Abstract
Carbon dots (CDs) is a kind of carbon nanoparticles with a plentiful of surface functional groups and tunable emission with different excitation wavelength. Broadly speaking, CDs include carbon nanodots, carbon quantum dots, graphene quantum dots, carbonized polymer dots. Due to the unique nature, they are explored for various applications in the bio-related fields such as bioimaging, sensor for ion and (bio)molecules, catalyst, LED and other fields. They are viewed as great alternative tracers to the current fluorescent biomarkers in personalized nanomedicine and surgery operation monitoring. In this review, we summarized the recent progress in the development of CDs, including improvement in fluorescence properties, two-photon fluorescence, and integration with other modalities as theragnostic agents. Specifically, we discussed the preparation of dual-modal imaging agents to improve the accuracy of diagnosis, the combination of imaging and targeting functionality for the effective accumulation of biomarkers, and the integration of imaging and therapeutic agents to effectively monitor the localization and concentration of therapeutic agents. Finally, the theragnostic agents composed of three functionalities (e.g. targeting, imaging, and therapy) were summarized to provide readers with future perspectives in this field.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Bioimaging covers a broad range of imaging modality, including optical imaging (OI), nuclear magnetic resonance (magnetic resonance imaging, MRI), computed tomography imaging (CT), positron emission tomography (PET) ultrasonic imaging, etc The images not only provide photography of biological matters, but also offer a great deal of molecular and spatial information depending on the types of imaging modality. Among these imaging modalities, fluorescent imaging technique carry significant weight, because of their high sensitivity, high resolution, affordable instruments and easy observation. A diverse set of fluorescent probes have been explored for fluorescent bioimaging, such as organic fluorescent molecules [1], quantum dots [2, 3], gold nanocluster [4, 5], aggregation-induced emission (AIE) nanoparticles [6, 7], near-infrared dyes or nanoparticles [8], and up-conversion nanoparticles [9–11]. Beyond bioimaging, these fluorescent probes can be combined with other functionalities to create dual/multi-imaging probes, targeted imaging units, or theragnostic agents to performance multiple functions at once [12].
Carbon dots (CDs), as the emerging fluorescent probes, have attracted much attention in various research fields since their discovery in 2004 [13, 14]. With the advancement in preparation and surface passivation methods, the fluorescent quantum yield (QY) of CDs have been improved to the levels comparable to other fluorescence probes [15]. The improved fluorescent properties have then led to extensive investigation of using CDs in bio-applications. Comparing with traditional fluorescent agents, CDs have several distinct merits. (i) A broad range of excitation wavelengths overcomes the limitation of traditional three channels in the fluorescent bioimaging device. (ii) CDs also exhibit super chemical and photo-stability due to the graphite core, which is a great advantage compared to the easily decomposed, commonly used organic molecular dyes. (iii) CDs have low cytotoxicity and excellent biocompatibility because their main composition, carbon, serve as natural sources for organism such as citric acid, urea, glucose, amino acid or other biomasses. Despite the advantages of CDs, the weak the long-wavelength emission (such as red) of CDs does limit their uses in bioimaging due to the low tissue penetration depth. Beyond the long wavelength emission in the IR and NIR region, the PL QY also another critical factor for the high contrast bioimaging. Further advancement is needed to improve the PL QY for high intensity or contrast image. Recently, several groups have been reported highly fluorescent red-emissive CDs with PL QY as high as 86% [16] and the emission peak was also extended NIR region (725 nm) [17]. This progress shined light of the further application of CDs in the bio-related applications [18]. In addition to the efforts of improving the PL QY of CDs, studies on two-photon emission of CDs have been reported [19]. The two-photon emission provides another potential route to overcome the low tissue penetration depth of the short-wavelength light source. NIR light has large penetration depth, which can be used as the excitation light and generate fluorescence in the visible light region for easy observation [17, 20, 21]. Furthermore, room temperature phosphorescence (RTP) properties of CDs were observed in the various organic, polymer or inorganic matrixes. Recently, Lin et al reported that heteroatom with favoring n → π* transition, such as P doped CDs, promoted intersystem crossing process, leading to ultralong lifetime RTP [22]. This property of CDs benefits the bioimaging without external excitation light. Based on these unique optical properties, fluorescent CDs are great potential candidates for fluorescent bioimaging. In addition, CDs also exhibit great potential on the sensor for ions [23–25] and (bio)molecules [26–28] and antibacterial materials, which introduced the positively charged quaternary ammonium groups or silver nanoparticles [29–33]. In this review, we are focusing on the fluorescent carbon dots used for fluorescent probes for bioimaging and related multiple function composites for biological applications.
2. Bioimaging
Generally, black carbon materials have excellent thermal and conductive properties due to their conjugated structures, and are treated as non-light emitting materials. On the other side, organic fluorescent molecules are carbon-based materials with heteroatoms like O, N, and S. Fluorescent CDs successfully combine the conjugated carbon backbone and heteroatoms, exhibiting some unique optical properties superior to others. For example, the excitation wavelength dependent emissions overcome the limitation of the fluorescent agents that can only be excited by specific excitation wavelength. The origins of the fluorescence of CDs are attributed to down-conversion (DC) or up-conversion (UC). Down-conversion refers to the conventional PL that is a low energy Stokes-shifted emission generated by a high energy light source. Up conversion makes use of sequential absorption of multiple low energy photons to produce higher energy anti-Stokes luminescence [34].
2.1. Down conversion emission
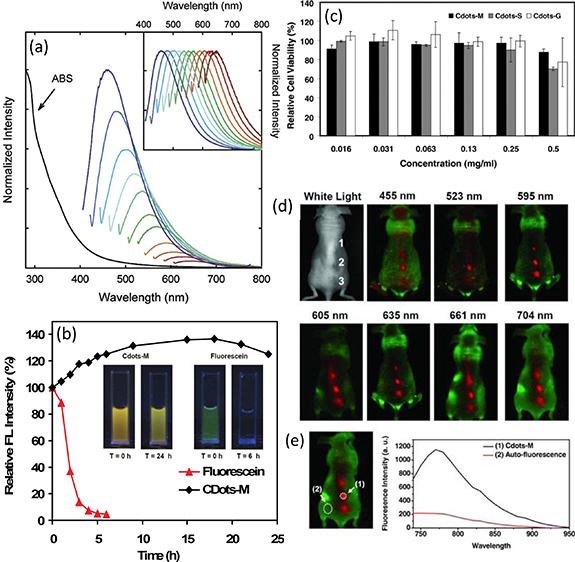
The PL QY of CDs with excitation dependent emission was greatly enhanced to ∼20% at 440 nm excitation by the surface passivation of PEG1500N reported by Sun's group [15], which opened the door for practical applications, such as the bioimaging (figure 1(a)). This application in bioimaging was further demonstrated by the same group by the in vitro and in vivo experiment [35]. Comparing with typical quantum dots (CdSe/ZnS), CDs showed comparable optical performance, low cytotoxicity, and superior biocompatibility [36]. For example, the MCF-7 cell viability kept at ∼80% with 100 μg ml−1 CDs solution treatment. No abnormity in major organs was observed on mice exposed up to 40 mg Kg−1 body weight for 28 d. CDs prepared from carbon nanotubes by chemical oxidation route exhibited much better photostability than normal organic dye molecules (figure 1(b)) [37]. The in vitro cytotoxicity tests of these CDs on the human embryonic kidney (HEK) 293 T cell line showed no significant reduction in cell viability even at ultra-high concentrations up to 0.5 mg ml−1 (figure 1(c)). Comparing to traditional dye, CDs have not only low cytotoxicity, but also excitation dependent emission that means the CDs can be excited by arbitrary wavelength. Although the emission intensity remains low in long-wavelength emissions, but the tissue autofluorescence background decreases as well at long-wavelength. Thus, the signal-to-noise ratio can be actually improved in the red and NIR excitation. The excitation dependent emissions allows for systematic investigation of CDs in in vivo bioimaging at different excitation wavelength ranging from 455 to 704 nm. A relatively high contrast was still clearly seen even excited at 704 nm (figure 1(d)). The in vivo biodistribution studies of CDs labeled by 125I (125I-CDs) indicated that preferable accumulation of CDs in the RES organs such as liver, kidney, and spleen after intravenous injection. The kidney uptake of CDs was relatively high at the early stage; suggesting CDs renal clearance. The great biocompatibility and easy excitation at long wavelength make them great candidates for bioimaging.
Figure 1. (a) The absorption (ABS) and luminescence emission spectra of PPEI-EI carbon dots in an aqueous solution. The emission spectral intensities are normalized to quantum yields (normalized to spectral peaks in the inset). Reprinted with permission from [35]. Copyright (2006) American Chemical Society. (b) Photostability comparison of Cdots-M and fluorescein under the high-brightness cold light source over time. Photos of Cdots-M and fluorescein under UV light before and after being exposed to the high-brightness cold light source. T refers to the time of exposure. (c) Relative viabilities of 293 T cells (normalized to the untreated control) after being incubated with Cdots at varying concentrations for 24 h. Error bars were based on standard deviations of triplicated samples. (d) In vivo fluorescence imaging in vivo fluorescence images of a Cdots-M-injected mouse. The images were taken under various excitation wavelengths at 455, 523, 595, 605, 635, 661, and 704 nm. Red and green represent fluorescent signals of Cdots-M and the tissue autofluorescence, respectively. B) Signal-to-background separation of the spectral image taken under the NIR (704 nm) excitation. The Cdots fluorescence was well separated from the tissue autofluorescence background. [38] John Wiley & Sons. (© 2012 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim).
Download figure:
Standard image High-resolution imageIn order to achieve high-resolution fluorescent bioimaging, both excitation and emission lights of CDs need to pass through the tissue. Therefore, the tissue penetration depth (TPD) is a critical factor for the fluorescent bioimaging. The NIR light (700–2500 nm) possesses higher TPD than visible light because the tissue scatters and absorbs less light at longer wavelengths [38, 39]. With deep tissue penetration goal in mind, developing methods to synthesize CDs with a longer wavelength and NIR emission has become a research focus in the field. Bhunia et al [40] reported the synthesis of blue, green, yellow and red emissive CDs from carbohydrates by thermal oxidation of concentrated H3PO4. A red emission at 600 nm of CDs with 7% PL QY was observed in the acidic solution when excited at 385 nm. However, the red emission was transferred to green emission in a neutral or basic solution. After functionalizing with PEG and TAT peptides or conjugated with folate targeting ligands, these functional CDs can effectively label the HeLa cells, indicated by fluorescent images. These cellular studies also demonstrated the low cytotoxicity of CDs at 200 μg ml−1.
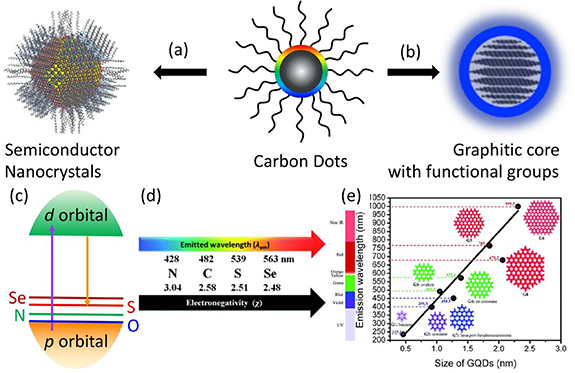
To achieve red and NIR emissive CDs, different routes have been developed according to the fluorescent mechanism. One route is treating CDs as a semiconductor nanoparticle, the bandgap of the semiconductor can be tuned by introducing heteroatom, which changes the valance band and/or conduction band position (figure 2(a)). Heteroatoms (B, N, O, P, S, Se and transition metal ions) have been employed to synthesize doped CDs with multiple color emission [41–47]. For examples, Yang et al [42] related the electronegativity of heteroatom to the emission wavelength of CDs where the lower electronegativity led to an increase in the valance band and a narrow bandgap (figures 2(b)–(c)). The narrow bandgap make the emission shift toward red. However, the concentration of dopant is another critical factor for the emission wavelength since the dopant can made the defect energy level into a deep energy level, Qu et al demonstrated that the green emission may be generated from high concentration Se doped CDs [48]. Another mechanism is treating CDs as nanoparticles with a graphite core and surface functional group shell (figure 2(b)) [49, 50], where the emissions of CDs can be regulated by changing the graphitization and functional groups. Chen's group simulated the fluorescence of CDs by treating CDs as conjugated molecules [51], where the effective conjugation length increases led to red shifts in emission peaks (figure 2(e)). On the other hand, the surface functional groups also caused red shift t in the emission of CDs. Citric acid as a common carbon source for CDs was extensively employed for the synthesis of CDs. Sun's group reported the emission of CDs prepared from citric acid and diethylenetriamine (DETA) in different solvents [52]. Furthermore, multiple color emissive CDs from blue to red were obtained by tuning the ratio of citric acid and urea in DMF and reaction temperature. The typical blue, green and red emitting CDs exhibit 52%, 35%, and 12%, respectively [53]. Rogach group also prepared the full spectrum emissive CDs from citric acid and urea in formamide by a two-step route [54]. In the above cases, the non-conjugated organic molecules were used for the carbon source. In 2015, Lin's group reported the blue, green and red emissive CDs from the phenylene diamine isomer [55]. After that, various conjugated molecules were introduced to synthesize red or NIR emissive CDs, such as e o-benzene diamine and dopamine [56–58]. The progress on the red and NIR emissive CDs greatly enhanced the bioimaging applications.
