature for the design of BMGs, such as the handbook by Kawazoe et al published in 1997 entitled -phase diagrams and physical properties of nonequilibrium alloys [82], and the handbook by Kawazoe et al published in 1997 entitled—nonequilibrium phase diagrams of ternary amorphous alloys [83]. In addition, different authors also built their own database by collecting data from the existing BMG literature, such as the database built by Long et al in 2009 [84], by Tripathi et al in 2015 [73], by Ward et al in 2018 [76], and by Zhou et al in 2021 [17]. Unfortunately, since people tended to collect good data (good glass-forming alloys) while discarded bad by tradition, the positive data for good glass-forming alloys significantly outnumbers the negative ones in most of the existing databases, as noted recently by Liu et al [71] and Zhou et al [17]. To date, it is still an ongoing research effort and challenge to develop reliable ML models for the discovery and development of next generation BMGs considering the inconsistency and round-up errors in the reported GFA data from different groups, and the imbalance between the positive and negative data in the available databases.
Figure 3. The schematics for developing a machine learning model with classification and/or regression algorithms; (a)–(e) Reprinted from [17]. CC BY 4.0. (a) The MG database with thousands of compositions from established data sources or literature. (b) The development of data descriptors. (c) Classification algorithms used in the study of glass-forming likelihood. (d) Regression algorithms for the prediction of GFA. (e) Contour maps of predicted glass-forming likelihood diagram based on the prediction results of classification model; (f) The development of MGs with high GFA through the prediction of regression model. Reprinted from [71], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageWith the established dataset, people have applied a variety of ML algorithms to study MGs with a good glass-forming likelihood [17, 71, 74–76, 78, 79, 85–87] (see figures 3(b), (c) and (e)) or a good GFA [17, 74–76, 87–92] (see figures 3(b), (d) and (f)). These mainly include support vector machine (SVM) [17, 74, 78, 79, 92], random forest (RF) [17, 66, 75, 76, 90], Gaussian process regression (GPR) [17, 74] and artificial neural network (ANN) [17, 71, 79, 87, 89]. However, to effectively train an ML model, one has to design proper 'fingerprints' or descriptors for their data (data featurization), as shown in figure 3(b). At the present time, data featurization is mostly based on the chemical composition of alloys [78, 87, 91] or by translating a chemical composition into empirical parameters guided by the aforementioned empirical rules [66, 71, 74–76, 88–90, 92], such as mean atomic size [74, 75, 79, 87], atomic size difference [71, 74, 79, 90], mean atomic volume [74, 90], mixing enthalpy [74, 75, 79, 87], ideal mixing entropy [71, 74, 75, 79, 87], mean electronegativity [75, 79, 87, 90], electronegativity difference [71, 79], valence electron concentration [71, 75, 79, 87] and calculated density [71, 75, 87]. If one considers all individual and collective attributes of constituent elements, the number of the data descriptors designed based on the empirical rules could reach 186 [76], which suggests the intrinsic complexity of the ML based design of BMGs. However, according to Ghiringhelli [93] and Zhang et al [94], this high dimensionality of data descriptors is not conducive to effective data mining, which can easily cause problems such as overfitting and high calculation cost. Through physical modeling guided ML, Zhou et al recently developed ML models with only 8 data descriptors [17]. Despite the challenges above mentioned, people have discovered a number of new glass-forming alloys, some of which can be cast in bulk forms (see table 1 for details).
Table 1. The up-to-date reported BMG/MG compositions discovered by machine learning.
| Composition | Year | Data descriptors | Algorithms | Dmax (mm) | References |
|---|---|---|---|---|---|
| Zr59.2Cu16.2Ni12.6Al9.6Hf2.2Ti0.2 | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 5 | [17] |
| Zr65Cu17.5Ni10Al7.5 | 2018 | 213 descriptors based on empirical rules | RF | 4.4 | [76] |
| Zr60Cu17.5Ni10Al7.5Ti5 | 2018 | 213 descriptors based on empirical rules | RF | 4.2 | [76] |
| Zr55Cu23Al12.5Ni7.5Ti2 | 2018 | 213 descriptors based on empirical rules | RF | 3.8 | [76] |
| Zr47Cu23.5Al15Ni11.5Ti3 | 2018 | 213 descriptors based on empirical rules | RF | 3.1 | [76] |
| Zr55Cu20Al15Co10 | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 3 | [17] |
| Zr54Ni16Cu14Ti10Al6 | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 3 | [17] |
| Zr55Cu20Co10Ti8Al7 | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 3 | [17] |
| Zr51Co39Al10 | 2021 | 52 descriptors based on chemical compositions | Correlation-based neural network | 3 | [87] |
| (Zr62Co32Al6)95Si5 | 2021 | 52 descriptors based on chemical compositions | Correlation-based neural network | 3 | [87] |
| (Zr63Co24Al13)95Ni5 | 2021 | 52 descriptors based on chemical compositions | Correlation-based neural network | 3 | [87] |
| Zr47Cu23Ni18Al10Ti2 | 2018 | 213 descriptors based on empirical rules | RF | 2.4 | [76] |
| Zr48Cu29.5Al18Ni4.5 | 2018 | 213 descriptors based on empirical rules | RF | 2.2 | [76] |
| Zr55Cu20Ti10Co10Al5 | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 2 | [17] |
| Zr45Cu20Ti10Al10Co10Hf5 | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 2 | [17] |
| (Zr58Co11Al31)95W5 | 2021 | 52 descriptors based on chemical compositions | Correlation-based neural network | 2 | [87] |
| ZrHfAlCo | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 0.05 | [17] |
| ZrHfAlCoCu | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 0.05 | [17] |
| ZrHfAlCoNiCu | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 0.05 | [17] |
| TiZrHfCo | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 0.05 | [17] |
| TiZrHfAlCo | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 0.05 | [17] |
| TiZrHfAlCoCu | 2021 | 8 descriptors based on physical models | Adaptive boosting, GPR, ANN, SVM, RF | 0.05 | [17] |
