Abstract
The protection of organic carbon through association with iron minerals (FeR) is an important factor in its stabilisation, long-term storage, and burial efficiency in marine sediments. However, large uncertainties still exist concerning the sources, lability, age, and composition of the organic matter associated with FeR in natural sediments. Therefore, the timing and environmental setting of the carbon-iron bonding process remain elusive. Here we use radiocarbon (Δ14C) and stable isotopes (δ13C) of downcore bulk sedimentary organic matter, benthic foraminifera and the organic carbon fraction bound to FeR to interrogate the source and age of the organic carbon pool associated with FeR in Arctic marine sediments. In the Barents Sea, we find that the organic carbon associated with FeR is younger overall than the bulk organic matter and is probably marine derived. The comparison to other investigations of OC-FeR origins reveals that in large parts of Arctic shelf regions FeR associated organic carbon is radiocarbon enriched and has a higher δ13Corg value compared to the bulk sediment, irrespective of sediment depth/age. Our findings suggest a rapid and preferential binding of fresh and marine organic matter with FeR. Hence, labile organic matter prone to decomposition is protected and stabilised, underlining the potential of the organic carbon–iron association as an efficient carbon burial mechanism.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Organic carbon sequestration in marine sediments is a major control on atmospheric CO2 and O2 concentrations over geological time (Berner 2003), while being a poorly constrained pathway of contemporary carbon burial (Regnier et al 2022). The majority (approximately 90%) of organic carbon deposited at the global seafloor is buried in shelf and slope sediments (Hedges And Keil 1995, Smith et al 2015). A set of physical, biological and chemical processes combine to control organic carbon preservation, including sedimentation rate (Müller and Suess 1979, Ingall and Vancappellen 1990), the presence and absence of oxygen (Pedersen and Calvert 1990, Canfield 1994, Hartnett et al 1998), selective preservation of biochemically unreactive compounds (Hatcher et al 1983, Burdige 2007), and the protection of organic matter through interactions with a mineral matrix (Mayer 1994, Hedges and Keil 1995, Hemingway et al 2019). However, the relative importance of these factors remains poorly constrained. Moreover, sedimentary organic matter on marine shelves consists of a diverse mixture of terrestrial and marine components that exhibit different ages, degradation states and maturities. A major challenge in evaluating the burial of organic carbon in marine sediments is the need to constrain degradation rates of these different organic components in relation to the environmental factors that control spatial and temporal changes in carbon sequestration (Arndt et al 2013).
Over the past four decades, enhanced atmospheric heat transport and inflow of Atlantic water have dramatically warmed the Arctic (e.g. Lind et al 2018). One of the most apparent signs of this warming trend and current global climate change is Arctic sea ice loss. For instance, the Barents Sea (figure 1) summer sea ice extent has drastically decreased by over 30% during the past decades (Meier et al 2014, Fetterer et al 2017). The ongoing transformation of the Arctic Ocean from an 'icy land' into an open ocean system forces the entire Arctic ecosystem to adapt and restructure, changing the Arctic carbon cycle, i.e. atmospheric CO2 uptake, pelagic-benthic coupling, organic matter sedimentation and long-term sequestration (Piepenburg 2005, Wassmann et al 2008, Arrigo and Van Dijken 2011, Wassmann 2011, Post et al 2013, Dalpadado et al 2014). However, future productivity and carbon burial in the Arctic and the Barents Sea remain uncertain, partly due to the challenges of linking of ongoing changes in the Arctic Ocean to organic carbon burial, sedimentary biogeochemical cycles and the marine ecosystems (Stein and Macdonald 2004, Wassmann 2011, Haug et al 2017).
Figure 1. Map of the western Barents Sea located north of Norway. Sampling locations (B13–B16) are shown by yellow circles and the oceanic polar front is depicted by the dotted black line (Oziel et al 2016). The white area indicates sea ice extent during the sampling campaign in July 2017 (Fetterer et al 2017).