Figure 2. Schematic of carbon dots can be treated as semiconductor nanocrystals (a) and nanoparticles composed of a graphitic core and functional groups (b). (c) The energy level diagram of semiconductor, their conduction band and valance band are composed of d orbital of transition metal ions and non-metal anion. The emission wavelength of non-metal atom doped CDs is related to the electronegativity of the dopant element. (d) The effect of the doped element electronegativity on emission wavelength of carbon dots [42]. (a)–(d) Reproduced from [43] with permission of The Royal Society of Chemistry. (e) Theoretical calculation of the emission wavelength dependence on the size of GQDs [51]. Reproduced from [52] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution image2.2. Up-conversion emission
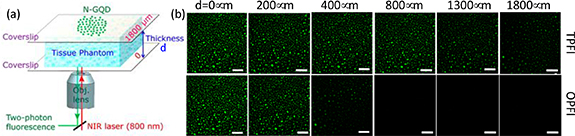
Photon up-conversion is a process in which the sequential absorption of two or more photons, leading to the light emissions at a shorter wavelength than the excitation wavelength. Up-conversion emission materials are ideal fluorescent agents for bioimaging because they can generate visible light emissions excited by TPD NIR light. CDs did not only exhibit down conversion emission but also showed up-conversion emission. Up-conversion bioimaging effective depresses the fluorescent background and provides a high contrast image. The CDs prepared from the top-down route and passivated by poly-(propionylethylenimine-co-ethylenimine) (PPEI-EI, with EI fraction ∼20%) can emit green light excited by argon ion laser (458 nm) and by femtosecond pulsed laser (800 nm) [19]. Furthermore, the human breast cancer MCF-7 cells were cultured and labeled both cell membrane and the cytoplasm with the passivated CDs. Beyond cell two-photon imaging, Kong et al [59] showed the 4'-(aminomethylphenyl) -2,2':6',2''-terpyridine (AE-TPY) modified CDs for two-photon imaging of living cells (A549) and living tissue at depth of 65–185 μm. Liu et al [60] modified the graphene oxide to generate N doped graphene quantum dots (N-GQDs) by hydrothermal treatment in the dimethylformamide (DMF). The two-photon absorption cross-section of N-GQD reaches 48 000 Göppert-Mayer units, which far surpasses that of the organic dyes and is comparable to that of the high-performance semiconductor QDs. They also demonstrated that the N-GQD can achieve a large TPD of 1800 μm (figure 3) which significantly extended the fundamental two-photon imaging depth limit. Jiang et al [55] prepared CDs from phenylene diamine isomers (o-, m- and p-), which exhibit green, blue and red emission. In addition, these CDs can generate yellow, green, and red emission excited by the laser of 800 nm. Lin's group [20] further developed NIR emissive CDs from glutathione formamide (3 wt%) solution, which can generate NIR emission (∼683 nm) by excitation of 850 nm. Lu et al [56] synthesized NIR CDs from dopamine and o-phenylenediamine by hydrothermal method. The two-photon luminescence spectra exhibit two peaks at 660 and 710 nm under 800 nm laser excitation. Those make the up-conversion emission shifted from green to red and even NIR. The above reports provided that the up-conversion emission under NIR-I windows (700–950 nm) excitation [61]. Li et al [21] demonstrated that the CDs prepared from citric acid and urea in DMF solution and modified with dimethyl sulfoxide (DMSO). Multiple-photon fluorescence is realized under the excitation of NIR-II windows (1100–1350 nm). The emission peaks at 654 and 766 nm are observed under the excitation laser of 1200 and 1400 nm. The progress in the optical properties make the CDs be a great candidate for potential fluorescent bioimaging agent and further turn into the practical application in the fluorescent imaging.
Figure 3. (a) Schematic of the setup used for two photo fluorescence imaging (TPFI) of N-GQDs in tissue phantom with different thicknesses. (b) The penetration depth of N-GQDs for TPFI (top panel) and one-photon fluorescence imaging (OPFI bottom panel) in tissue phantom (all scale bar: 100 μm) [60]. Reprinted with permission from [61]. Copyright (2013) American Chemical Society.
Download figure:
Standard image High-resolution image3. Multimodal bioimaging
Beyond the fluorescent bioimaging, A wide variety of bioimaging techniques (e.g. ultrasound, computed X-ray tomography, magnetic resonance imaging (MRI), and positron emission tomography) are commonly employed for clinical diagnostics and scientific research. While all of these methods use a characteristic 'energy-matter' interaction to provide specific details about biological processes, each modality differs from another in terms of spatial and temporal resolution, anatomical and molecular details, imaging depth, as well as the desired material properties of contrast agents needed for augmented imaging. On many occasions, it is advantageous to apply multiple complementary imaging modalities for a faster and more accurate prognosis. Since most imaging modalities employ exogenous contrast agents to improve the signal-to-noise ratio, the development and use of multimodal contrast agents are considered to be highly advantageous for obtaining improved imagery from sought-after imaging modalities [62].
3.1. MRI and FI dual modal imaging
MRI is a powerful, non-invasive imaging tool for diagnosis and post-therapy with great spatial resolution for in vivo imaging. The integration of MRI imaging and fluorescence imaging adds additional values of imaging quality by offering high sensitivity. Currently, there are three main types of MRI contrast agents, paramagnetic metal complexes, gadolinium (Gd) OR Mn complexes, nanoparticle-based T1 contrast agents, such as ultra-small iron oxide nanoparticles, Gd oxide nanoparticles, and Mn oxide nanoparticles. Integration of any type of MRI contrast agents with CDs will lead to bifunctional imaging agents.
In 2011, Srivastava and Gajbhiye [63] prepared the CDs from citric acid and L-lysine by calcination at 573–773 K. The as-prepared CDs exhibit an excitation dependent emission and maximum emission at 400 nm. To one's surprise, room-temperature magnetic studies revealed the ferromagnetic nature of CDs and the presence of an anti-ferromagnetic phase along with a ferromagnetic phase below 50 K. Although the magnetic properties are too weak to apply for MRI, it points out a new direction for the combination of optical and magnetic properties together. Furthermore, this group introduced citric acid iron oxides nanoparticles into the pyrolysis and carbonization reaction [64]. The iron oxide nanoparticles doped CDs composites (IO-CDs) were produced with both fluorescent and magnetic properties, which can be employed for magnetic resonance/fluorescence multimodal bioimaging. The cytotoxicity and cell imaging of IO-CDs demonstrated low cytotoxicity of these IO-CDs and good fluorescence imaging capability. In vivo magnetic resonance/fluorescence imaging also demonstrated that IO-CDs could be potential both T1/T2 contrast agents as well as an effective fluorescent biomarker. Zhou et al reported the Fe3O4@CD prepared from ferrocene in acetone with the presence of H2O2 by the solvothermal route [65, 66]. The 1D nanoparticle chain could be self-assembled under the magnetic field. In vitro experiment exhibited the Fe3O4@CD could be MRI (T2 relaxation) and fluorescent dual-modal bioimaging agent.
Beyond the iron oxides, the transition metal ions doped CDs also exhibit both fluorescence and ferromagnetic properties. Ji et al [67] synthesized Mn doped CDs (Mn-CDs) from citric acid, urea, and MnCl2 by the microwave-assisted route. The Mn-CDs displayed blue-green dual emission with changing the excitation wavelength from 340 to 460 nm. The r1 relaxivity of Mn-CDs can reach 6.23 mM−1 s−1 which is comparable to the most Mn-based nanoparticles [68, 69]. That makes the Mn-CDs suitable for acting as an efficient T1 contrast agent. In vivo MR imaging discloses a clear image of brain glioma region after injection for 30 min and 2 h. Ex vivo optical images exhibit the accumulation of Mn-CDs in the brain glioma region. In the same issue, Rub Pakkath et al synthesized TM2+ (Mn2+, Fe2+, Co2+, and Ni2+) doped CDs from Lemon juice, EDA and TM acetate salt by similar microwave method [46]. These TM doped CDs showed the excitation-dependent emission with decent PL QY of 35%–75% and the maximum r1 relaxivity of 0.34 mM−1 s−1 for Mn2+ doped CD. When 10 ml TM2+ doped CDs were injected into zebrafish, in vivo fluorescence, and magnetic resonance imaging confirmed TM doped CDs as suitable agents for T1-weighted contrast with bright green emission at the administrable quantities.
Comparing with transition metals oxides and ions, gadolinium (III) based contrast agents are commonly used in the clinical diagnosis. In 2012, Bourlinos et al [70] firstly demonstrated the GD(III) doped CDs combined fluorescence with a strong MRI T1 contrast agent. The Gd-CDs exhibit that excitation-dependent emission with maximum emission at 440 nm under excitation of ∼360 nm. The Gd-CDs displayed comparable signal enhancement in T1-weighted images to commercial Gadovist. On the contrary, CDs showed no such enhancement. Chen et al [71, 72] directly calcined gadopentetic acid (Gd-DTPA) at 300 °C for 2 h in the air to form a layer of inert carbon-coated Gd@C, which afford not only a high r1 relaxivity (5.88 mM−1 s−1) but also strong blue fluorescence with PL QY of 19.7% (figure 4(a)). Moreover, cRGD, a tumor-targeting peptide, was conjugated onto the Gd@CDs surface to obtain RGD-Gd@C. The inert carbon coating effectively prevents the leakage of Gd into the surrounding (figure 4(b)). The cytotoxicity of Gd-CDs was investigated in the presence of Ca(II) in the cell incubation media for the test of the particles' stability against transmetallation (figure 4(c)). There was no significant drop in cell viability for Gd@CDs concentration of 0–100 μg Gd ml−1. On the contrary, the Gd-DTPA showed an IC50 of 33.1 μg ml−1. In vivo MRI was carried out on the U87MG tumor-bearing mice. Strong contrast was observed in the bladder indicating that renal clearance of injected nanoparticles. After 4 h, signals in the normal tissues had receded to the normal level for both RGD-Gd@CDs and Gd@CDs injected animals. Meanwhile, there was a signal enhancement of 42.6 ± 0.08% in tumors of in animals injected with RGD-Gd@CDs compared to those injected with Gd@CDs (figure 4(d)). Harnessing the strong fluorescence of Gd@C-dots, we conducted immunofluorescent studies with the tumor tissues. Indeed, there was a good correlation between RGD-Gd@CDs and positive integrin β3 staining (figure 4(e)), confirming that the tumor retention was mainly mediated by RGD-integrin interaction. Afterward, various methods are developed for the preparation of Gd-CDs. Such as, hydrothermal route [73–76]. Shi et al [77] developed a post-modification route to modified the CDs with DTPA and form the Gd complexes with surface DTPA groups. Recently, Chen et al [78] reported the Gd@CDs prepared from p-phenylenediamine and Gd(NO3)3 in ethanol by hydrothermal route. The as-prepared Gd-CDs show strong emission at 570 nm, good Gd encapsulation (37.6%) and high r1 relaxivity (16 mM−1 s−1, 7 T). Those lead to high PTD for fluorescent imaging and high contrast for MRI.
Figure 4. Cytotoxicity and cell targeting of dual-modal bioimaging. (a) Photoluminescence intensity (ex/em 360/425 nm) change when Gd@C-dots were incubated in buffers of different pH values. (b) Gd release from Gd@C-dots over time. The nanoparticles were incubated in solutions with pH 5 or 7.4. #: The overall Gd concentrations in the solutions. (c) Cell viability, evaluated by MTT assays with U87MG cells. 2.5 mM Ca(II) was added to the incubation medium. (d) T1-weighted coronal MR images. Significant signal enhancement was observed in tumors of animals injected with RGD@Gd-dots. (e) Immunofluorescence histology study with tumor samples. A good overlap was observed between RGD-Gd@C-dots and positive integrin β3 staining. As a comparison, Gd@C-dots showed minimal tumor uptake. Red, integrin β3 (Cy5); blue, fluorescence from C-dots. Scale bars, 50 μm [71]. [71] John Wiley & Sons. (© 2014 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim).
Download figure:
Standard image High-resolution imageBeyond Gd doped CDs, Wang's group [79, 80] developed a new type of Gd based contrast agent Gd@C82, which exhibits a stronger MRI contrast than commercial Magnevist. The r1 relaxivities of Gd@C82 in water are 47 and 27 mM−1 s−1 at 0.5 T and 7.1 T, respectively. The corresponding r1 relaxivities of Magnevist are 4.5 and 3.5 mM−1 s−1 at 0.5 T and 7.1 T, respectively. After functionalization with the hydroxyl/amine group, Gd@C82 can emit blue light under UV excitation. Further, this group functionalized Gd@C82 with red emissive CDs and PEG and realize the bimodal imaging and photodynamic therapy. Wang et al [81] synthesized Gd2O3 nanoparticles and self-assembly with graphene quantum dots to obtain two-photon emission and MRI dual-modal bioimaging agents. Wang and Zhang et al [82] prepared B doped CDs as another new type of T1 contrast agent for MRI. Although the magnetization value of B-CDs is lower than that of Gd-based agents, it is a metal-free graphene-based material, which opens a new avenue to realize dual-modal bioimaging.