3. Additive Manufacturing (AM)
3.1. AM based on hot fusion
3.1.1. AM based on supercooled liquids.
In parallel to compositional design, AM has also been considered as an alternative approach to fabricate BMGs by joining MG feedstock in the form of powders, debris, or cut pieces [22, 95]. Considering the poor workability and inhomogeneous plastic flow of MGs below Tg [96], the AM techniques based on liquid state fusion (or hot fusion) have been developed and long utilized for achieving strong metallurgical bonding between MGs [29, 32, 35]. In principle, liquid state fusion of MGs can be achieved in the following two ways. First, one could join MGs by increasing the working temperature to a value between the glass transition temperature, Tg, and the onset crystallization temperature, Tx [96]. In this temperature range, also termed as supercooled liquid region (SCLR), metallic liquids flow in a non-Newtonian fashion [96], enabling long-range diffusion of atoms that leads to the bonding of MGs with the assistance of high pressure [97, 98]. Besides hot fusion in SCLR, AM can be also based on molten liquids. In a molten liquid, atomic diffusivity is significantly enhanced so that no external pressure is needed for hot fusion. In practice, hot fusion based AM of MGs can be achieved through SPS [21, 22, 35, 38–40, 95, 99–104], friction joining/welding [25, 26, 29, 105–109], thermoplastic compression [33, 98, 110, 111] and selective laser melting [23, 24, 48, 112–118].
While sintering or consolidation of solids can be traced back to the early works during 1920∼1950 [102], Kawamura et al developed the method of high-temperature sintering to produce BMGs in 1988 [35]. Back then, Kawamura and co-workers ground MGs ribbons about 20 μm thick into powders and were able to sinter these powders in their SCLR into a bulk rod with a diameter of 6 mm and height of 3.5 mm. In addition, Kawamura et al also developed other sintering methods for MGs, such as warm extrusion in 1994 [36] and pulsed current sintering in 1999 [21], also termed later as SPS [102]. As illustrated in figure 4(a), in a typical SPS process, MG powders are first filled into a vacuum chamber and are subsequently subject to a pulsed high direct current and pressure. During sintering, a local electrical breakdown can take place between powders, generating plasma to clean adsorbed gases and to break refractory oxide films [21]. As a result, the hot fusion of MGs in their SCLR through SPS can achieve nearly full densification within a short period of time and under low pressures [22, 35, 95, 102]. To date, SPS is still one of the most suitable and popular manufacturing trajectories for viscous or hot fusion of MG powders [100]. In order to achieve high densification in SPS, a greater pressure (normally ⩽500 MPa), longer holding duration, and a higher loading temperature are usually required [22, 38, 40], which however needs more expensive tooling (e.g. SiC or WC) [100, 103] and can also facilitate crystallization in MGs [39, 104]. While optimization of SPS for various MGs is still an open issue, people have successfully fabricated a variety of BMGs through SPS, as shown in figure 4(b), including Fe-based [22], Cu-based [104], Al-based [99], Mg-based [101], Ni-based [40], Ti-based [39], and Zr-based BMGs [38].
Figure 4. The schematic diagrams for the AM techniques based on supercooled liquids and the photo of a typical AMed BMG sample. (a) and (b) spark plasma sintering (SPS); (a) was reprinted from [125]. CC BY 4.0. (b) Reprinted from [100], Copyright (2016), with permission from Elsevier; (c) and (d) friction joining/welding; (c) Reprinted from [29], Copyright (2004) with permission from Elsevier; (d) Reprinted from [105], Copyright (2004) with permission from Elsevier; (e) and (f) thermoplastic compression; (e) Reprinted from [111], Copyright (2020), with permission from Elsevier; (f) Reprinted from [33], Copyright (2003), with permission from Elsevier.
Download figure:
Standard image High-resolution imageDifferent from SPS, friction welding, as patented in the 1890s [119], is able to join bulk samples of different shapes via a rotary, linear or orbital mode [120]. In 2001, Kawamura et al joined Pd40Ni40P20 rods by rotary friction welding [25]. As illustrated in figure 4(c), in a typical rotatory friction welding process, one workpiece is rotated around its central axis (left) while the other keeps stationary but in alignment with the rotating one (right). The friction heat generated at the interface leads to material softening and extrusion (figure 4(d)) in order to establish strong metallurgical bonding [25, 26, 29, 105, 106]. Through rotary friction welding, people already fabricated MG–MG [105, 107] and MG-crystalline composites [26] with a strong and tough welding interface [26, 105, 107]. Recently, friction stir welding [108] and friction stir spot welding [109] were also developed to join MGs.
On the other hand, while the viscous flow of MGs in their SCLR has long been considered as an avenue for thermoplastic forming and net-shape processing [121, 122], Kuo et al successfully adapted thermoplasticity to join BMGs in vacuum in 2010 [98]. Later, Schroers et al demonstrated that thermoplasticity can even join BMGs in air subject to a pressure that is sufficient to crush brittle native oxides [33], as illustrated in figures 4(e) and (f). In such a case, once two fresh BMGs are brought to contact, thermoplasticity enabled joining can take place within milliseconds or seconds [33]. In this way, people fabricated BMG honeycomb-like architectures [110]. To date, optimization of the thermoplasticity enabled joining is still a topic of active research [111]. Apart from the above-mentioned methods, we note that the joining of BMGs in their SCLR can be also realized via reactive foil welding [123] and small-scale resistance spot welding [124].