Download figure:
Standard image High-resolution imageSedimentary organic matter can be protected and stabilised via association with inorganic components (Mayer 1994, Hedges and Keil 1995, Hemingway et al 2019). Clay minerals are viewed as a major inorganic host for sedimentary organic matter, enhancing preservation (e.g. Mayer 1994, Kennedy et al 2002). However, as previously discovered in terrestrial soils, a chemical association between organic carbon and reactive iron oxides (nanoparticulate and amorphous phases of ferric (oxyhydr)oxides, e.g. ferrihydrite) could also play a central role in organic carbon preservation in marine sediments (Lalonde et al 2012). Organic carbon has a strong affinity to reactive iron phases (FeR), and the resultant association of organic carbon and iron (OC-FeR) is thought to promote long-term stabilisation and protection of sedimentary organic matter against microbial degradation (Lalonde et al 2012, Riedel et al 2013, Chen et al 2014, Chen and Sparks 2018). Investigations of global marine surface sediments revealed that the fraction of the total organic carbon bound to FeR (fOC-FeR) is on average 10%–20%, with values ranging from ∼0.5% to 40% (Lalonde et al 2012, Salvadó et al 2015, Ma et al 2018, Zhao et al 2018, Wang et al 2019, Faust et al 2020) and even up to 80% (Longman et al 2021). Moreover, recent investigations of the association between organic carbon and FeR following sediment burial showed a millennial-scale OC-FeR persistence, underlining the importance of OC-FeR as an important carbon sequestration mechanism (Faust et al 2021). However, large uncertainties still remain concerning the sources, lability, age and composition of the organic matter (preferentially) associated with FeR in natural sediments. In particular, the timing and environmental setting of the carbon-iron bonding process remains elusive—information that is crucial to better quantify the role of OC-FeR in the global carbon cycle, both in the past and into the future.
The source and age of organic carbon at the seafloor can be recorded by radioactive and stable carbon isotopes (14C and 13C) of sedimentary organic matter (e.g. Eglinton et al 1997, Tesi et al 2011, Goñi et al 2013). The stable isotope composition of sedimentary fOC-FeR has refined our understanding about different sources and cycling of organic carbon in marine sediments (Lalonde et al 2012, Salvadó et al 2015, Shields et al 2016, Ma et al 2018, Zhao et al 2018, Wang et al 2019). However, the various modes of chemical binding and physical associations between organic carbon and reactive metal phases have mainly been investigated in the uppermost (0–3 cm) horizon of the seafloor (Faust et al 2021). Hence, longer-term OC-FeR burial mechanisms in natural marine sediments are not well characterized. Recent work on sediment cores from the western Barents Sea (figure 1) revealed that a substantial fraction of the OC-FeR pool is probably allochthonous, suggesting that the OC-Fe association is generated prior to deposition at the seafloor and not, for example, during authigenic precipitation of iron (oxyhydr)oxides at the Fe(II)/(III) redox boundary in the sediments (Faust et al 2021). To interrogate the source and age of the organic carbon pool associated with reactive iron in Arctic marine sediments, here we use radiocarbon and stable isotopes of downcore bulk sedimentary organic matter, carbonate (benthic foraminifera) and chemically extracted organic carbon bound to reactive iron.