3.2. Multimodal imaging
Besides the MRI/FI dual-modal imaging, computed tomography (CT) is another common bioimaging technique in the clinical diagnosis. Zhang et al synthesized Iodine (I) doped CDs (I-CDs) from iodixanol and glycine [83] or citric acid [84] by hydrothermal route at 180 °C for 3 h. The aqueous I-CDs solution exhibited excitation-dependent and stable emissions with a maximum at 475 nm. I-doped CDs further displayed superior X-ray attenuation properties and excellent biocompatibility in vitro. After intravenous injection, I-doped CDs were distributed throughout the body datable by X-ray CT imaging and excreted by renal clearance. Gd/Yb@CDs were synthesized from Na2EDTA, GdCl3, YbCl3, and L-arginine by hydrothermal method [85]. Gd/Yb@CDs exhibited blue emission, and higher longitudinal relaxivity (r1 = 6.65 mM−1 s−1) and excellent X-ray absorption performance (45.43 HU l g−1). This system is a great example of achieving multimodal imaging by a simple nanoparticle system. Bao et al [86] modified S-doped CDs with Cu2+ and PEG-SH to obtain the PEG-CuCDs, which exhibited blue emission and high photothermal efficiency of 41.3%. The PEG-CuCDs could be an agent for FI, photothermal imaging, and photoacoustic imaging. Sun et al [87] conjugated Ce6 (Chlorin e6) onto the red emissive amino rich CDs to form Ce6-RCD, which exhibited red emission at ∼640 nm and photothermal conversion efficiency of 46% with 671 nm laser irradiation. The Ce6-RCD could be a multimodal bioimaging agent for FI, PT, and PA. Also, they (PEG-CuCDs and Ce6-RCDs) can be employed for photothermal and photodynamic therapy agents.
4. Fluorescent CDs with targeting function
4.1. Fluorescent CDs conjugated with targeting unit
Compared to other types of fluorescence probes, one of the key advantage of the CDs is the ability of naturally carrying part of the functionality of original molecules for the synthesis. It is a great advantage, because it allows for direct targeting or conjugation with other functions without the needs of chemical functionalization.
Targeting function is an effective way of increasing accuracy and validity for the diagnosis of tumor cells. When CDs is used as a fluorescent bioimaging agent, it has no selectivity and marks many kinds of cells. At the point of diagnosis, it is highly expected to stain the specific cell such as tumor cells. Thus, targeting function needs to be endowed onto fluorescent CDs to selectively label the specific cells [89]. Table 1 briefly summarized the fluorescent CDs linked with targeting units. Li et al [90] synthesized transferrin modified CDs-PEGNH2 (Tf-CDs) through bioconjugation reaction. HeLa cell line was employed for the in vitro imaging to evaluate the cellular uptake and targeting. Tf, a serum glycoprotein (80 kDa), has been known for its ability in targeting cancer cells due to the overexpression of Tf receptors on cancer cell membranes [91]. Tf conjugated CDs and CDs with the equivalent concentrations were added into the HeLa cells culture solution. After 2 h of incubation, HeLa cells were shown to internalize the Tf-CDs much more efficiency than non-Tf-conjugated CDs. When the cells were pretreated with free Tf and then cultured with Tf-CDs, the pretreatment of cells with free Tf cause a sharp decrease in the targeting efficiency for Tf-CDs, suggesting saturation of Tf receptors with free Tf. These strongly prove a Tf-receptor-mediated cellular uptake of the TF-CDs. Folate receptor (FR) is well known to be upregulated in a variety of human cancerous cells [92]. Folic acid (FA) is an ideal ligand for the specific FR detection, drug delivery, and cancer targeting because it is readily internalized into the cell through FR mediated endocytosis with non-immunogenicity. Song et al [88] prepared the amine-terminated CDs from glucose and 4,7,10-trioxa-1,13-tridecanediamine by microwave method. And then CDs-FA was obtained by modifying the CDs with FA through bioconjugation reaction (figure 5). The mixture of NIH-3T3 and HeLa cells as normal and cancerous cells were co-cultured in the dish with a CDs-FA solution. Here, NIH-3T3 cells are relatively large, fibroblastic appearance and lacking FR, whereas HeLa cells are smaller, near round morphology and overexpress FR. After incubating for 6 h, HeLa cells produced bright fluorescence, whereas NIH-3T3 cells do not, indicating that CDs-FA is suitable for the discrimination of FR-positive cancerous cells from the normal cells. (Figure 5(c)) Green emissive CDs were prepared from glucose and poly(acrylate sodium) and then modified with FA by reflux reaction [93–97]. The as-prepared CDs exhibit green emission and targeting on the cancerous cells. Aptamers are small oligonucleotides that are selected to bind tightly and specifically to a target molecule [98]. Small molecules like aptamers targeting tenascin C (Tnc), nucleolin, and Muc1 proteins have been successfully conjugated with various nanoparticles due to their advantages such as high binding affinity to targets, short residual time in the blood, low immune reaction and easy processing in production against antibodies [99]. Lee et al [100] developed a thiol-terminated C-Dots from glycerol (SH-CDs) conjugated with a maleimide-terminated TTA1 (mal-Tnc) aptamer targeting Tnc proteins. The aptamer conjugated SH-CDs showed a great specificity for targeting glioma and cervical cancer cells. Aptamer possesses biological and chemical advantages like the absence of immune risk, ease of production and high binding affinity to targets [101, 102]. That is another potential way to develop targeting fluorescent agent. Recently, Gao's group has reported a series of results on the construction of fluorescent CDs with targeting function. Blue emissive CDs were prepared from silk by the hydrothermal method and modified by PEGylation [103]. In vitro cellular uptake study demonstrated that both CNP and PEG-CNP showed higher uptake intensity by H9c2 cells (a heart cell line) than that by human umbilical vein endothelial cells. In vivo results demonstrated that CNP could target the heart, in which the fluorescent intensity was much higher than that in other organs, even liver, and spleen. Furthermore, Green emissive CDs prepared from glucose and glutamic acid by the pyrolysis route [104]. Angiopep-2 is one such ligand whose receptor is low-density lipoprotein receptor-related protein-1 (LRP1) that is overexpressed in glioma cells [105]. After PEGylation, PEG-CDs was modified by angiopep-2 to form An-PEG-CDs. The serum stability experiments indicate that An-PEG-CDs have similar stability as PEG-CDs due to PEGylation significantly improved their stability (figures 6(a)–(c)) [104]. Hemocompatibility was carried out using fresh 2% blood red cells (figures 6(d)–(f)). After 12 h incubation, the hemolysis rates of PEG-CDs and An-PEG-CDs were approximately 14%, which was almost the same compared to PBS, suggesting that PEG-CDs and An-PEG-CDs could not induce hemolysis. The endosomes colocalization assay showed relatively high colocalization of An-PEG-CDs with endosomes after 1 h incubation (figure 6(g)), while the colocalization was considerably decreased after 4 h incubation and most of An-PEG-CDs were located in cytoplasm rather than endosomes, suggesting that enhanced cellular uptake of An-PEG-CDs was mediated by endosomes (figure 6(g)). After i.v. injected into mice through tail while the intensity of mice injected with An-PEG-CDs was much higher than that of PEG-CDs group suggesting that modification of angiopep-2 could improve the glioma targeting effect of PEG-CDs (figures 6(h) and (i)) [104].
Figure 5. (a) Synthesis route of the conjugation of FA–NHS to the passivated C-dots with TTDDA. (b) Schematic illustration of the uptake of CDs–FA by HeLa cells. In the absence of free FA (top), more CDs–FA can bind to FR, leading to the entrance of more C-dots–FA into the cell and thereby much brighter fluorescence. In the presence of free FA (bottom), the competitive binding of FA to FR decreases the entrance of CDs–FA, thus producing rather weak fluorescence. (c) Fluorescence images of different cell samples. The cell mixture of NIH-3T3 and HeLa cells after incubation for 6 h in the presence (A) and absence (B; control) of CDs–FA (50 μg ml−1). (C) Only NIH-3T3 cells incubated with CDs–FA (50 μg ml−1) at 37 °C for 6 h (another control). The DIC images of the corresponding samples are shown below (D)–(F). In the image (D), the white arrows indicate NIH-3T3 cells. Scale bar, 20 μm [88]. Reproduced from [89] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageFigure 6. Serum stability of CDs, PEG-CDs, and An-PEG-CDs. (a) Time-related stability of 100 μg ml−1 of CDs, PEG-CDs, and An-PEG-CDs incubated with 10% FBS. (b) Time-related stability of 100 μg ml−1 of CDs, PEG-CDs, and An-PEG-CDs incubated with 50% FBS. *p < 0.05 vs PEG-CDs and An-PEG-CDs. (c) Stability of 100 μg ml−1 of CDs, PEG-CDs, and An-PEG-CDs incubated with different concentrations of FBS for 12 h. *p < 0.05. (d) Hemocompatibility of CDs, PEG-CDs, and An-PEG-CDs. Time-related hemolysis rates of 5 mg ml−1 of CDs, PEG-CDs, and An-PEG-CDs. *p < 0.05 vs PEG-CDs and An-PEG-CDs. (e) Concentration related hemolysis rates of different concentrations of CDs, PEG-CDs, and An-PEG-CDs incubated with red blood cells for 8 h. *p < 0.05 vs PBS. (f) Image of red blood cells incubated with 5 mg ml−1 of CDs, PEG-CDs, and An-PEG-CDs for 8 h. (g) Colocalization of particles with endosomes after C6 cells incubated with 125 μg ml−1 of PEG-CDs and An-PEG-CDs for 1 or 4 h. The bar represents 10 μm. (h) Ex vivo imaging of glioma bearing brain. (i) Semiquantitative fluorescent intensity of brain and glioma. *p< 0.05 [104]. Reproduced from [89] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageTable 1. CDs with targeting function prepared with chemical linking targeting unit with CDs.
| Types of CDs | Optical properties | Targeting unit | Receptor | Biological tests | Ref. |
|---|---|---|---|---|---|
| CD-PEG-Tf | EWDE (460-550 nm) | Tf | Tf receptor | Hela cells | [90] |
| CDs-FA | Ex. 460/Em. 520 nm | FA | FA receptor | Hela cells | [88, 93–97] |
| CDs-TTA1 | EWDE(460–600 nm) | TTA1 | Tenascin C | Glioma and cervical cells | [98, 100] |
| PEG-CDs | Ex. 345/Em. 434 nm | PEG | Hindering effect of PEG | H9c2 heart line | [103] |
| An-PEG-CDs | EWDE (440–560 nm) | An | LRP1 | Glioma | [104] |
| RGD-PEG-CDs | EWDE(440–560 nm) | cRGD | Integrin αvβ3 | MCF-7/ADR cells/glioma | [106, 107] |
| CTB-CDs | Ex. 360/Em. 443 nm | CTB | GM1 ganglioside | Nerve cell membrane and nervous tissue | [109] |
| HA-CDs | Ex. 360/Em. 470 nm | HA | CD44 receptor | Cancer cells | [110–112] |
| TPP-CDs | EWDE (400-570 nm) | TPP | Mitochondria | HeLa cells | [113] |
Abbreviations: EWDE: Excitation Wavelength Dependent Emission; PEG: polyethylene glycol; Tf: Transferrin; FA: Folic acid; TTA1: maleimide-terminated Targeting tenascin C aptamers; An: angiopep-2; LRP1: low density lipoprotein receptor-related protein-1; cRGD: cyclic Arginyl-Glycyl-Aspartic acid (cRGD) peptides; CTB: Cholera toxin B; HA: Hyaluronic acid; TPP: Triphenyl phosphonium.
CDs can be easily conjugated with targeting molecules for specific localization. For example, cyclic Arginyl-Glycyl-Aspartic acid (cRGD) peptides modified CDs showed effective accumulation on membrane surface and cytoplasm of tumor cells [106, 107] . It is well known that cRGD specifically targets the integrin receptors which overexpress on the tumor cells while RGD recognize cell surface receptor integrin αvβ3 of all cells [108]. Cholera toxin B (CTB), a non-toxic, cell binding moiety of cholera toxin, is a highly sensitive retrograde neural tracer since it can bind to the GM1 ganglioside of the nerve cell membrane and nervous tissue with abundant gangliosides. Zhou et al [109] developed a novel fluorescent neural tracer: CTB conjugated CDs (CTB-CDs), which were taken up and retrogradely transported by neurons in the peripheral nervous system of the rat companying with high photoluminescent intensity, good optical stability, and non-toxicity. Hyaluronic acid (HA) was used for the functionalization of nitrogen-doped carbon quantum dots (HA-CQDs) that provide robust fluorescent dots for use in confocal microscopy and flow cytometry. The results proved that HA-CQDs can be used as a new biocompatible cell-specific targeting probe for imaging of CD44 receptor-over expressed in cancer cells [110–112]. Besides targeting the specific cells, high selectivity on the organelle is another way to achieve the targeting function. Wang et al [113] have selected the triphenyl phosphonium (TPP) head groups as the targeting moiety to deliver the fluorophore to mitochondria, where these lipophilic cations can selectively accumulate in the organelle due to the large membrane potential gradient [114]. The TPP was chemically linked with fluorescent CDs, which results in the TPP-CDs has higher accumulation on the mitochondria than the non-conjugated CDs. Laurylamine functionalized CDs (L-CDs) are obtained via the conjugation of laurylamine with CDs prepared from citric acid and urea [115]. The as-prepared L-CDs provide clear and bright imaging results for lysosome and endoplasmic reticulum, respectively.