3.1.2. AM based on molten liquids.
Aside from SCLR, AM can be also carried out by heating MGs above their melting temperatures. As atomic diffusion is greatly enhanced in this temperature range, which enables metallurgic bonding even without external pressure, the challenge becomes how one can avoid crystallization in the fusion zone and/or the heat-affected zone during cooling [30, 32, 33]. In the 2000s, Kawamura et al successfully weld BMGs by heating up their temperatures into the range of molten liquids through electron-beam welding [27, 28, 126], spark welding (pulse-current welding) [32], and explosive welding [31, 127, 128]. In these early attempts, the welding time was kept short, merely 10−2∼101 s [29, 32] and some of the weldments appeared as hard as their master alloys [27, 28, 32]. However, these early methods could merely fabricate crystal-free weldments among a few good glass formers, such as Zr- and Ti-based BMGs [27, 28, 31, 32, 126–130], which had a critical size larger than 10 mm. According to Shen et al [129], the cooling rates in these methods were estimated to be around 103 K s−1, which may not be sufficient for joining marginal glass formers without crystallization.
By comparison, laser melting is considered more suitable to join BMGs in their molten liquid state [30], owing to its high energy density, narrow spot size, and deep weld penetration [131]. In 2006, Li et al successfully laser-welded two pieces of Zr45Cu48Al7 BMGs without causing crystallization at the cooling rate well above 103 K s−1, as calculated by finite element simulations [30]. Subsequently, in order to further improve the quality of laser welding, people upgraded the laser sources by employing those with a higher power [132], a deeper penetration (e.g. fiber laser) [133], and a shorter welding duration (e.g. pulsed laser) [134, 135]. With the updated lasers, weldments could attain almost the same tensile strength as the corresponding base alloys [135, 136]. Despite these successful applications of laser welding, laser-BMG interactions, which involve heating, cooling, and crystallization, in a typical laser welding process are not fully understood from a theoretic viewpoint [131, 135], which to a certain degree hinders the wide use of laser welding.
Apart from laser welding, laser based 3D printing is another attractive AM technique to produce BMGs. According to the recent review article by Zhang et al [24], the up-to-date laser based 3D printing techniques to fabricate BMGs include selective laser melting (SLM), laser engineered net shaping, thermal spray 3D printing, laser foil 3D printing, fused filament fabrication, and laser forward transfer 3D printing. Among these 3D printing techniques, SLM may be the most popular method to be reported from 2013 to 2021 [137]. By comparison, SLM enables a high cooling rate (104∼106 K s−1) [23, 138], a better dimensional precision with a laser spot of 102∼103 μm [137, 139], and good control of geometric complexities [112]. Figure 5(a) sketches one typical operational mechanism of SLM, for which thin layers of MG powders are paved onto a base plate subject to the scanning melting of a laser beam guided by the galvano mirrors (scanner) in order to build a 3D structure layer by layer (figure 5(b)). At the current moment, the main issues with SLM-ed BMGs are to reduce their porosity while increasing their volume fraction of amorphous structures. To address these issues, people proposed three strategies which are related to laser energy densities, laser scanning trajectory, and MG powders, in order to improve the quality of 3D printed BMGs [24, 112–114, 140]. In comparison to cast bulk alloys, 3D printed BMGs are generally less stiff (lower elastic modulus), softer (lower strength), and less ductile [24] owing to the presence of micro-pores [140] and the precipitation of brittle intermetallic [48, 115, 116]. While these defects (pores and precipitates) are detrimental to the general structural applications, they are conducive to the functional applications of SML-end BMGs [48], such as for catalysis [117] and bioimplants [118].
Figure 5. (a) The schematic diagram and (b) the photo of printed BMG structures with selective laser melting (SLM). Figure (a) Reprinted from [24], Copyright (2021), with permission from Elsevier; (b) Reprinted from [112], Copyright (2017), with permission from Elsevier.
Download figure:
Standard image High-resolution image3.2. AM based on cold fusion
Recently, a fast cold fusion technology has been proposed for MGs based on high-frequency ultrasonic vibration processing, namely ultrasonic AM, which can bypass the defects generally induced by thermal effects, such as crystallization, oxidation, and element segregation [141]. As shown in figure 6(a), the ultrasonic vibration loading with a frequency of 20 kHz is applied on the single kind of MG ribbons or mixed MG ribbons, and then the dense bulk MG with metallurgical bonding can be rapidly obtained within 1 s [34]. It should be noted that the joining temperature of MGs during the ultrasonic AM is much lower than Tg, indicating that a new bonding mechanism dominates the cold fusion rather than anchoring or adhesion in SCLR [142]. The surface activation analysis of MGs by dynamic scanning probe microscopy shows that the mobility of the atoms on the surface is significantly influenced by the activation frequency, as shown in figure 6(b), the viscoelastic loss tangent (tanδ) correlating with internal friction almost increases to four times as the driven frequency shifting from 200 Hz to 70 kHz, this high tanδ value under ambient temperature is close to that of bulk samples near Tg. Meanwhile, the corresponding surface viscosity decreases to three orders of magnitude as the activation frequency increases to 70 kHz. These results demonstrate that the high-frequency vibration significantly improves the surface atom mobility, and thus enhancing the atomic diffusion in the bonding interface, which enables the fast cold fusion of various MG ribbons to synthesize composites with metallurgical interfaces among different alloy systems, such as Pd–Zr, Pd–La and Pd–Zr–La MG systems (figures 6(c) and (d)). In addition, by utilizing this reliable bonding behavior, the composites of MGs and other materials can also be fabricated by ultrasonic AM [143]. As shown in figure 6(e), the Zr-based MG ribbons mixed with high entropy alloy (HEA) ribbons are subjected to the ultrasonic loading for several seconds, then the bulk composite sample with a tunable proportion of rigid amorphous phase and soft crystalline phase can be facilely achieved (figure 6(f)). This design strategy produces an exceptional toughening effect due to the synergic deformation mechanisms of shear bands and dislocations.
Figure 6. (a) The schematic diagram to fabricate single- and multi-phase bulk sample by ultrasonic cold joining of the MG ribbons, (b) The MG interfaces activation and bonding mechanism under ultrasonic vibration, (c) Bulk composite sample of Zr-MG and HEA synthesized by ultrasonic joining, (d) The micro-sectional morphology of the HEA/MG composite, and the corresponding TEM diffraction images at different positions, (e) The shear bands formed in the MG matrix in the fracture surface of the composite under compression loading, (f) The strong embedding effect of HEA particles in the MG matrix observed in the fracture surface, (g) Stress-strain curve of HEA/MG composite obtained by ultrasonic additive manufacturing, comparing to that of the single HEA and MG, (a)–(g) adapted from [143] (2020) (© 2022 Springer Nature Switzerland AG. Part of Springer Nature.). With permission of Springer.