2. Material and methods
2.1. Study area
The Barents Sea is located between 70 and 81° N off the northern Norwegian coast and is the largest of the six pan-Arctic shelf seas. It covers an area of 1.6 million square kilometres with an average water depth of 230 m (Carmack et al 2006). For a detailed descriptions about the modern climate setting and ecosystem of the Barents Sea, we refer to extensive overviews and reviews published during the past two decades (Loeng et al 1997, Wassmann et al 2006, Jakobsen and Ozhigin 2011, Smedsrud et al 2013, Dalpadado et al 2014, Jørgensen et al 2015). In brief, the oceanic circulation pattern of the western Barents Sea is dominated by the relatively warm northward-flowing North Atlantic Current (temperature 2 °C–8 °C, salinity >35‰) and cold Arctic currents (Spitsbergen and Persey; temperature <0 °C, salinity <35‰) entering the Barents Sea from the northeast. The relatively sharp boundary between these water masses forms the oceanographic polar front (figure 1) (Harris et al 1998) which is mainly determined by the shelf bathymetry and is, therefore, relatively stable from year to year (Drinkwater 2011). The northern Barents Sea is seasonally ice-covered with maximum and minimum ice coverage in March–April and August–September, respectively. The heat content of the Atlantic water keeps the southern Barents Sea permanently ice-free. River runoff into the Barents Sea is very limited. Only one larger river, the Petchora River, enters directly into the south-eastern Barents Sea in Russia. Rivers on the Kola Peninsula, on Svalbard and in Norway are small. Thus, sediment discharge through river inflow is low (e.g. Politova et al 2020) and the main processes responsible for Barents Sea surface sediment distribution are re-deposition by winnowing from shallow banks into troughs and depressions, and deposition from sea ice. Hence, sedimentation rates are generally low, 0.04–2.1 mm y−1 since the last glacial period (Faust et al 2020), but can be much higher proximal to glacier outlets, for instance in places close to Svalbard. The present ecological setting, as in all Arctic shelf seas, is characterized by very pronounced seasonal fluctuations in insolation and primary production. Despite the relatively short duration of the growing season in the Arctic, the Barents Sea is a high productivity shelf area where 40% of the total primary production of the Arctic Ocean takes place (Sakshaug 2004). Throughout the western Barents Sea, oxygen penetration was repeatedly analysed by direct measurements and indirect indicators (e.g. pore water profiles), and was found to be between ∼2 and 6 cm below the sediment–water interface (Vandieken et al 2006, Nickel et al 2008, Freitas et al 2020, Stevenson et al 2020, Faust et al 2021). In addition, 210Pb profiles and direct measurements of benthic faunal activity show a distinct sediment mixed layer in the Barents Sea that extends to a sediment depth of approximately 2 cm (Carroll et al 2008, Solan et al 2020).
2.2. Sediment sampling
Four sediment cores were collected by using a multi-corer along a south-north gradient in the western Barents Sea during the Changing Arctic Ocean Seafloor cruise (JR16006) in summer 2017 (figure 1). Sediment cores from station B13, B14, B15, B16 (supplementary table S1) were sliced in 0.5 cm intervals from 0 to 2 cm and in 1 cm intervals thereafter. Samples were stored in plastic bags at −20 °C immediately after recovery on-board the Royal Research Ship James Clark Ross. Prior to any chemical sediment analysis, all samples were freeze-dried and homogenized by gentle grinding using an agate mortar and pestle. Between two and four downcore samples from each sediment core were selected for radioactive carbon-14 content (Δ14C) and stable isotopic δ13C signature analysis (supplementary tables S2 and S3).
2.3. Organic carbon extraction and analysis
To quantify the amount of organic carbon bound to iron (oxyhydr)oxides in our samples, we applied a method described in detail by Lalonde et al (2012) and Salvadó et al (2015). Briefly, 0.25 g of sediment was transferred into 30 ml centrifuge tubes. Fifteen millilitre of a solution containing 0.27 M trisodium citrate (Na3C6H5O7·H2O) and 0.11 M sodium bicarbonate (NaHCO3) was added, mixed and heated to 80°C in a water bath; 0.1 M sodium dithionite (Na2S2O4; 0.25 g) was added to the mixture, temperature was maintained at 80°C, and the tube was shaken every 5 min. After 15 min, the mixture was centrifuged for 10 min at 4000 rpm, the supernatant was decanted, and 200 μl of 12 N HCl were added to prevent Fe(III) precipitation. The remaining sediment samples were rinsed three times with artificial seawater and then freeze-dried. To quantify potential organic carbon loss unrelated to metal oxide dissolution, a control experiment was conducted: A 0.25 g aliquot of each sample was treated the same way as the reduction experiment, but the complexing and reducing agents (sodium citrate and sodium dithionite) were replaced with sodium chloride to reach a solution of the same ionic strength. All samples were weighed after the experiment to account for mass loss.
Organic carbon content of the bulk sediment before and after the reduction and control experiments was analysed on decarbonated samples using 10% (vol.) HCl, rinsed three times and dried overnight at 50°C. Organic carbon content was determined with a LECO SC-144DR combustion analyser at the University of Leeds, UK (Faust et al 2021). The certified reference material LECO 502-062 and blanks were included in every batch, and results are given in weight percentage. The relative error of the organic carbon analysis was ±1.7%. To account for the mass loss during the extraction experiment we applied the mass balance calculation of Salvadó et al (2015).