4.2. Self-targeting fluorescent CDs
The targeting function can be added by modifying the CDs with the molecules with targeting function through one or multiple steps of the chemical reaction. Thus, the one-step route was developed by adding the targeting molecules into the CDs preparation reaction. Sharker et al [116] synthesized HA-CDs from HA dehydrated and carbonized by concentrated H2SO4 with ∼1.5 min. HA was partially carbonized to form CDs core and with HA surface groups. In vitro and in vivo results displayed higher fluorescent intensity was observed in the cancerous cells (MDAB and A549 cells) than that in the normal cells (MDCK cells), due to the overexpression of the CD44 receptor on the surface of the MDAMB and A-549 cells. HA sodium salt can be directly carbonized to form CDs with fluorescence and targeting properties by hydrothermal route [117]. Chondroitin sulfate (CS) is an anionic linear chain attached to specific scaffolds, forming a major extracellular matrix on the cell surface. Wang et al produced the excitation-dependent emission CDs from CS, which exhibit high uptaking of SAS cells [118]. FA and tris(hydroxymethyl)amino methane are heated to form FR targeting CDs (FR-CDs) with PL QY of 77% and super photo and thermal stability [119]. With the presence of free FA, PL intensity of FR-CDs inside the SKOV3 cells decreased over 85% indicating that the uptake of FR-CDs effectively depressed by free FA. FR-CDs are also internalized into cells through the FR associating cellular uptake mechanism.
It is easy to understand the targeting function originated from the known targeting molecules because the targeting molecules are partially carbonized to form fluorescent CD with targeting molecules as surface functional groups. However, it is reported that non-targeting molecules can be used for constructing the targeting CDs as well. Zheng et al [120] proposed the concept of self-targeting CDs from L-aspartic acid and D-glucose. The as-prepared CD-Asp exhibits not only excellent fluorescent properties and good biocompatibility but also exhibit free penetration through the blood-brain barrier (BBB) and targeting the brain glioma tissue. Comparing with the normal cells (L929 cell line), the CDs-Asp exhibits high up-taking for the C6 cells. After injection from the tail vein, the CDs-Asp rapidly accumulated to the glioma tissue within 15 min (figure 7). They proposed targeting function may originate from the RGD-like functional groups on the surface of CDs, which were from L-aspartic acid and glucose during the carbonization process. That provides a simple way to achieve high contrast fluorescent imaging agents. Similar works are summarized in the table 2. Qian et al synthesized the CDs from glutamic acid and glucose by the thermolysis route [122]. As-prepared CDs possess good serum stability and hemocompatibility with low cytotoxicity. In vitro results indicated that the CDs could be efficiently taken up by bEnd.3 cells in a concentration- and time-dependent manner. In vivo results showed that it could be used for noninvasive brain imaging due to its high accumulation in the brain region, which was demonstrated by in vivo imaging and ex vivo tissue imaging. They also found the CDs from the pyrolysis of glycine, which also has fluorescent emission properties and targeting the C6 glioma cells [121]. Wang et al [123] synthesized polymer-coated N doped CDs (pN-CDs) from N-methyl-2-pyrrolidone. The pN-CDs exhibited typical excitation-dependent emission with a maximum at 420 nm. In vitro and in vivo results indicated that the U251 glioma cells exhibit extra high cellular uptake implying that pN-CDs could be potential self-targeting CDs. However, the exact chemical composition and targeting receptor on the glioma remain unclear. Si modified CDs can be prepared from 3-aminopropyl)tri-methoxysilane (APTMS) and glycerol by hydrothermal route [170]. The excellent optical properties such as excitation-dependent emission, photostability and biocompatibility are observed in the Si-CDs. Beyond that, the selective stain of mitochondria was demonstrated in various cancerous cells. However, the normal cellular uptake remains relatively low level. The modification results in a positive zeta-potential for Si-CDs, mitochondria exhibit a large membrane potential of up to −180 mV, leading the accumulation of cationic species. Recently, m-phenylenediamine and L-cysteine were employed as a carbon and nitrogen source for N-CDs which exhibit unique selectivity on the nucleus of cells [171]. High contrast nucleolus fluorescent imaging can be reached by as-prepared N-CDs.
Figure 7. (a) Schematic synthetic route of green emissive CDs from D-glucose and L-aspartic acid (b) LSCM images of C6 (A–C) and L929 (E–G) cell lines pretreated with CD-Asp for 1 h at 37 °C, under excitation of 405, 488, and 555 nm. The scale bar is 20 μm. Flow cytometric profiles of C6 (D) and L929 (H) cells treated with CD-Asp for 1 h. (c) In vivo imaging of glioma-bearing mice after tail intravenous injection of CD-Asp. Whole-body distribution of CD-Asp as a function of time after injection [120]. Reprinted with permission from [121]. Copyright (2015) American Chemical Society.
Download figure:
Standard image High-resolution imageTable 2. FI of CDs with targeting function prepared by one-step method.
| Reactant# | Optical properties | Targeting unit | Receptor/biological test | Key finding | Ref. |
|---|---|---|---|---|---|
| HA → HACDs | EWDE (380–500 nm) | HA | CD44 receptor/MDAB and A549 Cells | The remaining HA parts in HA-FCN via carbonization process were selective to simultaneously deliver the target molecules and for imaging applications. | [116, 117] |
| CS → CSCDs | EWDE (418–500 nm) | CS | CD44 receptor/SAS Cells | CSCDs prepared from CS retained CS properties to simulate ECM, and thus promoted the invasion and proliferation for SAS cells. CSCDs created nanoscale topography for SAS cells and enhanced the expression of their MMPs genes. | [118] |
| FA and Tris → FACDs | Em. 395 nm | FA | FA receptor/BMSC, MCF7, MDA-MB-231 and SKOV3 cells | Even though pterin moiety of folic acid is destroyed, the FR targeting ability is retained, while a much better QY and stability properties are demonstrated. | [119] |
| L-Asp and D-Glc → CDs or glycine→ CDs | EWDE (450–600 nm) | cRGD like groups | Integrin αvβ3/C6 cells Glioma | Targeting function may originate from the RGD-like functional groups on the surface of CDs, which were from L-aspartic acid and glucose during the carbonization process. | [120, 121] |
| Glu/Glc | EWDE (420–500 nm) | Glc receptor and Glu receptor | bEnd.3 cells/glioma | In vitro, the CDs could be efficiently taken up by bEnd.3 cells in a concentration- and time-dependent manner. In vivo, CDs could be used for noninvasive brain imaging due to its high accumulation in brain region. | [122] |
| NMP | EWDE (420–500 nm) | EPR effect | U251 glioma cells | In vitro and in vivo results indicated that the U251 glioma cells exhibit extra high cellular uptake. The pN-CDs could enter glioma cells in vitro and mediate glioma fluorescence imaging in vivo with good contrast via elevated passive targeting based on an enhanced permeability and retention (EPR) effect | [123] |
#The reactants were directly carbonized to form fluorescent CDs. CS: Chondroitin sulfate.Abbreviations: HA: hyaluronic acid; CS: chondroitin sulfate; FA: folic acid; Tris: tris(hydroxymethyl) amino methane; L-Asp: L-Aspartic acid; Glc: glucose; Gly: glycine; Glu: Glutamic acid; NMP: N-methyl-2-pyrrolidone.
5. Fluorescent CDs with therapeutic function
5.1. Fluorescent CDs conjugated with chemical therapeutic agents
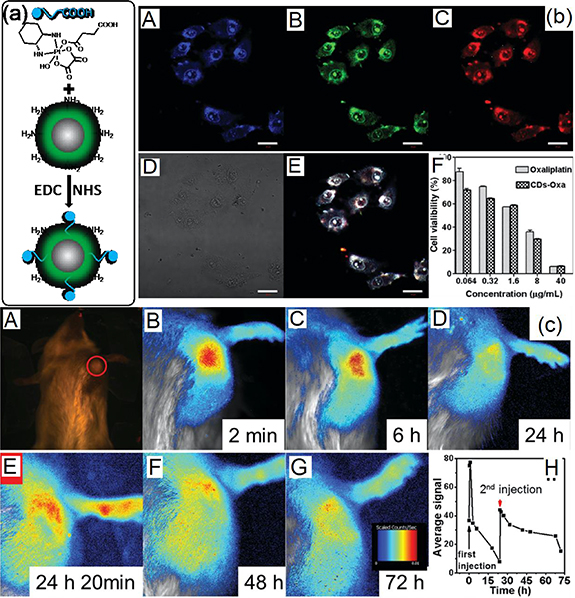
Many methods have been applied to treat tumors, such as surgical operation, chemotherapy, radiation therapy, immunotherapy, targeted therapy and so on. (www.cancer.gov/about-cancer/treatment/types). Chemotherapy can be used to treat cancer, lessen the chance of recurrence, stop or slow its growth. These types of chemotherapeutic drugs have been developed: alkylating agents, plant alkaloids, antitumor antibiotics, antimetabolites, topoisomerase inhibitors, and miscellaneous anti-neoplastic. For example, platin salts (carboplatin, oxaliplatin, and cisplatin); vinca alkaloids, anthracyclines (doxorubicin, DOX), the folic acid antagonist (Methotrexate, MX) and others. It is helpful to understand the metabolic pathway and quantity monitor of medicine by combining chemotherapeutic medicine with fluorescent bioimaging agents. That will give much more information to the physician for making the decision on how much and how long chemotherapy is given and chemotherapy resistance. Table 3 exhibits the recent progress on the fluorescent CD with therapeutic function. In 2014, Zheng et al [124] firstly modified oxaliplatin by succinic anhydride to produce Oxa-COOH according to the literature. And then Oxa-COOH was conjugated on the amine terminal CDs surface to achieve CD-Oxa nanomedicine (figure 8). In vitro MTT assay indicated that the cytotoxicity of CD-Oxa and oxaliplatin is close (half maximal inhibitory concentration, IC50 = 3.4 μg ml−1). After incubation with CD-Oxa for 1 h, HeLa cells can be directly imaged by confocal fluorescence microscopy. Direct injection of CD-Oxa into the tumor site of KM mice. PL emission from the injected CD-Oxa could be readily detected under the blue light excitation. The Intensity of PL emission strongly related to the concentration of the CD-Oxa. Thus, the second injection time and quantity can be estimated by the PL intensity. That is beneficial to avoid the side effects of excess medicine. At the same time, the tumor volume decreased by extending the treatment time.
Figure 8. (a) Synthetic scheme for CD-Oxa by bio-conjugation. (b) A–D: Confocal fluorescence images of HeLa cells treated with CD-Oxa and imaged under 405 nm (A), 488 nm (B), 555 nm (C) excitation and bright-field (D). E: Overlay image of (B–D). The scale bars correspond to 20 μm. F: Cell viability of HepG2 human cancer cells after incubation with oxaliplatin (II) or CD-Oxa for 48 h, determined by MTT assay. (c) In vivo fluorescence images of mice bearing H22 liver cancer after the first intralesional injection of CD-Oxa. A: 0 min (taken under white light; the red circle marks the position of tumor), B: 2 min, C: 6 h, D: 24 h, E: 24 h 20 min (the second injection), F: 48 h and G: 72 h. All taken under blue light unless otherwise stated. H: Semiquantitative fluorescence intensities of the tumor area determined at different times. The red arrow indicates the 2nd injection [124]. [127] John Wiley & Sons. (© 2014 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim).