Download figure:
Standard image High-resolution imageTheoretically, the dynamic evolution of amorphous atomic structure under ultrasonic vibration can be described by a phenomenological model consisting of densely packed clusters (elastic matrix) and loosely packed clusters (liquid-like units) in MGs [144, 145]. Those liquid-like units can largely absorb the high-frequency vibration energy and enhance the mobility of the atoms due to the nature of viscoelasticity and low stiffness, which can loosen the densely packed clusters and extend the liquid-like regions. This variation of the amorphous structure will induce significantly softening behavior and viscous flow of MGs [146–149]. Based on this mechanism, the ultrasonic AM process can be applied to the fast cold fusion of BMGs at a very low pressure. Thus far, various pairs of BMGs including Pt–Pt, Pt–La, La–La, Pd–Pd, and Zr–Zr have been successfully bonded by ultrasonic vibration, under short processing time (<1 s) and low loading (<1 MPa) (figures 7(a) and (b)). Most importantly, the interface temperature captured by infrared thermal imaging during the ultrasonic joining is far below the Tg of the selected MGs [150, 151]. The high-resolution CT detection and SEM observation confirm the metallurgical bonding of BMGs (figures 7(c)–(e)), and the bonding interface retains the amorphous atomic arrangement (figures 7(f) and (g)). The molecular dynamics simulation demonstrated that the surface atoms require less energy to pass through the damaged oxide layer and diffuse to another side than that of the bulk interior (figure 7(h)). Thus the interface atom diffusion can be significantly accelerated to realize rapid bonding at an ambient temperature. Besides, the high instantaneous strain induced by ultrasonic loading can effectively break the rigid oxidation layer that acts as a barrier for forming reliable bonding between interfaces. And the subsequent viscous flow of MGs under ultrasonic vibration is also beneficial to the removal of oxidation debris, producing pristine MG surfaces for metallurgical bonding. The ultrasound assisted cold fusion strategy provides a new solution for the rapid AM of MGs and synthesizes novel MG matrix composites with tunable functions.
Figure 7. (a) The schematic diagram of ultrasonic joining of BMGs; Reprinted from [150], Copyright (2020), with permission from Elsevier. (b) Various pairs of BMGs bonded by ultrasonic cold fusion; Pt–Pt-based MG (S1), Pt–La-based MG (S2), La–La-based MG (S3), Pd–Pd-based MG (S4), Zr–Zr-based MG1 (S5), and Zr–Zr-based MG2 (S6). (c)–(e) SEM image of bonding interface of La–La-based MG, Pt–La-based MG, and Pd–Pd-based MGs, respectively. (f)–(g) High-resolution TEM and SAED pattern of the bonding interface of Pt–La-based MG obtained by ultrasonic cold fusion. (h) Molecular dynamics simulation of atomic mobility of MGs showing that the activated energy required for the surface atoms is lower than that in the bulk; (b)–(h) Reprinted from [151], Copyright (2021) © 2022 Springer Nature Switzerland AG. Part of Springer Nature.). With permission of Springer.
Download figure:
Standard image High-resolution imageNow, we would like to discuss the pros and cons of different AM techniques. Although hot fusion based AM techniques (e.g. laser based 3D printing and SPS) have been well established after years of dedicated research, which can be easily integrated into existing production lines for industrial scale mass production [22, 38–40, 99, 101, 104] and were already proven to be applicable to a wide range of MG compositions and geometrically complex shapes [23, 24, 112], the MG bulk structures and/or products so fabricated usually exhibit poor strength, low densification and even undesirable crystallization. By comparison, the cold fusion based AM techniques are just emerging and their applicability to different MG compositions is yet to be confirmed. However, the issue of undesirable crystallization at high temperatures can be mitigated in cold fusion of MG powders or ribbons. Here, we note that the bulk MG structures fabricated through cold fusion can nearly retain the density and strength of their feedstock materials [34], which is promising and warrants further research.
4. Recent applications of chemically complex MGs
4.1. HEMGs for refrigeration
The notion of HEMGs is related to HEAs [57], the latter of which is expected to exhibit a simple solid solution phase because of a high configurational entropy of mixing resulting from the mixing of multiple principal elements. To HEMGs, this so-called high entropy effect is more related to the confusion principle [57], which assumes that viable crystallization could be interrupted because of the competition of multiple crystallization processes in a multi-principal element alloy, hence leading to a stable metallic liquid and ultimately vitrification when supercooled. Compared to conventional MGs, such as Zr- and Fe-based MGs, HEMGs do not have a unique base element and therefore their configurational entropy of mixing is expected to be greater.