The δ13C and Δ14C of organic carbon associated with reactive iron were determined by difference from the control and the reductive leach residue and compared to bulk untreated sediments (supplementary tables S2 and S3). In more detail, bulk sediment samples before and after the reduction and control experiments were moistened with a small amount of deionised water, covered by glass fibre filter papers and placed into a sealed glass desiccator vessel together with a beaker of concentrated hydrochloric acid to hydrolyse any carbonate in the sample over three days via acid fumigation at the Natural Environment Research Council Radiocarbon Facility. Decarbonated samples were freeze-dried before combustion to CO2 in a sealed quartz tube. After combustion, sample CO2 was cryogenically recovered on a vacuum line. An aliquot of recovered sample CO2 was used to measure δ13C on a dual inlet stable isotope mass spectrometer (Thermo Fisher Delta V). This value was used for normalisation of the measured sample 14C/13C ratio. A second aliquot of sample CO2 was prepared to graphite and the sample 14C/13C ratio was measured by accelerator mass spectrometry at the Scottish Universities Environmental Research Centre AMS laboratory. The 14C enrichment of organic carbon in each sample was calculated as Δ14Corg based on the relative difference between the isotope ratio of the absolute international standard (relative to the year of measurement, 2021) and the age-corrected sample isotope ratio, where the latter was first normalised to-25% δ13C Vienna Pee Dee Belemnit (VPDB), using the measured δ13Corg value described above (Stuiver and Polach 1977). The fraction modern (F14C) is also reported where F14C = (Δ14Corg + 1000)/991.448 based on the correction factor for measurement in 2021. To quantify potential 14C contamination from the organic carbon extraction process, three process standards, modern (TIRI Barley Mash; TBM), background (Anthracite) and carbon free silica sand, were separately processed alongside the natural sediment samples in both the control and the reaction experiment. The aliquots of Anthracite standard show slightly elevated values above the laboratory blank (i.e. 14C added during sample treatment), indicating an addition of a small amount of modern carbon during the extraction procedure. Accordingly, we applied a background correction to samples and TBM standards of a value of F14C = 0.006 ± 0.001. This value was obtained via the average of measured values for Anthracite processing blanks. This addition of a small quantity of carbon during processing is consistent with results for silica blanks, which showed a carbon content of 0.01%–0.02%.
δ13C and Δ14C of the organic carbon bonded to reactive iron phases (δ13C-FeR; Δ14C-FeR; supplementary tables S2 and S3) were calculated assuming that the isotopic composition of the control sample (Δ14C-FeR control) is comprised of a mixture of organic carbon from reactive iron (the unknown Δ14C-FeR) and carbon forming the residue after the reductive removal of reactive iron (measured Δ14C-FeR reaction), such that mass balance leads to equations (1) and (2):


where fOC-FeR is the fraction of total organic carbon bond to reactive iron from Faust et al (2021).
To determine the 14C carbonate contents of benthic foraminifera, providing constraint on the 14C activity of marine dissolved inorganic carbon (DIC) at time of deposition, aliquots of bulk sediment samples were washed through a 63 μm sieve before being dried at 50°C, and microfossils were picked using a Leica MZ12 binocular microscope. All benthic foraminifera used for radiocarbon analyses were a mixture epifaunal or shallow infaunal species, dominated by rotaliids (>90%). Pyrgo and other miliolid species, which have previously been associated with anomalous radiocarbon measurements in the Arctic (Ezat et al 2017), were not analysed. The radiocarbon activities of the carbonate microfossils were obtained via the Mini Carbon Dating System at the Bristol Radiocarbon Accelerator Mass Spectrometry facility. Carbonate specimens were acidified to release CO2 (<100 μgC), which was measured directly without graphitisation (Tuna et al 2018).