Download figure:
Standard image High-resolution imageTable 3. CDs with therapeutic functions.
| Types of CDs | Properties (em/ex) | Therapeutic agents | Biological test | Key finding | Ref. |
|---|---|---|---|---|---|
| CDs-Oxa-Pt or CDs-Pt(iV)@PEG (PAH/DMMA) | EWDE (450–550 nm) | Oxaliplatin for ChT | HeLa/HepG2 Cells and H22 Liver cancer | The drug can be tracked and monitored by fluorescent signal, which helped customize the injection time and dosage of the medicine | [124, 125] |
| DOX/PEG-Chitosan@CDs | Ex. 409/Em. 518 nm | DOX for ChT | Loading DOX into the hybrid gel via π–π stacking | Two-photon fluorescence and NIR photothermal regulate the release of the DOX. | [126–130] |
| CDs-Pt(IV)-DOX | EWDE (450–540 nm) | Cisplatin and DOX for ChT | A2780 and A2780cis cancer cells | The internalization process of CD-Pt(IV)-DOX by cancer cells could be monitored through multicolor emission. The intracellular and reductive conduction and weakly acidic promote the release of Pt(IV) and DOX. It conquers cisplatin resistance in the cancer treatment. | [131] |
| Nic-CB-CDs | EWDE (400–450 nm) | Nic for ChT | MCF-7 cells | The host-guest chemistry between cucurbit [6] uril and niclosamide makes the delivery of the hydrophobic drug feasible. In vitro assessments in human breast cancer cells indicate approximately two-fold enhancement in IC50 of drug. | [132, 133] |
| CDs-Ce6 | Em. 525 nm for CDs and Em. 668 nm for CDs-Ce6 | Ce6 for PDT | MGC803 cells/gastric cancer | Its fluorescence can be enhanced by FRET between CDs and Ce6. The synthesized multifunctional nanocarrier platform is effective for simultaneous enhanced-PFD and PDT of gastric cancer tumor in vivo. | [134, 135] |
| CDs-Porphyrin (CDs-TMPyP) | Em. 415 nm for CD and Em. 670 nm for CDs-TMPyP | TMPyP for PDT | HeLa Cells | CDs can work as donor to transfer the energy to TMPyP by FRET with an efficiency of 45%. The TMPyP act as PDT and FRET for two-photon emission(TPE). In vitro PDT Killing was achieved with CDs-TMPyP by TPE of 700 nm fs laser. | [136] |
| CDs from Por or graphite rod | EWDE (400–600 nm) | CDs for PDT | HepG2/H22 cells and U251 human glioma cells | In vivo therapeutic effects validate that CDs can suppress the increase of solid tumor. It acts as both fluorescent agent and photodynamic agent | [137–139] |
| CDs prepared from polythiophene | Em. 680 nm | CDs for PDT | HeLa cells/breast cancer | The CDs exhibit a combination of properties, including broad absorption from vis to NIR, Deep red emission, good aqueous dispersity, high photo and pH stability and good biocompatibility. CDs also showed a high 1O2 generation yield greater than 1.3. | [140, 141] |
| CDs/MnO2-PEG or Mn-CDs from Mn-Por | Em. 690 nm | CDs for PDT MnO2 for catalyzing H2O2 decomposition | HeLa cells/breast cancer 4T1-luc cells | CD/MnO2-PEG exhibited (1) the quenched fluorescence, weak 1O2 generation, and low MRI signal in the normal physiological environment; (2) enhanced fluorescence, 1O2 generation, and MRI signal in the tumor micro environment (TME); and (3) low toxicity and complete clearance from the body. The CDs/MnO2-PEG s can be applied as pH/H2O2-driven, turn-on nanotheranostics for the concurrent bimodal MR/FL imaging and oxygen-elevated PDT of solid tumors. | [142–146] |
| CDs from deoxyadenosine monophosphate | EWDE (450–550 nm) | CDs for PDT | High fluorescent CDs (PL QY of 144 12.4%) with excellent photostability. The CDs exhibited promising 1O2 generation with quantum yield of 1.20. | [147] | |
| CDs from spinach | Ex. 405/Em. 677 nm | CDs for PDT | HeLa/7702 cells Tumor bear mice | The CDs-Cu nanocomposites by binding CDs with copper ions, which are used as fluorescence probes and photosensitizers for in vivo near-infrared fluorescence imaging of biothiol and dual-enhanced PDT of tumor. Under NIR irradiation, the prepared CDs-Cu generated large amounts of ROS which can be used for PDT of tumors. | [148] |
| CDs/WS2 from gemini glucose surfactant capped WS2 NR | EWDE (Ex 375/Em. 460 nm) | WS2 for PTT | HeLa cells | The WS2-CDs enable both cell imaging by exploiting the multicolor fluorescence properties of the CDs as well as PTT through NIR-induced localized heating by the WS2 nanorods. CDs provided the capability of FI and WS2 exhibited NIR photothermal behaviors. | [149] |
| Au NR@SiO2-CDs | Em. 675 nm | Au NR for PTT | B16-F0 cells/B16-F0 tumor-bearing nude mice | The SiO2 layer has two functions: improving the stability of the Au NRs and avoiding the fluorescence quenching of CDs. Au NR@SiO2-CDs showed high sensitivity and good spatial resolution of FL/PA imaging to guide the PDT/PTT treatment through i.v. administration. The combination of PDT and PTT proved to be more efficient to kill cancer cells. | [150] |
| CDs from the polythiophene benzoic acid (PBA) | Em. 640 nm | CDs for PDT/PTT | B16-F0 cells/B16-F0 tumor-bearing nude mice | CDs exhibited PDT (η = 0.27)/PTT (η = 36.2%) for 630 nm laser irradiation. The CDs enriched in the nuclei of cells. CDs worked as a red-light-trigger cancer theragnostic agent with imaging-guided PDT/PTT. | [151] |
| S, Se codoped CDs | Em. 731 and 820 nm | CDs for PTT | HeLa, A549 and KB cells | CDs exhibited excitation wavelength-independent NIR emissions with peaks at 731 and 820 nm, a PTT conversion efficiency of ∼58% and a large two-photon absorption cross section (∼30 045 GM). | [152] |
| CDs from CA and urea | Em. 650 nm | CDs for PTT | The CDs exhibited NIR imaging and PTT (η = 54.2%). They found the new NIR absorption peaks is resulted in an increasing amount of pyrrolic N. | [153] | |
| CDs from CA in formamide | Em. 640 nm | CDs for PTT | MCF-7 and HeLa cells | The CDs exhibited the red emission with a PL QY of 22.9%, low toxicity, TPE fluorescence and high PT efficiency of 43.9% under irradiation of 671 nm laser. | [154] |
| CDs from CA and PEI in formamide and conjugated with Ce6 | Em. 640 nm | Ce6-CDs for PDT and PTT | HeLa, MCF-7 and 4T1 cells/tumor-bearing Balb/c nude mice | The Ce6-CDs complexes showed synergetic PTT and PDT effect under a single NIR laser (671 nm) source. The photothermal conversion efficiency reach 46%. In addition, the Ce6-CDs also exhibited fluorescent, PA and PT imaging properties. | [87] |
| CDs aggregated from CA and urea | Em. 450 nm. Strong absorption from 470–1000 nm | CDs for PTT | HeLa cells | This blue supra-CDs exhibited a broad absorption peak in the NIR region which is responsible to the high photothermal conversion efficiency 52%) under 732 nm laser of 0.5 W cm−2. | [155, 156] |
| CDs from watermelon | Em. 900–1200 nm | CDs for PTT | HeLa cells/HeLa cells in the back of Balb/c nude mice. | The designed CDs with 900–1200 nm luminescence possess high quantum yield (QY-0.4%), which have proven to be effective probes for in vivo NIR-II bioimaging, and PTT conversion efficiency of 30.6% triggered by laser of 808 nm. | [157] |
| Cu, N-CDs for EDTA.2Na | EWDE 450 nm. Absorption band at 726 nm | Cu,N-CDs for PTT | B16 Cells/Balb/c nude mice bearing B16 melanoma tumors | Cu, N-CDs exhibit both fluorescent imaging and synergistic PTT and PDT effect under NIR laser (808 nm) irradiation. | [86, 158] |
Abbreviations: Em.: Emission; Ex.: Excitation; Oxa: Oxaliplatin; ChT: Chemical therapy; DOX: doxorubicin; Nic: niclosamide; CB: cucurbituril; Ce6: chlorin e6; PDT: photodynamic therapy; TMPyP: 5, 10, 15, 20-tetrakis(1-methyl 4-pyridino) porphyrins; Por: porphyrins; PTT: photothermal therapy; CA: citric acid.
However, CDs based drug nanocarrier can easily get rid of the body to lower their therapeutic effect due to their small size and surface properties. Normally, PEGylation or negatively charged drug carriers can extend blood circulation time PEGylation serves as a stealthy layer to prevent the drug carriers from rapid clearance by the immune system [172] and negatively charged property can withstand protein adsorption originated from electrostatic repulsion [173]. However, PEGylation can also impede the uptake of drug carriers by cancer cells, and negatively charged drug carriers cannot easily enter into cancer cells due to electrostatic repulsion with the negatively charged cell membrane, resulting in decreased therapeutic efficiency [174]. To provide CDs-Pt(IV) with charge-convertible property in mildly acidic tumor extracellular microenvironment, Feng et al designed and synthesized dimethyl maleic acid (DMMA) and PEG functionalized poly(allylamine) (PEG-(PAH/DMMA)) [125]. In the weak acid tumor microenvironment, CDs-Pt(IV)@PEG-(PAH/DMMA) can change from negative charge at normal physiological condition (pH 7.4) to positive one at tumor extracellular microenvironment (pH 6.8). Thus, it exhibited higher cytotoxicity to the tumor cells than the normal cell. Besides that, CDs-Pt (IV)@PEG-(PAH/DMMA) also displays multicolor bioimaging prolonged circulation time in blood, effective accumulation at the tumor site, enhanced internalization by cancer cells, facilitated endosome escape and controlled intracellular drug release. That greatly promotes the practical application of CD-based drug nanocarrier. Wang et al [126] synthesized fluorescent CDs from glucose by the hydrothermal route and encapsulated by poly(ethylene glycol) dimethacrylate and chitosan to form PEG-Chitosan@CDs hybrid nanogel. DOX was loaded into the PEG-Chitosan@CDs through the intermolecular interactions (hydrogen bonding and/or π-π stacking). The PEG-Chitosan@CDs hybrid nanogel exhibits two-photon fluorescence and NIR photothermal/pH sensitive volume phase transition to regulate the DOX release. The hybrid nanogels enable combined chemo-photothermal treatment to provide a high therapeutic efficacy due to their highly synergistic effect. The blue, green, and red emissive CDs were prepared from sugar and ascorbic acid by hydrothermal reaction. DOX can also directly loaded on the surface of CDs for localized cancer therapy by simply mixing these CDs and DOX [127, 128]. DOX can be released in the tumor acid microenvironment [129, 130]. Chemotherapeutic DNA-modifying cisplatin and topoisomerase II-inhibiting DOX were conjugated on the full-color emissive CDs via a pH sensitive hydrazone bond [131]. Cisplatin prodrug was activated to cytotoxic cisplatin under intracellular reductive microenvironment and the hydrazine bond wad hydrolyzed to release DOX in the intracellular weakly acidic condition. This combination offer a promising fluorescent CD-based co-adminstration for combat cisplatin resistance in the cancer treatment. Amine capped CDs were modified digitonin and then conjugated with MX. In vitro results indicates that enhanced cytotoxicity is observed in the CDMX [175]. Niclosamide (Nic) is an FDA-approved anthelminthic drug that has been acknowledged to demonstrate anti-neoplastic properties via the active inhibition of multiple pathways modulated in cancer stem cells [176]. Ostadhossein et al [132] designed a host-guest system to load the hydrophobic drug into cucurbituril (CB) which is a macrocyclic cavitand compound with hydrophilic surface and hydrophobic cavity (figure 9). Hydrophobic Nic can be encapsulated inside of CB to form complexes, which chemically bonded on the CDs surface. The coculture of MCF-7 cells (human breast cancer cell) with various combinations reveals that CDs, CB6, and CB6-CDs did not induce any cytotoxic effect after 48 h of incubation. IC50 of Nic, CB6-Nic, and Nic-CB6-CDs are ∼45, 28, and 21 μM, respectively. The anti-tumor capability of Nic was verified in vivo in a xenograft generated from MCF-7 cells in athymic nude mice. 40 μl of PBS, CB6-Nic and Nic-CB6-CDs solution were intratumorally injected into the different tumors. The tumor growth could be depressed using CB6-Nic and CDs-CB6-Nic cases. The nanocarriers equipped with defined functionality for tailored interaction through 'host-guest chemistry', and biodegradable properties can ferry poorly soluble agents to selectively target cancer cells with stem-like properties and controllably release their payload in response to the environment, thus resulting in an improved antitumor activity in vivo. Pei et al [133] linked the β-cyclodextrin (β-CD) on the CDs to form β-CD-CDs, which further DOX can be loaded via host-guest complexation. The DOX -loading capacity can reach 39.5% and release in the weakly acidic tumor microenvironment. To deliver the water-insoluble drug and monitor the release process, Zhai et al [177] added CDs and ketoprofen into the polyvinylpyrrolidone (PVP) and electrospun to form the nanofibers. The release amount and rate of drug and CDs will keep the dependence on the concentration encapsulated in the PVP nanofibers.