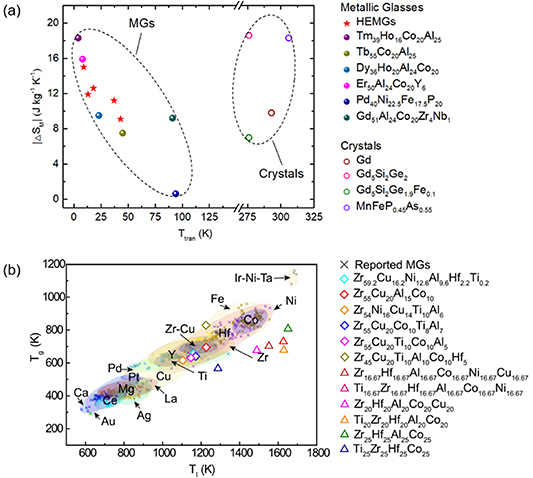
Indeed, the first report of HEMGs can be traced back to the early work of Ma et al in 2002 [152], two years before the publication of the seminar works about HEAs by Yeh et al [153] and by Cantor et al [154] in 2004. In this 2002 paper, Ma and co-workers developed a series of Ti–Zr–Hf–Cu–M (M=Fe, Co, Ni) HEMGs [152]. After that, however, the research of HEMGs was seemingly shadowed by that of conventional MGs until a surge of renewed interest was seen in 2011 [59, 62]. Up to date, a variety of HEMGs were discovered with their critical size ranging from micro- to centi-metres [155]. Although the study of HEMGs is still in its infancy, people already discovered some interesting HEMGs, such as the Ti20Zr20Cu20Ni20Be20 HEMG [63] with the critical diameter of 3 mm and its variant (by introducing Hf) with the critical diameter of 15 mm [61], the Sr20Ca20Yb20(Li0.55Mg0.45)20Zn20 HEMG with an extremely low Tg and elastic modulus that can be imprinted even at room temperature [59], and the Fe25Co25Ni25Mo5P10B10 HEMG with a high refrigeration capacity (RC) because of its large magnetic entropy change (ΔSM) [156]. Here, it may be worth noting that, compared to the prototypical magnetocaloric alloy Gd5Si2Ge2 with |ΔSM| = 18.6 J (kg K)−1 and RC = 305 J kg−1, the Ho20Er20Co20Al20RE20 (RE = Gd, Dy, and Tm) HEMG reported by Huo et al [157] has |ΔSM| = 15.0 J (kg K)−1 and RC = 627 J kg−1, which is twice of that of Gd5Si2Ge2. The excellent magnetocaloric effect makes these HEMGs an attractive candidate alloy for magnetic refrigeration. Nonetheless, the GFA of the magnetocaloric HEMGs is poor. Li et al [158] replaced Ho in the above HEMG and obtained the magnetocaloric Er20Dy20Co20Al20RE20 (RE = Gd, Tb and Tm) HEMGs with an improved GFA. Significantly, we note that the transition temperature (Ttran) of these HEMGs is much lower than 100 K, in contrast to Ttran > 273 K for typical crystalline magnetic refrigerants [159]. This behavior implies a promising application of these HEMGs as a potential refrigerant alloy in the helium and hydrogen liquefaction temperature range (figure 8(a)). Recently, Zhou et al [17] developed a series of HEMGs through ML, of which the primary crystalline products show outstanding thermal stability, as shown in figure 8(b). Compared to conventional MGs, the liquidus temperatures (Tl) of these newly developed HEMGs are much higher than what the empirical rule from conventional MGs suggests Tl/Tg ∼ 2/3. This high thermal stability of the primary crystalline products in the HEMGs may be attributed to a high entropy effect.
Figure 8. (a) A comparison of the magnetic entropy changes (|ΔSM|) under 5T and transition temperature (Ttran) among MGs and crystalline magnetic refrigerants [158, 159]. (b) Summary of Tg versus liquidus temperature of various MGs; Reproduced from [17]. CC BY 4.0.
Download figure:
Standard image High-resolution image4.2. Uranium-based MGs for nuclear energy
Uranium and its alloys are valuable nuclear fuel materials, but their applications are usually hindered by poor corrosion resistance due to the high chemical activity of uranium. In order to improve their corrosion resistance, amorphization of U alloys by rapid liquid quenching was explored with different means, such as splat quenching, arc-furnace quenching, and vacuum melting spinning, by people in the US in the 1970s, such as Ray et al [160], Giessen et al [161], and Drehman et al [162], who reported glass formation in uranium-TM (transition metals: V, Cr, Mn, Fe, Co, Ni, Pd, Ir, Os) binary systems and U–Cr–V ternary system [163]. However, glass transition could not be easily observed in the calorimetric analysis of these U-based alloys. Notably, the heat release upon crystallization of the U-TM MGs was determined to be in a range of 3∼7 kJ mol−1, which is comparable to those of some ordinary MG formers. Also, people only performed a preliminary evaluation of the corrosion resistance of the U70Cr30 and U70V30 MGs in 1976 by merely examining if there was any change in their surface color [160].
To tackle the issue of global warming, nuclear energy as a source of clean energy provides a feasible solution to the world. Compared to the conventional ceramic UO2 fuel, which has a relatively low thermal conductivity and low U content (just ∼33 at. % U), metallic nuclear fuels are more attractive because of their higher thermal conductivity and richer U concentration. In addition, we note that amorphous metallic fuels contain abundant free volumes (e.g. extra volumes between constituent elements), which leads to a stronger fission gas capacity than their crystalline counterparts. Recently, the group led by Huang et al [164–179] performed a systematic study of U-based BMGs. By means of multi-component alloying and micro-alloying, they developed a few ternary or pseudo-ternary MG systems, such as U–Co–Al [173, 179], (U, B)–Co–Al [173], U–Fe–Sn [178], and U–Fe–Al [164]. With melt spinning, glassy samples can be prepared in a large compositional range for the U–Co–Al or U–Fe–Al systems [164, 179]. Notably, these newly designed U-based MGs showed Tg > 600 K, Tx > 630 K, and Tg/Tl > 0.6, among which U60Fe27.5Al12.5 may be considered as the best glass former with Tg = 635 K, ΔTx (Tx – Tg) = 48 K, Tg/Tl = 0.612. Apparently, the GFA of U60Fe27.5Al12.5 is comparable to that of some Zr-, La-, and Mg-based BMGs [164]. At the fundamental level, crystallization of U-based MGs seemed to be governed mainly by crystal nucleation with the activation energy of 210∼240 kJ mol−1 [168, 176]. Here, it is worth mentioning that Al-containing U-based MGs displayed excellent corrosive resistance in neutral solutions as a consequence of the amorphous structure and the formation of a compact aluminum oxide film. To make U-containing BMGs, sphere-dispersed glass matrix composites were successfully synthesized in-situ with suction-casting and tilt-casting, as seen in figure 9. These composites can be manufactured for a wide composition range of U (from 3 to 40 at.% ) and in a dimension of at least 15 mm in diameter. A typical U30.03Zr28.83Ti9.66 Ni7.00Cu8.75Be15.73 composite exhibited a compressive fracture strength of 1.7 GPa and high corrosion resistance in both neutral and alkaline media [174].