3. Results and discussion
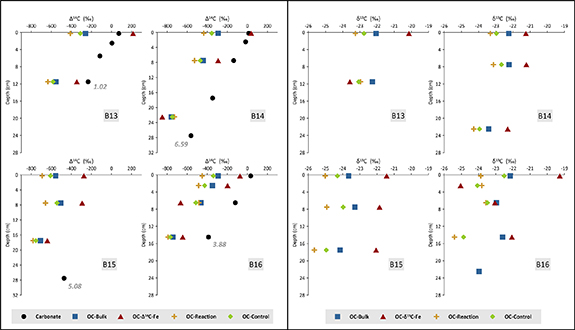
To evaluate the age and origin of the organic carbon bound to reactive iron phases (OC-FeR) over long time scales of about 6 kyr before present (BP) (figure 2), we analysed Δ14C and δ13C signatures in sediment samples (Δ14Corg and δ13Corg) and in the organic carbon fraction bound to FeR (Δ14C-FeR and δ13C-FeR). To place these in the context of the radiocarbon activity of the marine dissolved inorganic carbon reservoir at the time of deposition, we compare the organic matter to the Δ14C content of benthic foraminifera (carbonate). In summary, we first discuss the origin of the bulk organic matter and show that its mainly marine-derived, but is significantly aged prior to deposition. We then compare our bulk Δ14Corg and δ13Corg signatures with Δ14C-FeR, δ13C-FeR and Δ14C carbonate, finding that OC-FeR in the Barents Sea is younger overall than the bulk organic matter and probably marine derived. Finally, we relate our findings to other investigations of OC-FeR origins and reveal that in large parts of Arctic shelf regions iron associated organic carbon is radiocarbon enriched and has a higher δ13Corg value compared to the bulk sedimentary, irrespective of sediment depth/age, which indicates rapid sequestration of contemporary organic carbon.
Figure 2. Downcore Δ14Corg (left) and δ13Corg (right) values in the sediment residuals of the control and extraction experiment and bulk sediment. These are shown relative to the Δ14C content of benthic foraminifera (carbonate). The isotopic signatures of the organic carbon fraction bond to FeR (Δ14C-FeR and δ13C-FeR) are calculated by mass balance (equations (1) and (2)). Grey numbers indicate uncalibrated radiocarbon dates of the lowest biogenic carbonate measurement in kyr BP.
Download figure:
Standard image High-resolution image3.1. Origin of the bulk organic matter
The stable carbon isotope signature of organic matter (δ13Corg) in marine sediments reflects the isotopic composition of the carbon source and the fractionation between 12C and 13C during photosynthesis (Hayes 1993). As the contribution of C4 plant types is insignificant in the Arctic region (Collins and Jones 1986, Still et al 2003), δ13Corg can be a reliable proxy to identify marine versus terrigenous organic matter in Barents Sea sediments (e.g. Schubert and Calvert 2001). Marine organic carbon is isotopically enriched in 13C compared to terrestrial C3 plant material (Arthur et al 1985) and typical endmember values are −20.1‰ and −26.1‰ for marine and terrigenous organic matter, respectively, in the Barents Sea region (Knies and Martinez 2009, Pathirana et al 2014). Our results show that δ13Corg signatures of all bulk untreated surface sediment samples vary between −22.1‰ and −23.7‰ (average −22.5‰) suggesting a bulk organic matter pool more dominated by marine organic matter (Martens et al 2021 and ref. therein). The δ13Corg values show a slight decrease with sediment depth (figure 2 and supplementary figure 1). The decline in δ13Corg is correlated with a Δ14Corg decrease (r = 0.8; supplementary figure S2) which occurs as total organic carbon contents and related biomarker records documenting relatively steady first-order organic matter decay with depth (Stevenson et al 2020, Faust et al 2021). This indicates no significant changes in the type of organic matter deposited at the seafloor, hence, we attribute these changes to a preferential degradation of labile marine organic matter, and not to a gradual change in organic carbon sources over time.
14C is commonly utilized for age determination of the carbon-bearing component. The 14C signature of marine organic matter (Δ14Corg) is a marker for modern, pre-aged or fossil carbon and, therefore, provides some information about the organic matter source, especially if combined with δ13Corg signatures. Marine surface sediments from circum-Arctic shelf regions reveal a substantial range of published Δ14Corg values, outlining large-scale differences in organic carbon sources to the present seafloor (Martens et al 2021). The Laptev and East Siberian Seas receive substantial carbon contributions from remobilization of thawing permafrost or other older deposits. In contrast, terrigenous organic carbon input in the Kara Sea is mainly contemporary (i.e. recent plant cover), and Barents and Chukchi Sea sediments are dominated by modern carbon derived from marine primary producers (Martens et al 2021).