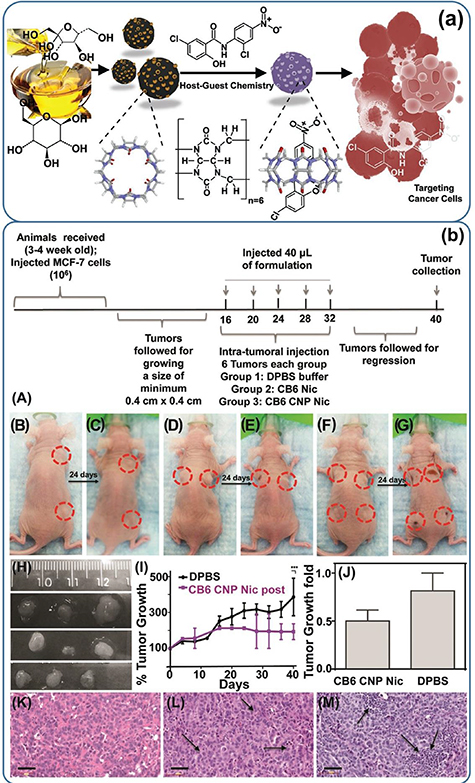
Figure 9. (a) Schematic representation of preparation, loading mechanism, and treatment of cancer cells. The CNP coated with CB6 was loaded with STAT-3 inhibitor (niclosamide) to effectively treat cancer cells via apoptosis. (b) In vivo results on a xenograft mouse model. (A) Timeline of the experiment; representative animals with tumors before and after treatment with (B), (C) DPBS; (D), (E) CB6 Nic and (F), (G) CB6 CNP Nic. (H) Tumors collected after sacrificing the animals treated with DPBS (top line); CB6 Nic (middle line) and CB6 CNP Nic (bottom line). (I) Tumor growth curves with time and (J) fold change. H&E images of tumor sections for treatments with (K) DPBS; (L) CB6 Nic and (M) CB6 CNP Nic. An unpaired t test was performed between % tumor growth values in animals with DPBS treatment and CB6 CNP Nic to reach a two-tailed p-value < 0.001, represented as ***. Scale bar is 50 μm [132]. [135] John Wiley & Sons. (© 2016 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim).
Download figure:
Standard image High-resolution imageCancer immunotherapy can not only initiate immune cells to systematically target and kill specific tumor cells with no harm to normal tissues but can also make immune cells memorize tumor antigens to avoid recurrence, which other traditional or emerging therapies cannot achieve. Dendritic cells (DCs) play a vital role in cancer immunotherapy since they specialize in recognizing and processing antigens. After that, activated mature DCs will migrate to the lymph nodes and present a combination of the antigen peptide and the major histocompatibility complex (MHC) molecule to activate T cells which can target tumor cells expressing this antigen and then induce effective cytotoxic T cell (CTL) responses. Luo et al synthesized the nanocomposite of uniform-sized CDs and tumor model antigen protein ovalbumin (OVA), which could efficiently stimulate the maturation of DCs and corresponding T cell proliferation [178]. This method developed an original CD-based vaccine and may open the door to the exploitation of CDs in cancer immunotherapy.
5.2. Fluorescent CDs conjugated with phototherapy agent
5.2.1. Fluorescent CDs conjugated photodynamic therapeutic (PDT) agent
Recently, photo-therapy has been developed such as photodynamic is that the reactive oxygen species (ROS) including singlet O2 or free radicals can be produced to kill the tumor cells by photosensitizer under the photoirradiation. Another is the photothermal therapy, which converts light energy to thermal energy to make the temperature overpass the survival limit of the cell. The biggest advantage is that the effective area of therapy is limited within the light irradiation area. It does not affect outside of light irradiation even in the presence of the medicine. Green emissive CDs was combined with chlorin e6 (Ce6) together by conjugation to form the nanomedicine [134, 135]. Ce6 as a typical photosensitizer can generate ROS under light irradiation and emit red light under green light excitation. In this combination, the emission of Ce6 is enhanced by Forster resonance energy transfer (FRET) from CDs, which is beneficial to the contrast of the NIR bioimaging. On the other hand, CDs enhanced the tumor-homing ability based on the enhanced permeability and retention (EPR) effect, which improved the therapeutic efficiency. Besides, CDs helped to improve the stability, water solubility and lower the toxicity. Porphyrin is one of the photosensitizers used for photodynamic therapy (PDT). Wang et al [136] linked the 5, 10, 15, 20-tetrakis(1-methyl 4-pyridino) porphyrins (TMPyP) to CDs to form CDs-TMPyP by electrostatic force. The emission band of CDs was overlapped with the absorption of TMPyP so that the FRET happens between CDs and TMPyP. CDs Can realize the two-photon bioimaging, the more important CDs have a high two-photon absorption cross-section, which promotes the two-photon photodynamic therapy of TMPyP. Li et al [137] used the mixture of porphyrin and chitosan to prepare the porphyrin-based CDs by a single-step hydrothermal method, which exhibits blue emission and photodynamic effect. Markovic et al [138] demonstrate the CDs produced from graphite by electrochemical oxidation not only exhibited the green emissive fluorescence but also the generated ROS to kill the U251 human glioma cells under the irradiation of 470 nm laser. This result settled the foundation on the CD used as a photodynamic agent. Furthermore, they also demonstrate CDs can be used for photodynamic anti-bacterial effect [139]. Hsu et al [179] demonstrated that the CDs prepared from green tea with high-temperature treatment exhibited excitation-wavelength and the high inhibition efficiency of the growth of cancer cells (MCF-7 and MDA-MB-231) and low toxicity to the normal cells (MCF-10A) they found the H2O2 can be generated with the presence of CDs in the tumor cell. It did not happen in the normal cells and catechin cases. Catechin is the main component of green tea. They did not disclose the generation mechanism of H2O2.
Ge et al [140] started from polythiophene to prepare the deep-red emissive CDs by hydrothermal route. The as-prepared CDs exhibit a broad absorption and emission at 680 nm. The electron spin resonance (ESR) signal identified the ROS generation by CDs under irradiation. In vitro experiments displayed the HeLa cells can be stained by CDs and showed strong red fluorescence. The photodynamic activity was demonstrated by monitoring the morphology variation of HeLa cells in the presence of CDs under laser irradiation. Figure 10 showed the in vivo imaging after injecting the CDs solution into the back of nude mice. The injection site displayed a much higher fluorescence intensity than the background signal from the mice. The PDT therapy effect is shown in figure 10. The tumor volume dramatically decreased after 10 d of treatment. However, the tumor volume kept increasing in the cases of only CDs and only light. Individual CDs nanoparticles have a small size so that they easily transport in and excrete out of the body. That results in a short EPR time in the body and lowers the therapy efficiency. Making the particles with ∼100 nm in size and tuning the surface charge will enhance the EPR time in the body. Jia et al [141] prepared the CDs nanosphere with a size of ∼100 nm through the ionic self-assembly with positive charge CD and negative charge sodium dodecyl benzene sulfonate (SDBS). Further, the CDs spheres were passivated with HOOC-PEG-NH2 to reduce in vivo cytotoxicity and increase blood circulation time. Besides that, the CDs nanospheres make the adsorption and emission shift toward the red due to the formation of aggregates. These are beneficial to the circulation and fluorescent imaging of CDs. As known, the tumor environment is hypoxia, which limits the generation of 1O2 and lowers the photodynamic efficiency. On the other hand, the tumor microenvironment is acidic (pH = 6.5–6.9) and high H2O2 concentration (0.5 nmol/104 cells h−1). To solve the hypoxia problem, Wang's group [142] constructed the CDs/MnO2, MnO2 can not only be reduced into Mn(II) by acidic H2O2 as an T1 contrast agent for MRI but also generate oxygen within tumor to enhance the PDT efficiency. Magnetofluorescent Mn-CDs were prepared from Mn(II) phthalocyanine [143] and metal porphyrin [144–146]. After cooperative self-assembly with DSPE-PEG, the Mn-CDs aggregates can be used for bimodal contrast agent for MRI and fluorescence imaging agent. More interestingly, the Mn-CDs aggregates can not only effectively produce 1O2 (quantum yield of 0.40) but also highly catalyze H2O2 to generate oxygen. Nucleic acids are macromolecules that store genetic information and enable protein production, which are composed of phosphate, sugar and nitrogenous base. Thus, the nucleic acid can be used as precursor for fluorescent CDs. Zheng et al [147] developed high fluorescent CDs (PL QY of 12.4%) with excellent photostability which is 91.9% under continuous UV excitation for 30 min from deoxyadenosine monophosphate. And the same time, the CDs exhibited promising singlet oxygen generation with quantum yield of 1.20, which is higher than that of conventional photosensitizer Rose Bengal (0.75). Liu et al [148] used the chlorophyll extracted from spinach as precursor to synthesize NIR emissive CDs by thermolysis. The as-prepared CDs were further bound to the Cu2+ to form quenched fluorescence Cu-CDs complexes, which can be used as a fluorescence nanoprobe and highly efficient photosensitizer for in vivo NIR fluorescent imaging of biothiol and dual-enhanced PDT of tumor.
Figure 10. (a) The schematic synthetic route of red emissive CDs based on polythiophenes. In vivo imaging and PDT [180]. Reprinted with permission from [180]. Copyright © 2015 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. Bright-field image (b) and red-fluorescence image (c) after subcutaneous injection of GQDs in different areas. (d) Time-dependent tumor growth curves (n = 5) after different treatments. P < 0.05 for each group. The excitation wavelength was 502–540 nm, and the collected fluorescence channel was 695–775 nm. (e) Photographs of mice after various treatments on the 1st, 9th, 17th and 25th day (PDT: GQDs + light irradiation; C1: GQDs only; C2: light irradiation only) [140]. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Nature, Nature Communication, 'A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation', Jiechao Ge et al (2014).
Download figure:
Standard image High-resolution image5.2.2. Fluorescent CDs conjugated with photothermal therapeutic (PTT) agent
Photothermal therapy (PTT) is another effective strategy to disrupt tumors, therefore, integration of CDs with photothermal agents leads to excellent theragnostic agents. For example, WS2 has been demonstrated as a potential PTT agent [181]. Nandi et al [149] attached a gemini-glucose surfactants on the amine capped WS2 nanorods surface. After converting the surfactant into CDs, a theragnostic agent system was generated, where CDs provided the capability of fluorescent imaging and WS2 exhibited NIR photothermal behaviors. Gold nanorods also exhibit excellent photothermal behaviors with the potential of serving as PTT and photoacoustic (PA) agent. Jia et al [150] constructed a photo theragnostic agent based on gold nanorod@SiO2-CDs by introducing a thin layer of SiO2 between Au nanorods and CDs (figure 11). The SiO2 layer has two functions: improving the stability of the Au nanorods and avoiding the fluorescence quenching of CDs. This novel Au NR@SiO2-CDs photo theragnostic agent showed high sensitivity and good spatial resolution of FL/PA imaging to guide the PDT/PTT treatment through i.v. administration. The PDT and PTT were demonstrated to kill cancer cells under relatively low dose of laser irradiation (<0.5 W cm−2). Ge et al [151] reported red emissive CDs from the polythiophene benzoic acid (PBA) as carbon source. Besides the fluorescent emission at 640 nm with PL QY of 3.5%, these CDs also displayed ROS (1O2) generation under 635 nm laser irradiation. The efficiency of 1O2 generation was about 0.27. In addition, these CDs exhibited tunable photothermal effects. The temperature of the aqueous solution of CDs increased from 7.2 °C (20 μg ml−1) to 30.2 °C (200 μg ml−1) under 2 W cm−1 of 635 nm laser irradiation. The photothermal conversion efficiency reached to 36.2%. The as-prepared CDs enriched in the nuclei of the cells, making them great biomarkers for the nuclei. In vivo and in vitro results indicated that CDs worked as a red-light-trigger cancer theragnostic agent with imaging-guided PDT/PTT. Lan et al [152] raised the photothermal efficiency to 58% by synthesizing S, Se co doped CDs, which exhibited a broad absorption at 526 nm. Moreover, the CDs had a large two-photon absorption cross section (∼30 045 GM), which allowed NIR emissions and the photothermal conversion of the CDs through the two-photon excitation (TPE) mechanism. Permatasari et al [153] demonstrated CDs with the photothermal conversion efficiency of 54.2% prepared from CA and urea. They found the new NIR absorption peaks is resulted in an increasing amount of pyrrolic N. That is the reason why high photothermal efficiency can be achieved. Although the above reports reported various CDs with high photothermal conversion efficiency (>50%), the visible light are employed as the excited laser in the most cases, which limits the practical application due to low tissue penetration depth of visible light. Sun et al [154] synthesized red emissive CDs from citric acid in formamide with microwave method. The CDs exhibited the emission at 640 nm with a decent PL QY of 22.9%, low toxicity, two-photon excited fluorescent and high photothermal conversion efficiency of 43.9% under irradiation of 671 nm laser. They prepared red emissive CDs from CA and PEI in the formamide and conjugated with Ce6 as a photosensitizer [87]. The Ce6-CDs complexes showed synergetic PTT and PDT effect under a single NIR laser (671 nm) source. The photothermal conversion efficiency reach 46%. In addition, the Ce6-CDs also exhibited fluorescent, photoacoustic and photothermal imaging properties. A single complexes achieves multimodal imaging and multitherapy. Li et al [155, 156] found that the yellow CDs from CA and urea can aggregate and form blue supra-CDs in the high humidity environment (RH > 60%). This blue supra-CDs exhibited a broad absorption peak in the NIR region which is responsible to the high photothermal conversion efficiency 52%) under 732 nm laser of 0.5 W cm−2. Li et al [157] showed the CDs from the biomass-watermelon, which showed the NIR II imaging and photothermal conversion efficiency of 30.6% triggered by laser of 808 nm. It is well known that the d orbits of Cu(II) can split under the ligand field with specific geometry, generating higher degenerate e.g. orbits and lower degenerate t2g orbits [182]. The splitting energy, energy gap between e.g. and t2g, may match the energy of NIR region when proper ligand field is formed. In various coordination modes of Cu(II), the N-Cu-N coordination is one kind of coordination mode that can lead to NIR absorption of Cu(II) nanomaterials. Guo et al [158] prepared Cu, N-CDs from EDTA.2Na and CuCl2 by hydrothermal route. A broad absorption band was observed over 500–900 nm corresponding to the dz2 → d22x-y transition of square-planar Cu(II) complexes. Cu, N-CDs exhibit both fluorescent imaging and synergistic PTT and PDT effect under NIR laser (808 nm) irradiation. To achieve high photothermal efficiency, Bao et al [86] introduced the Cu ions into S doped CDs and obtained CuCDs nanosheets. The CuCDs nanosheets exhibit high photothermal efficiency of 41.3%. The CuCDs were further coated with PEG-SH and fluorescent molecules to achieve multimodal (photoacoustic, photothermal and fluorescent) imaging guided cancer therapy. The CuCDs nanosheets exhibited laser-triggered cytosolic delivery, lysosomal escape and nuclear-targeting nature. To enhance the cellular uptake or the EPR, Hou et al [183] conjugated the carboxylate CDs with PLGA-b-PEG-NH2 through the bioconjugation. The CDs modified block copolymer can further self-assembly into micellar nanoparticles. The CDs aggregates were embedded inside the micellar nanoparticles. The aggregation increased the photothermal efficiency to 5.1% under irradiation of 671 nm laser. Wang et al [184] loaded CDs and DOX into chitosan nanogel and further cross-linked the nanogels by EDTA to stabilize the CDs. The nanogel permeated into the tumor tissue and slowly released the chemical drug. In addition, the nanogel also exhibited photothermal function from the NIR irradiation.