Figure 9. The photograph of U-based glass matrix composites; Reprinted from [174], Copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution imageCompared to other MGs, U-based MGs commonly display low liquid fragility (m) values of 20∼28, indicating that they are strong glass formers. Furthermore, unlike other MGs, the elastic modulus of the U–Co–Al MGs seemingly does not obey the simple rule of mixture, the latter of which suggests that the elastic properties of MGs should be the weighted average of those of their constituent elements. Such two phenomena are interesting and worth further research, which might be attributed to the electronic structure of U, such as their 5f electrons. In general, these U-based MGs with a high U concentration (>60 at. %) exhibit the merits of high strength, good corrosion, and irradiation resistance, which endows them with great potential as a new fuel material in next generation nuclear fuel reactors. Therefore, we expect that the U-based MGs could play a pivotal role in the battle against global warming and also in helping various countries, such as China, to achieve carbon-neutral development. Besides, the elements making up U-based MGs usually have a large atomic weight difference, which is quite appealing in the fundamental study of MGs, such as the correlation between nano-scale heterogeneity and mesoscale properties.
4.3. Copper based MGs for antibacterial surfaces
Despite the ever-increasing investment in the medical and healthcare field, the threat from hospital-acquired infections (HAI) remains significant. Moreover, the adverse effects of HAI can be further aggravated by the emergence of antibiotic-resistant bacteria. According to predictions in recent reports, the global costs of antibiotic resistance will reach 100 trillion $ and 10 million lives annually in 2050. To tackle such a severe situation, in addition to discovering novel antibiotics, other supplemental antimicrobial agents are also urgently needed. As an effective approach, antimicrobial metal materials such as copper and silver have been widely used since ancient times to limit microbial activity. In comparison to silver, the high antibacterial effect coupled with its relatively low cost have made copper the preferred material for touch surfaces, and Cu element has already been implemented in some healthcare settings [180]. However, the poor wear resistance of copper limits its long-term applicability, which could be addressed by developing Cu-based BMGs [181], because they can unify the antimicrobial effectiveness of copper and high hardness, excellent wear and corrosion resistance, and smooth surface of BMGs.
In the past decades, researchers have demonstrated that some types of Cu-based BMGs exhibit good antibacterial activity, such as Cu50+X(Zr44Al6)50−X (X = 0, 3 and 6) [182], Cu48Zr42Ti4Al6 [183], and Cu(62∼64)–Hf(36∼38) [181], and their antimicrobial effectiveness depends on the Cu content. For example, González et al [182] indicated that the higher Cu content results in a higher reduction in colony-forming units for Escherichia coli (gram-negative) and Bacillus subtilis (gram-positive) after 60 min of contact time for the Cu–Zr–Al BMGs. The improvement of antimicrobial effectiveness was ascribed to the higher concentration of Cu ions released from Cu-based BMGs, because the Cu ions can eliminate bacteria with three possible mechanisms [184, 185]: (a) the interference to the permeability of cell membrane; (b) the degradation of genomic and plasmid DNA, and (c) the generation of reactive hydroxyl radicals through a Fenton-type redox reaction which can cause oxidation of proteins and lipids.
The studies previously described have been mostly focused on optimizing the chemical composition of materials to control their antimicrobial effectiveness. Nevertheless, other factors, including surface morphologies [186] and oxidation [187], crystalline phases content [188] are also at work. For instance, Villapún et al [187] demonstrated that the oxidation can increase the antimicrobial performance of the Cu-based BMGs, and the improvement can be explained by the fact that interphase boundaries could constitute the easy diffusion paths for Cu ions release, while the needle-shape structure of oxides could trigger mechanosensitive channels to promote the migration of copper ions into the cell. Furthermore, Villapún et al also reported that the surface texture can improve the antimicrobial ability of Cu55Zr40Al5 BMGs. Thus, it is believed that the surface modification can be adopted as an effective post approach to enhance the antimicrobial performance of Cu-based BMGs.
Although the bulk samples are useful as a screening method to investigate the effects of chemical composition and surface structure of Cu-based BMGs for desired antimicrobial performance, once the most promising compositions and surface structure are selected, they have to be produced as coatings in order to exploit their antimicrobial effectiveness in the healthcare sector. Eskandrany et al [189] coated the Cu50Ti30Ni20 film onto the SUS304 alloy by cold spraying technology and demonstrated that Cu50Ti30Ni20 film can remarkably inhibit colony formation of E. coli compared with bare SUS304. Based on the above literature review, it can be confirmed that the Cu-based BMGs are promising candidates for antimicrobial application, but the studies on Cu-based BMGs concerning their antimicrobial performance are still in their infancy.
4.4. Iron based MGs for electric transformers
Fe-based MGs have been widely used in electrical and electronic devices, including transformers, motors, etc, owing to their low coercivity (Hc), high permeability (μe), and low core loss [190]. Their typical boundary-free and long-range-disordered microstructure results in an ultra-low pinning effect on the magnetic domain and superior soft-magnetic properties [191]. Compared with the commercial Si-steel, the relatively lower Bs limits the wide application of commercial MGs, therefore motivating us to explore new alloy and process design strategies [192–197]. The conventional alloy design concept based on a homogeneous microstructure and high glass formability (GFA) has approached the limit to improve magnetization (Bs) of MGs [194–197] considering the trade-off between GFA and Bs as shown in figure 10(a). From the application viewpoint, the improvement of Bs without the sacrifice of GFA is important for the miniaturization of electric devices with attractive energy-saving and high-efficiency advantages.