The organic carbon Δ14Corg signatures of our bulk untreated Barents Sea sediment samples vary between −260‰ and −760‰ and decrease with sediment depth due to the time-dependant decay of 14C (figure 2, supplementary figure S1). Bulk Δ14Corg values in the first centimetre of each core are in accordance with published Δ14Corg data of bulk surface sediments from the western Barents Sea, which range from −245‰ to −504‰ (n = 11) (Martens et al 2021). Sediment cores from stations B13, B14 and B16 show very similar Δ14Corg values (−260‰, −292‰, −291‰) in the first centimetre. At station B15, Δ14Corg is lower (−558‰) as is δ13Corg. Due to a very shallow mean bioturbation depth (<2 cm) at all investigated stations (Carroll et al 2008, Solan et al 2020), the presence of old bulk organic matter at the seafloor can be explained by a combination of generally low sedimentation rates in the Barents Sea (figure 2), leading to long residence times of material at the seafloor (Griffith et al 2010), and the lateral transport of material across the shelf, which could include pre-aged organic matter from terrestrial or marine sources (e.g. Vonk et al 2014).
3.2. Evidence for young and marine OC-FeR in Barents Sea sediments
Dissimilatory iron reduction is an important process in the anaerobic degradation of organic matter in marine sediments. Iron (oxyhydr)oxides are reduced, and Fe2+ is released into the pore waters (Burdige 1993). Following upward diffusion out of the iron reduction zone, this Fe2+ is oxidised mainly by molecular O2, but also by NO3 − and solid Mn (oxyhydr)oxides (Burdige 1993). This oxidation usually occurs in the upper centimetres of a shelf sediment profile below the oxygenated, strongly bioturbated surface sediment layer, and leads to an authigenic enrichment of sedimentary FeR (Froelich et al 1978) below the sediment-water interface. Organic carbon has a strong affinity to such freshly precipitating Fe(III) phases (e.g. ferrihydrite), and it has therefore been proposed that coprecipitation of organic carbon with or adsorption to, this authigenic FeR within the sediment is the main OC-FeR coupling process that promotes the stabilization of sedimentary organic matter (Lalonde et al 2012, Riedel et al 2013, Chen et al 2014, Barber et al 2017, Chen and Sparks 2018). However, several recent investigations indicate that the fraction of the total organic carbon content bound to FeR (fOC-FeR) is not generally controlled by FeR availability (Sirois et al 2018, Faust et al 2020, 2021). Downcore fOC-FeR profiles in combination with pore water composition and sedimentary FeR contents reveal that iron redox cycling and associated authigenic FeR formation within the sediment are less important for the coupling of FeR to organic carbon than assumed (Faust et al 2021). Indeed, substantial amounts (>10%) of the total organic carbon content is bound to FeR at the sediment-water interface above the zone of authigenic FeR precipitation. This raises the question of how much of the OC-FeR is allochthonous, i.e. formed in the overlying water column, in sea ice, or on land; and how much is autochthonous, i.e. formed by biogeochemical processes within the sediments.