Figure 11. (a) Schematic illustration of GNR@SiO2-CDs as a phototheragnostic agent for dual-modality FL and PA imaging-guided synergistic PDT/PTT therapy. (b) Real-time in vivo FL images (Ex = 635 nm), and (c) PA images of tumors obtained after i.v. injection with GNR@SiO2-CDs in nude mice at different time points (from 0 to 72 h). (d) Maximum temperature within the tumor region of the four groups recorded by an IR camera after different treatments; (e) the change curve in temperature at the mice tumor sites of the four groups as a function of irradiation time; (f) time-dependent tumor growth curves (n = 5) observed after different treatments (n = 5, P < 0.05 for each group); (g) the relative body weights of the nude mice increased continuously after different treatments; (h) representative photographs of the four groups after different treatments; (i) H&E stained images of tumors of the four groups after different treatments [150]. Reproduced from [11] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageCDs exhibit not only fluorescent imaging capability, but also phototherapy nature including photodynamic and photothermal, that is beneficial to the photothermal and photoacoustic imaging. Thus, CDs provide a strong potential for multimodal imaging and multiple therapy. This greatly promote the practical applications of CDs in the nanomedical fields.
6. Theragnostic agent based on CDs
To monitor the distribution of drugs and reduce potential damage to the healthy organ, theragnostic agents, so-called smart carriers, were developed, which integrate targeting, imaging, and therapeutic functions into one carrier. The targeting unit directs the smart carriers to selectively accumulate at the tumor site and reduce the drug distribution at undesired locations. The imaging component provides diagnostic and tracking function, allowing for direct monitoring of tumor drug localization, drug concentration, and drug metabolism. These multifunctional smart carriers have the potential to help doctors in disease diagnosis and personalized medicine realization. The therapeutic unit accomplishes the most important function to kill tumor cell. Table 4 presents the progress on the constructing CDs based theragnostic agents. Tang et al [159] constructed the first prototype of the theragnostic carriers based on CDs (figure 12). FA and DOX were loaded onto the CDs surface through electrostatic and π-π stacking interactions. The CDs emitted green light with emission peak at 498 nm when excited at 405 nm where the emitted light from CDs excited DOX with a near red light emission at 595 nm through Forester resonance energy transfer (FRET) between CDs and DOX. The CDs served as both drug carriers and donors of FRET. When the DOX was combined with CDs, the red light was emitted from the system. The green emission will be observed when the DOX was dissociate from the CDs carrier in the tumor microenviroment. The system facilitates the real-time monitoring the drug release based on the fluorescent signal. In addition, the two-photon imaging of CD-DOX at tumor tissue of 65–300 mm has been demonstrated under the excitation of 810 nm. FA was also covalently attached on the PEGylated CDs for targeting the cancer cells overexpressing FA receptors. Thus, the theragnostic nanocarriers can target cancer cells. Fahmi et al [160] synthesized magnetofluorescent nanoparticles by bio-conjugated fluorescent CDs to the magnetic MnFe2O4 nanoparticles. Furthermore, DOX and 4-carboxyphenylboronic acid were loaded on the MnFe2O4 nanoparticles. The DOX can aggregate onto the CDs through π-π stacking, which worked as chemotherapeutic agents. The 4-carboxyphenylboronic acid on the surfaces of MnFe2O4 nanoparticles through –COOH chelating, which act as a cancer cell-specific targeting ligand. This combination exhibited T2—weighted MR and green fluorescent in vitro and in vivo dual modal imaging. It also showed the controlled released manner of DOX at low pH values and inhibition of HeLa cell proliferation as well. Thus, it has potential to act as an effective theragnostic agents.
Figure 12. (a) schematic representation of the surface coupling chemistry for FRET-CDot-DDS. (b) Proposed mechanism of the FRET-CDot-DDS for drug delivery. CDot acts as both a drug carrier and a donor, and DOX acts as an acceptor. When DOX is released from the CDot surface, the FRET between CDot and DOX is terminated, recovering the fluorescence of CDots. (c) Time-dependent release profiles of DOX from CDot-FA-DOX at different pH values: 7.4 (blue), 6.0 (green), and 5.0 (red). The release profile of free DOX molecules at a pH of 7.4 is also displayed (black) for comparison. (d) Growth inhibition results for HeLa cells treated with free DOX, CDot-DOX, and CDot-FA-DOX with concentration of 0–5.0 μg ml–1 after 24 h incubation [159]. [175] John Wiley & Sons. (© 2013 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim).
Download figure:
Standard image High-resolution imageTable 4. Typical theragnostic agent based on CDs.
| Complexes | Imaging unit | Targeting unit | Therapy unit | Key finding | Ref |
|---|---|---|---|---|---|
| FA-DOX-CDs | CDs for FI | FA | DOX | The red light was emitted from the system through the FRET effect between CDs and DOX. The green emission will be observed when the DOX was dissociate from the CDs carrier in the TME. The system facilitates the real-time monitoring the drug release based on the fluorescent signal. The two-photon imaging of CD-DOX at tumor tissue of 65–300 mm has been demonstrated under the excitation of 810 nm. | [159] |
| CDs-MnFe2O4-DOX-4-carboxylphenylboronic acid | CDs and MnFe2O4 for FI and MRI | 4-carboxyphenylboronic acid | DOX | The 4-carboxyphenylboronic acid on the 161 surfaces of MnFe2O4 nanoparticles through -COOH chelating, which act as a cancer cell-specific targeting ligand. This combination exhibited T2—weighted MR and green fluorescent in vitro and in vivo dual modal imaging. It also showed the controlled released manner of DOX at low pH values and inhibition of HeLa cell proliferation. | [160] |
| CDs-DOX loaded into PEG-Citosan nanogels | CDs for FI | PEG for EPR effect | DOX for ChT; CDs for PTT | The nanogels were pH sensitive, allowing for 162 pH-controlled drug release. The CDs exhibited two-photon fluorescence bioimaging and NIR light drug release and photothermal for synergistic therapy. PEG conferred EPR effect for the accumulation of nanogel at the tumor site. | [126] |
| CDs/DOX/BCPVs/siRNA | CDs for FI | siRNA | CDs for PTT DOX for ChT | The heat generated by laser light by the complexes easily damage the cells which triggered the release of DOX from complexes. | [161] |
| CD-DOX-FA-mesoporous silica NPss | CDs for FI | FA | DOX for ChT | DOX and CDs were loaded into the mesoporous silica nanoparticles and conjugated FA on the surface of nanoparticles. This design ensures (1) targeted bio-imaging, (2) prevention of premature drug release and (3) bio-responsive drug release | [162, 163] |
| CDs-Pt(IV)@PEG-PAH/DMMA | CDs for FI | PEG for EPR effect | Cisplatin for ChT | Several advantage of the combination: (1) negative charge/PEGylation to have 117 prolonged circulation time in blood, (2) accumulation at the tumor site through EPR effect, (3) enhanced tumor cell internalization (4) less side effect in normal physiological condition, (5) controlled release of cisplatin in the reductive cytosol of cancer cells, (6) multiple color bioimaging of CDs. | [125, 164] |
| CDs-PEG-FA/ZnPc or R780 iodide or Ce6 | CDs for FI | FA for FR | ZnPc for PDT IR780 iodide for PTT; Ce6 for PDT | The targeting of ZnPc/CDs-PEG-FA was evaluated by monitoring the blue and red fluorescence of CDs (λex/λem = 358/461 nm) and ZnPc ((λex/λem = 647/665 nm). The PDT on the HeLa cells was also demonstrated by laser irradiation (660 nm, 30 mW cm−2). | [165–167] |
| Heavy-chain ferritin (HFn)-CDs-DOX | CDs for FI | HFn for targeting | CDs for PDT DOX for ChT | HFn nanocages can be used as vector coupled with dual functional CDs on the surface of ferritin and encapsulating the chemotherapeutic drug DOX. | [168] |
| Au cube/Mesoporous SiO2-MnCDs/DOX-RGD | Mn for MRI CDs for FI | RGD for targeting | CDs for PDT DOX for ChT Au cube for PTT | RGD act as an active targeting effect in this combination. The Au cube, Mn doped CDs functionalize as multiple imaging agent of PT, FI and MRI for diagnosis and guidance of therapy. Under 635 nm irradiation, the composites in situ generate oxygen and amplifies the content of 1O2 for PDT in the TME. Au Cube exhibits 65.6% PT conversion efficiency under 808 nm irradiation the PT induced the DOX release as well. | [169] |
| CDs from HA | CDs | CDs | CDs | The targeting ability of these CDs was attributed to the partial preservation of HA functionality, which has high specific affinity for CD44 receptors overexpressed on the surfaces of many cancer cells. The CDs exhibited not only strong emission at 454 nm upon the excitation at 390 nm but also with the ROS production of CDs for PDT | [117] |
Abbreviations: FI: Fluoroscent imaging; MRI: magnetic resonance imaging; FA: flolic acid; FR: folic acid receptor; ZnPc: Zinc phthalocyanine; HFn: Heavy-chain ferritin; HA: hyaluronic acid; 1O2:singlet oxygen.
Wang et al [126] constructed a PEG-chitosan@CDs hybrid nanogels by integrating branched PEG, chitosan, CDs and DOX into a single nanoparticle (figure 13). The nanogels were pH sensitive, allowing for pH-controlled drug release. The CDs exhibited two-photon fluorescence bioimaging and NIR light drug release and photothermal for synergistic therapy. PEG conferred EPR effect for the accumulation of nanogel at the tumor site. Yang et al [161] loaded DOX and small interfering ribonucleic acid (siRNA) onto CDs surfaces and capsulated with biocharged polyester vectors (BCPVs) to form CDs/DOX/BCPVs/siRNA. The heat generated by laser light by the complexes easily damage the cells which triggered the release of DOX from complexes. Prasad et al [162, 163] used mesoporous silica nanoparticles as a carrier. DOX and CDs were loaded into the mesoporous silica nanoparticles and conjugated FA on the surface of nanoparticles. This design ensures (1) targeted bio-imaging, (2) prevention of premature drug release and (3) bio-responsive drug release. Feng et al [164] linked the targeting RGD peptide on the CDs-Pt(IV) based on the Zheng's work [124]. The drug nanocarriers showed effective uptake by cancer cells through RGD-integrin αvβ3 (ligand–receptor) interaction. Upon the internalization, the loaded cisplatin (IV) prodrug was reduced to cytotoxic cisplatin in reductive cytosol of cancer cells to exhibit therapeutic effects. Furthermore, they wrapped the CDs-Pt(IV) with PEG functionalized poly(allyamine) (PEG-(PAH/DMMA)) (figure 14) [125] to achieve several advantage (1) negative charge/PEGylation to have prolonged circulation time in blood, (2) accumulation at the tumor site through EPR effect, (3) enhanced tumor cell internalization due to the charge convertible property in tumor extracellular microenvironment, positive charge at tumor site (pH = 6.8) (4) less side effect in normal physiological condition, (5) controlled release of cisplatin from cisplatin (IV) prodrug in the reductive cytosol of cancer cells, (6) multiple color bioimaging of CDs. In the above progress, CDs mainly act as a bio-imaging agent for diagnosis and/or drug carrier. Chemotherapy—such as DOX or Pt work as anticancer medicine. However, the chemotherapy has toxic and side effect on the normal organ and tissue.