Figure 10. (a) Dependences of GFA and Bs on the ferromagnetic element (Fe(Co, Ni)) content in different alloy systems, showing the GFA and Bs limits in the Fe-based metallic glass. (b) Atomic number and radius of the component elements in Fe(Co, Ni)SiBPC alloys (inset shows the mixing enthalpy). (c) Other influences of Co/Ni alloying; (a)–(c) [200] (2021) © 2022 Springer Nature Switzerland AG. Part of Springer Nature.). With permission of Springer. (d) Fe content of the electric steels and commercial MGs [198]. (e) Image of annealed Fe85.7Si2.3B9.7P1.5C0.8 ribbon after bending for 180°. (f) SEM image of the shear zone in the bending region; (d)–(f) [198] (2017) © 2022 Springer Nature Switzerland AG. Part of Springer Nature.). With permission of Springer.
Download figure:
Standard image High-resolution imageRecently, Wang et al reported novel alloy design concepts based on the high-entropy, cluster stabilization, and magnetic-intercoupling enhancement mechanisms [194, 198, 199] [shown in figures 10(b) and (c)]. By using these new approaches, Fe82.7–85.7Si2–4.9B9.2–11.2P1.5–2.7C0.8 MGs with a distinctly high Fe content of 93.5–95.5 wt.% were readily developed [198] (see figure 10(d)). However, it was subsequently found that the high Fe content does not lead to an expected super-high Bs, and it appears that the maximum Bs is attained when the Fe content reaches about 80%, such as in the Fe84.2Si2.1B11P1.9C0.8 alloy [198] as illustrated in figure 10(a). As inspired by these results, Zhao et al changed to a new strategy for a stronger magnetic-interaction by incorporating Co/Ni in the high Fe-content alloys and developed Fe71(Co, Ni)15B9.5Si2P2C0.5 alloys with a high Bs value up to 1.86 T via the fourfold combinational approaches [200]. This strategy should apply to a wide range of Fe-based MGs in overcoming the Bs-GFA trade-off. Moreover, it is worth noting that the high Fe-content alloy ribbon is ductile and can withstand 180° bending prior to fracture, which is desirable for engineering applications as shown in figures 10(e) and (f) [198, 201].
4.5. Refractory MGs for applications under extreme conditions
Over past decades, refractory metallic glasses (RMGs) have attracted increasing attention owing to their great potential for use in many applications, such as military, aerospace, energy production, etc [202–204]. Similar to other types of MGs, RMGs exhibit high strength, high hardness, and high resistance with respect to wear or corrosion [204]. More importantly, RMGs display extremely high thermal stability against crystallization at high temperatures (>700 °C) [204]. As a result, RMGs could be an ideal candidate material for use in a highly corrosive and high temperature environments, such as in molten salts or supercritical water often used in nuclear power plants [205, 206].
Being one prototypical RMG, iridium-based MGs (e.g. Ir–Ni–Ta–(B)) were developed recently by Li et al [19] using combinatorial methods. According to [19], the iridium-based Ir–Ni–Ta–(B) MGs were found to possess a high Tg, Tx, good GFA, and high-temperature strength in addition to high resistance against corrosion and oxidation. According to the recent study [19], the boron-containing Ir–Ni–Ta based MG exhibits a high Tg of 889 °C and a wide SLR of 136 °C, indicative of their superior thermal stability. Moreover, we note that, while the Ir–Ni–Ta MGs can be synthesized into 2 mm-diameter glassy rods in a wide compositional range that contains 20%–35% Ir, 35%–40% Ta, and 25%–40% Ni, the addition of B can significantly enhance its GFA with the critical diameter increased to 3 mm [6]. Also, as the temperature rises near Tg, the Ir–Ni–Ta system exhibits unprecedented strength of >4 GPa, unlike other conventional Ir- and Co-based BMGs that show notable drop in strength. Moreover, similar to other typical high-temperature alloys such as Inconel 718, these Ir–Ni–Ta MGs can retain their high-temperature strength even at high temperatures [19].
Recently, it was reported that the Ir–Ni–Ta MGs had excellent mechanical and chemical properties at micro-/nano-scales. For instance, Wang et al [207] reported that Ir–Ni–Ta MGs displayed a large plastic strain up to 35% in micro-compression with the micropillar size ranging from ∼500 nm to ∼5 μm; and its yielding strength can reach up to 7 GPa, remarkably higher than those of most metallic materials. Furthermore, the enhancement of deformability for Ir–Ni–Ta MGs at a small scale has been rationalized in terms of the competition between the cracked-induced fracture instability and the intrinsic shear instability. In light of these results, it was suggested that the Ir–Ni–Ta MGs might be used in next generation micro-devices that are expected to deploy at high temperatures or under extreme conditions [207].
Furthermore, Wang et al [208] found that the Ir25Ni33Ta42 RMG nanofilm exhibits high intrinsic activity and superior stability at an ultralow Ir loading of 8.14 μg cm−2 for hydrogen evolution reaction (HER) in 0.5 m H2SO4. The Ir-based RMG film is believed to be the most active HER heterogeneous catalysts in terms of an overpotential of 99 mV for a current density of 10 mA cm−2, a small Tafel slope of 35 mV dec−1, and high turnover frequencies of 1.76 and 19.3 H2 s−1 at 50 and 100 mV overpotentials respectively. When compared to other catalysts, such as MGs, sulfides, phosphides, and precious-metal-containing catalysts (see figure 11), the HER performance of the Ir25Ni33Ta42 RMG film is exceptionally good, which may be attributed to a synergistic effect of alloy composition and amorphous structure [208].