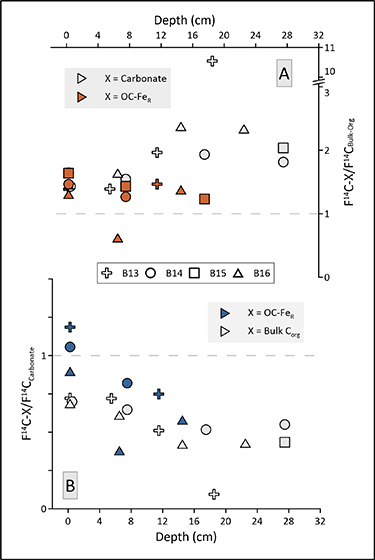
To further validate and characterise a potential allochthonous OC-FeR source in Arctic marine sediments, we compare our bulk Δ14Corg and δ13Corg signatures with those of Δ14C-FeR and δ13C-FeR as well as Δ14C content of benthic foraminifera. To identify the type and amount of organic carbon bound to FeR we conducted an iron oxide extraction (reaction experiment) based on the method originally developed by Mehra and Jackson (1958), modified for marine sediments by Lalonde et al (2012). To account for organic carbon released during the reaction experiment that was not related to iron phases, we conducted a sodium chloride extraction (control experiment) without the complexing and reducing agents trisodium citrate and sodium dithionate. The comparison of the δ13Corg and Δ14Corg values between the reaction experiment and control experiment reveal slightly more depleted values in the control experiment at station B13 (11.5 cm), B14 (22.5 cm) and B16 (2.5 cm, 6.5 cm; figure 2). Such differences have been reported before (e.g. Salvadó et al 2015) and they indicate that in some cases the sodium chloride treatment, washed out labile unbound organic matter. This could be an effect of different organic matter sources, degradation state or liberation of very labile OC-FeR during sodium chloride treatment (Fisher et al 2020). Nevertheless, following extraction treatment of our sediment samples, the isotopic signatures of Δ14Corg and δ13Corg in the solid residues of the control versus reaction experiments show clear differences from the bulk sediment samples at all stations (figure 2). The reaction and control experiment residues have mostly lower (more negative) Δ14Corg and δ13Corg values. It follows that the treatment liberated organic carbon that was relatively enriched in 13C and 14C from the sediments and selectively liberated organic matter with a more marine and younger isotopic signature. When we calculate the δ13C and Δ14C signatures of the organic carbon bound to reactive iron phases (δ13C-FeR; Δ14C-FeR, equations (1) and (2)), we find they are considerably higher than the respective values in most of the studied bulk samples (figure 2). To further investigate these isotopic disequilibria between organic carbon fractions of the bulk, control and reaction residues, we calculate the respective 'fraction modern' (F14C) offsets (see Soulet et al 2016 for details) of the contemporaneous carbon reservoirs of F14C-FeR, bulk organic carbon and carbonate (benthic foraminifera; figure 3).
Figure 3. Comparison of fraction modern values (F14C) between carbon phases measured at the same sediment depth for each station. (A) F14C of benthic foraminifera (carbonate) and F14C of OC associated to reactive iron (equation (1)), OC-FeR, relative to the F14C values of bulk organic carbon. Values >1 are younger (14C-enriched) than the bulk organic matter). (B) F14C values of OC-FeR and bulk organic carbon relative to the F14C of carbonate. Values <1 show older (14C-depleted) signatures compared to the benthic foraminifera phase.
Download figure:
Standard image High-resolution imageBenthic calcareous fossils (e.g. foraminifera) are commonly used to derive age-depth relationships for marine sediments over the last ∼50 ka. In comparison to bulk organic carbon, which represents a mixture of organic carbon from various sources, of various ages and different degradation states, the calcareous material of benthic organisms reflects bottom-water radiocarbon activity (i.e. a single carbon source) that is not significantly influenced by processes within the sediment. Thus, radiocarbon ages based on Δ14C content of benthic calcareous fossils are assumed to reveal the most accurate ages of sediment deposition (e.g. Skinner and Bard 2022). The 14C offsets of F14C-FeR and benthic foraminifera (carbonate) in relation to bulk organic carbon show that over time (downcore), the offsets between carbonate and bulk organic carbon show an increasing trend (figure 3). As bioturbation activity is low at all investigated stations (Solan et al 2020, Faust et al 2021), we assume that this is a function of decomposition and preferential degradation of fresh and young marine organic matter in the bulk sediment. However, we cannot completely exclude the possibility that the observed offset change is caused by a variation in local 14C reservoir effect i.e. bottom water dissolved inorganic carbon age changes e.g. due to oceanographic variability. The F14C-FeR offset is (apart from sample B16; 6.5 cm) always >1 and is relatively stable, ranging between 1.23 and 1.64. Thus, the organic carbon bound to iron is considerably younger than the bulk organic carbon. Moreover, the F14C-FeR offset relative to biogenic carbonate shows that F14C-FeR at the top of the sediment cores is of similar age, or even younger, than carbonate. The downcore decrease in the FeR-carbonate offset could be related to the loss of some fresh and labile organic carbon bound to iron. Alternatively, the type/source of the organic carbon bound to FeR could have changed in the past. But irrespective of the reason, our F14C-FeR data show that the organic carbon bound to iron is young in all samples, indicating rapid and lasting sequestration of CO2 and a direct link to contemporaneous terrestrial and/or marine primary productivity.