Figure 13. Schematic illustration of biocompatible PEG-chitosan@CDs hybrid nanogel for multifunctional applications in biomedical field, with CD representing for carbon dot [126]. Reprinted with permission from [126]. John Wiley & Sons. (© 2015 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim).
Download figure:
Standard image High-resolution imageFigure 14. (a) Schematic illustration for the preparation of charge-convertible CDs-based drug nanocarrier CDs–Pt(IV)@PEG-(PAH/DMMA). pHe means tumor extracellular pH. (b) Schematic illustration for the drug delivery process of CDs–Pt(IV)@PEG-(PAH/DMMA): (1) negative charge/PEGylation to afford prolonged circulation time in blood, (2) accumulation at the tumor site through the EPR effect, (3) responsiveness to tumor extracellular pH, (4) effective uptake by cancer cells, (5) facilitated endosome escape by 'proton sponge' effect and controlled cisplatin release from cisplatin (IV) prodrug under reductive cytosol, and (6) cisplatin binding with DNA to exhibit cytotoxicity. Cell viability of A2780 cells after incubating with (c) CDs–Pt(IV)@PEG-(PAH/DMMA) and (d) CDs–Pt(IV)@PEG-(PAH/SA) at pH 7.4 and 6.8 at 37 °C. (e) Photographs of mice and excised tumors from representative euthanized mice. (f) H&E stained tumor slices after 14 d treatment from different groups. The error bars are based on the standard errors of the mean (SEM) of five tumors per group. p values: ***p < 0.001 [125]. Reprinted with permission from [128]. Copyright (2016) American Chemical Society.
Download figure:
Standard image High-resolution imageTo reduce side effects of chemotheraptic drugs, phototherapeutic agents were integrated with CDs to create theragnostic agents. Choi et al prepared CDs from the α-cyclodextrin by chemical oxidation reaction (figure 15) [165], followed by FA conjugation onto the surface of carboxyl capped CDs through H2N-PEG-NH2. Furthermore, the photosensitizer for phototherapy–zinc phthalocyanine (ZnPc) was attached on the CDs-PEG-FA through π–π stacking interaction. The targeting of ZnPc/CDs-PEG-FA was evaluated by monitoring the blue and red fluorescence of CDs (λex/λem = 358/461 nm) and ZnPc (λex/λem = 647/665 nm). The intense fluorescence of CDs in the cytoplasm was observed after internalization of CD-PEG-FA/ZnPc, whereas no fluorescence signals were detected in the cases of CDs-PEG and FA saturated HeLa cells. The photodynamic therapy on the HeLa cells was also demonstrated by laser irradiation (660 nm, 30 mW cm−2) for 10 min. Significant cell death was observed in CD-PEG-FA/ZnPc, whereas other control samples did not exhibit measurable photodynamic activity. In vivo results demonstrated the effective bioimaging and photodynamic activity. Li et al [166] integrated the IR780 iodide, a typical photothermal agent, with FA modified CDs through π–π stacking interaction. This combination exhibited an improved photostability, an enhanced tumor-targeting ability, and a high photothermal conversion efficiency of 87.9% to effectively eradicate tumors upon 808 nm laser irradiation. Xue et al [167] synthesized blue emissive CDs from the biomass-lychee exocarp and modified with H2N-PEG-NH2 to form CDs-PEG through bioconjugation reaction. The CDs-PEG further conjugated with chlorin e6 (Ce6) as PDT unit and transferrin (Tf) as a targeting unit to form CDs-PEG-Ce6-Tf. Targeted NIR fluorescent imaging of tumor tissue was achieved through the FRET between CDs and Ce6. In addition, Ce6 can releases the singlet oxygen (1O2) by 650 nm light irradiation to kill cancer cells.
Figure 15. (a) Schematic illustration of the preparation of carbon nanodots (CD) from α-cyclodextrin and targeted photodynamic therapy with folic acid functionalized carbon nanodots loaded with zinc phthalocyanine (CD-PEG-FA/ZnPc). (b) Fluorescence of ZnPc (excited at 660 nm) in tumor was imaged after 12 h injection of CD-PEG-FA/ZnPc, CD-PEG/ZnPc, and CD-PEG-FA (0.5 mg of ZnPc kg−1 mouse). (c) CD-PEG-FA/ZnPc suspensions were injected into tail veins of tumor-bearing mice and the fluorescent signals were obtained at various time points (1, 2, 6, 12, 24, and 48 h). (d) Ex vivo fluorescence images of major organs of mice. The fluorescent signals corresponding to ZnPc (excited at 660 nm) from major organs, tumor, and skin were obtained after 12 h of i.v. injection of CD-PEG-FA/ZnPc and CD-PEG/ZnPc into tumor-bearing mice. FA-conjugated CD delivered and released ZnPc to tumor effectively, in contrast with the CD lacking FA. (e), (f) Relative tumor volumes measured over time after the tumor-bearing mice were treated with various CD derivatives. Tumor-bearing mice were separated into 6 groups: (i) PBS control; (ii) CD-PEG-FA without irradiation; (iii) CD-PEG-FA with irradiation; (iv) CD-PEG/ZnPc without irradiation; (v) CD-PEG/ZnPc with irradiation; (vi) CD-PEG-FA/ZnPc without irradiation; (vii) CD-PEG-FA/ZnPc with irradiation (n = 4 for each group). Irradiation was performed 2 using a 660 nm laser at 0.3 W cm−1. Tumor volumes were measured over 10 d. It is notable that no significant increase in tumor volume was observed for 8 d in mice treated with CD-PEG-FA/ZnPc with irradiation. P values were calculated by Student's t-test: * for p < 0.05, n = 4 [165]. [182] John Wiley & Sons. (© 2014 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim).
Download figure:
Standard image High-resolution imageTo further enhance the therapeutic efficiency, multiple therapeutic strategies can be integrated into a single system. Yao et al [168] designed a sensitive bio-imaging and effective treatment system by employing heavy-chain ferritin (HFn) nanocages as vector coupled with dual functional CDs on the surface of ferritin and encapsulating the chemotherapeutic drug DOX. The photodynamic therapeutic effects of CDs was demonstrated by the laser irradiation of 532 nm. HFn exhibited the targeting function on the tumor cells. Both in vivo and in vitro results indicated that the complexes have great potential biomedical application in the targeted chemo- and photodynamic therapy effect. Zhang et al [169] loaded DOX and Mn doped CDs prepared from Mn(II)-phthalocyanine into the mesoporous silica-coated gold cube-in cubes (CCmMC/DOX). pS-PEG2000-RGD was modified the surface of CCmMC to form RGD-CCmMC/DOX. In this combination, RGD act as an active targeting effect. The Au cube, Mn doped CDs functionalize as multiple imaging agent of photothermal, fluorescence and MRI for diagnosis and guidance of therapy. Under 635 nm irradiation, the composites in situ generate oxygen and amplifies the content of singlet oxygen (1O2) for PDT therapy in the tumor microenvironment. Au cube exhibits 65.6% photothermal conversion efficiency under 808 nm irradiation the photothermal induced the DOX release as well. Thus, the composites combined the chemo- and phototherapy together.
The preparation of theragnostic agents normally involves the integration of multiple functionalities into a single system. The functionality of each individual component can greatly influence the preparation process. For example, CDs prepared with HA, a negatively charged polysaccharide, showed specific targeting to cancer cells [117]. The targeting ability of these CDs was attributed to the partial preservation of HA functionality, which has high specific affinity for CD44 receptors overexpressed on the surfaces of many cancer cells [117]. The CDs from this facile approach not only exhibited strong emission at 454 nm upon the excitation at 390 nm and had the strong affinity on the CD44 receptors, allowing preferred accumulation of CDs at the tumor site. In conjunction with the ROS production of CDs for PDT, this single system is now equipped with three functions-fluorescence, targeting and therapy.
Very recently, Gong et al [185] use the CDs as the smart drug carrier to construct theragnostic agents (figure 16) based on mitochondrial redox homeostasis and the balance between reactive oxygen species and antioxidants-glutathione (GSH). Typically, H2O2 is produced within mitochondria and reducing species like GSH is consumed to maintain the balance. The design strategy of this study was to level up the concentration of oxidative species (H2O2) by depletion of GSH. Then, the ROS generator promoted the conversion of oxidative species into ROS to kill the cancer cells. In this study, atomically dispersed Au were packed on CDs carriers, which were further modified with triphenylphosphine (TPP) and cinnamaldehyde. TPP induced nanocarriers to accumulate in mitochondria and the atomically dispersed Au effective depleted GSH to promote the rising of ROS concentration while the CDs provided the capability of fluorescent imaging.
Figure 16. (a) Schematic illustration of the synthesis process of CAT-g. (b) TEM image of carbon dots. Experiments were repeated three times. (c) TEM image of carbon-dot-supported Au with a Au loading of 0.5 wt% (Au/CDs1). Experiments were repeated three times. (d) TEM image of carbon-dot-supported Au with a Au loading of 15.3 wt% (Au/CDs2, CAT-g). Experiments were repeated three times. (e) TEM image of carbon-dot-supported Au with a Au loading of 45.7 wt% (Au/CDs3). Experiments were repeated three times. (f) High-angle annular dark-field (HAADF) image of carbon dots. Experiments were repeated three times.(g) HAADF image of Au/CDs1. Individual Au atoms are outlined by red cycles. Experiments were repeated three times. (h) HAADF image of Au/CDs2 (CAT-g). Experiments were repeated three times. (i) HAADF image of Au/CDs3. Experiments were repeated three times. (j) Design of MitoCAT-g to amplify oxidative stress in mitochondria. MitoCAT-g is composed of carbon-dot-supported atomically dispersed gold (CAT-g) with surface modifications of mitochondrion-targeting TPP and ROS-generating CA. (k) After MitoCAT-g endocytosis, CA is released at the acidic pH value found in endosomes and then generates ROS in mitochondria. After CA release, the resulting TPP–CAT-g particles target mitochondria and deplete GSH by forming Au–S bonds with GSH. Elevation of ROS and depletion of GSH amplify the oxidative stress in mitochondria and cause apoptotic cell death [185]. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Nanotechnology, 'Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifer for cancer treatment', Ningqiang Gong et al, Copyright © Springer Nature (2019)
Download figure:
Standard image High-resolution image7. Conclusion and outlook
CDs have been extensively explored for the medical application due to their low cytotoxicity and excellent biocompatibility. Basically, they can work as a fluorescent probe for bioimaging and present high-resolution imaging observed by bare eyes for diagnosis and navigation in the operation. They further act as a carrier for the loading of other functional units, like targeting and/or therapeutic unit because of abundant functional groups on the surface. Recently, the researchers discovered the photothermal and photodynamic natures of CDs, which extend the potential applications in the therapeutic field. In other words, CDs are not only bioimaging agents but also phototherapeutic agents. The photothermal property also can be employed for the photothermal and photoacoustic imaging. Considering these properties, CDs have great potential in the personalized theragnostic nanomedicine. It can integrate the multiple functions into one system. However, there is also some serious issue to be solved for the practical application. To promote the emission in the long wavelength, especially in the red light region, various aromatic compounds are employed for the synthesized for red emissive CDs. Although CDs were treated as low toxicity and high biocompatibility, these conclusions were based on the CDs prepared in top-down route and/or citric acid, glucose, and amino acid reaction system. The biological effect of red emissive CDs from aromatic compounds need to be further clarified. These carbon sources may result in strong cytotoxicity in the animal body.
- (a)Tissue penetration depth is a critical factor for the clinical diagnosis. NIR window I and II have low light absorbance Thus, developing two-photon fluorescence of CDs turned to be an attractive research field.
- (b)Besides the two-photon fluorescence, the room temperature phosphor (RTP) is another effective route. If the materials have a large RTP lifetime (a few hours), it can be used as a biomarker that can be excited out of the body, then it can emit the light without excitation light, which can avoid the autofluorescence.
- (c)CDs have exhibited not only the fluorescent properties but also targeting and therapeutic nature, which may achieve CDs based theragnostic agent by itself. However, the report in this field just emerged in recent 2 years. More and more results may be reported in the near future.
Acknowledgment
This work was financially supported by the Beijing Municipal High Level Innovative Team Building Program (No. IDHT20180504), Beijing Outstanding Young Scientists Program (BJJWZYJH0201910005017), the National Natural Science Foundation of China (Nos. 21805004, 21671011, 21872001 and 51801006), Beijing Natural Science Foundation (Nos. KZ201710005002 and 2192005), China Postdoctoral Science Foundation (No. 2018M641133), Beijing Postdoctoral Research Foundation (No. 2018-ZZ-021) and Chaoyang District Postdoctoral Research Foundation (No. 2018-ZZ-026). These funding agencies are acknowledged.