Figure 11. (a) Tafel slope versus the overpotential at 10 mA cm−2 for the Ir25Ni33Ta42 MG film or HER in 0.5 M H2SO4 and other existing MG catalysts for HER. (b) turnover frequency (TOF) values averaged over all surface sites of the Ir25Ni33Ta42 MG film compared with the Ir film, Pt film, and other highly active HER catalysts, including molybdenum sulfide-based catalysts, transition metal phosphides, and precious-metal-containing catalysts; [208] John Wiley & Sons. [© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution image4.6. MG coatings for healthcare
Although the structural applications of BMGs are hindered by their low temperature brittleness, low dimensional MGs (e.g. thin films [47], nanowires [209], nanotubes [210], nanomembranes [211]) attracted a great deal of interest in different fields, such as nano-devices [212], microfabrication [213], bio-implants [214], even healthcare [47] and electrocatalysis [215]. Owing to the plasticity size effect [216, 217], low dimensional MGs are generally ductile and stretchable. For instance, compared with conventional metallic and ceramic coatings, MG thin films possess an excellent combination of strength and ductility, as seen in figure 12(a) [218]; therefore, they are considered as an ideal candidate coating material for structural applications. In addition, some metallic-glass thin films, such as Ti50Cu42Ni8, are bio-compatible and were already proven to have no cytotoxicity effect on biological cells [45]. By alloying with Cu and Ag, the metallic-glass thin films (e.g. Zr61Al7.5Ni10Cu17.5Si4) even show outstanding antimicrobial capabilities [219]. This unique combination of mechanical and biomedical properties renders metallic-glass thin films with a great potential for applications in the biomedical and healthcare industries.
Figure 12. (a) A comparison plot of ductility versus yield strength of various coating materials; Reprinted from [218], Copyright (2020), with permission from Elsevier. (b) The wound healing after seven days for hairless skin grafting; Reprinted from [221], Copyright (2019), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIn 2017, Cai et al [214] coated the commercial Ti–6Al–4V, the widely used alloy for dental implant, with the Zr60.14Cu22.31Fe4.85Al9.7Ag3 thin film. As a result, the fretting resistance of the coated dental implant increased by almost 3 times with a decreasing bio-corrosion rate. On the other hand, due to the absence of crystalline microstructures and defects, metallic-glass thin films can attain a much smoother surface than crystalline films, particularly after appropriate annealing [220]. Making use of this attribute, people can improve the smoothness and sharpness of surgical blades by coating them with metallic-glass films. For instance, Chu et al [221] coated cutting blades with the Zr53Cu33Al9Ta5 metallic-glass thin film, which could reduce the cutting force of microtome blades by ∼51%. Meanwhile, the metallic-glass coatings can reduce the increase in the roughness of dermatome blades from 70% to only 8.6% after their use in a split-thickness skin graft surgery for hairless skin. Meanwhile, the wounds left by the MG-coated blades were noticeably smaller, as seen in figure 12(b), which could be attributed to the smoother surface and thus a lower coefficient of friction. Apart from surgical blades, metallic-glass films can be also coated onto medical needles, which are another common tool that needs a low insertion force and hence a less resultant trauma. Up to date, metallic-glass thin films have already been coated on dental needles [222] and tattoo needles [223]. A good example is the 304 stainless-steel dental needles coated with the Zr53Cu33Al9Ta5 metallic-glass thin film. After coating, the insertion (or retraction) force of a 304 stainless-steel dental needle was reduced by ∼66% (or ∼72%) when the needle was inserted into (or retracted from) a polyurethane rubber block [222]. Notably, this beneficial effect came along with the reduction in the coefficient of friction by one order of magnitude. As tattooing became a fashion recently worldwide, non-sticky tattoo needles, which can retain tattoo patterns while diminishing skin injuries, became attractive. Recently, Chu et al [223] coated tattoo needles with the Zr62.4Cu22.4Al9.8Ni5.4 thin films, which showed less tissue adherence after insertion and improved performance by preventing 57% spread of pigments to surrounding skins. With the MG-coated needles, one can attain very fine tattoo lines and make a high-resolution tattoo pattern. Most importantly, the use of the MG coated needles can lead to the closure of trauma within 2 h after insertion and only five days are needed for entire healing, which is significantly faster than that created by bare needles.
Before moving to the summary, we note that, despite the excellent thermoplastic formability and castability of MGs, most of them contain precious and/or rare-earth metals, such as Pd, Au, Ag, Zr, Hf, Be, and others , as shown in figure 13(a). Therefore, the price of MG-made products could be much higher than those made of conventional alloys. This poses an issue of economic viability for any real applications of MGs. Figure 13(b) compares the estimated material cost of MGs in comparison to that of Ti–Ni alloys, Al alloys and steels. Evidently, the materials cost of MGs could be several orders of magnitude higher than those conventional alloys. Undoubtedly, these extremely high price makes MGs unaffordable or replaceable in conventional applications, particularly considering their low temperature brittleness under tension [42]. In addition, MGs can easily crystallize at temperatures above Tx, which can lead to crystallization induced embrittlement [122]. Therefore, they are not suitable for high temperature structural applications unless one could significantly increase Tg and Tx and design the so-called high temperature MGs, as discussed in [19].
Figure 13. (a) The prices of elements in forming MGs. (b) A material cost comparison between different types of BMGs and conventional alloys.
Download figure:
Standard image High-resolution image5. Summary
To sum up, we provide an overview in this work on the recent development of chemically complex MGs by focusing on the new methods for alloy design, AM, and some new and interesting applications. Since the 1960s, people have developed over 5000 MG compositions including over 800 BMGs. However, with the approach of big data, we expect that one could discover and develop more new MG compositions with excellent GFAs at a much faster pace with higher efficiency. Putting aside GFAs, AM provides an alternative way to produce bulk sized MGs for industrial applications. After 30 year continuous development, we now have a variety of AM methods established based on the principle of either hot or cold fusion. Compared to cast MGs, manufactured MGs are not restricted in size and geometry by their GFAs, therefore more amenable to structural and functional applications. With these new tools and developments, we anticipate that one may face another surge of renewed interest in chemically complex MGs in near future over a very wide range of applications.
Acknowledgments
Y Y acknowledges financial support provided by the Research Grants Committee (RGC), the Hong Kong government, the General Research Fund (GRF) with Grant Nos. CityU11200719 and CityU11213118, and also by the City University of Hong Kong through an internal grant with Grant No. 7005438.