3.3. Wider evidence for contemporary iron associated organic carbon
Previous work on OC-FeR coupling in marine sediments further afield, has focused primarily on bulk carbon content, and/or δ13Corg (δ13Corg-FeR) in surface sediments (Lalonde et al 2012, Barber et al 2014, Ma et al 2018, Zhao et al 2018, Wang et al 2019). In accordance with our bulk δ13Corg and δ13Corg-FeR findings, a global data set of sediment from various depositional environments, including freshwaters, estuaries, river deltas, shelf sediments and the deep sea, shows that, in most cases, δ13Corg-FeR is enriched in 13C relative to the bulk organic matter (Lalonde et al 2012). The authors attributed the isotopic shift towards higher values to selective association of certain types of organic matter rich in proteins and carbohydrates, as these are more likely to establish protective inner-sphere complexes with reactive iron phases. Follow-up studies on surface sediments from the shelf areas of China indicated more complex δ13Corg-FeR signatures with values ranging from −49‰ to −4‰ (mean −23‰) (Zhao et al 2018, Wang et al 2019). Their finding of larger regions with substantially enriched and/or depleted δ13Corg-FeR values relative to bulk organic matter, and a trend of more enriched values towards the continental margin, indicated a preference for terrigenous organic carbon binding with iron. Presuming that the organic carbon–FeR bounding occurs mainly in the sediment, the authors suggested that the observed δ13Corg-FeR variability was caused by selective sequestration and release of 13C-depleted organic carbon either during the OC-FeR binding process or by its reduction under anoxic conditions (Wang et al 2019). Our downcore δ13Corg-FeR signatures from the Barents Sea do not support the assumption of preferential binding of terrigenous organic carbon in the oxic part of the core, although the slight downcore decrease (13C depletion) might indeed be related to selective release of 13C-enriched organic carbon. But the driving force(s) for the OC-FeR binding is still not known and it could be physical, chemical and/or biological mechanisms that initiate an isotopic fractionation. This hinders the assignment of the δ13Corg as organic carbon source indicator.
A more robust attempt to identify the type and origin of the organic carbon bound to FeR in marine sediment is a dual-carbon isotope approach similar to ours. To the best of our knowledge, only one study used both δ13Corg and Δ14Corg to evaluate the origin of the OC-FeR in marine sediments (Salvadó et al 2015). They showed that along the Eurasian Arctic shelf, δ13Corg-FeR and Δ14Corg-FeR signatures point towards an older and more terrestrial source in the Laptev Sea, probably related to coastal erosion and thawing permafrost. However, in areas where marine phytoplankton is an important sedimentary organic carbon source, e.g. in the East Siberian Sea and towards the outer shelf areas, their data imply a younger and marine plankton-dominated source of the organic carbon bound to FeR. Thus, the δ13Corg-FeR and Δ14Corg-FeR spatial variability in Chinese and Eurasian shelf sediments implies that the origin of the organic carbon varies distinctly with proximity to land and is related to the dominant organic matter source (marine versus terrigenous), of the bulk sedimentary composition.
Remarkable though is that, disregarding the spatial variability of the δ13Corg-FeR and Δ14Corg-FeR signatures in Eurasian Arctic shelf surface sediments, a re-examination of these data reveals that the δ13Corg and Δ14Corg offsets between bulk and FeR-bound organic carbon show F14C offsets >1 and enriched δ13Corg values compared to the bulk sediment composition the East- and West-East Siberian Sea (figure 4). In a similar way, the four sediment cores from the Barents Sea have organic carbon bound to FeR that is enriched in 13C and 14C compared to the bulk organic carbon content, irrespective of sediment depth/age. These findings indicate a rapid and preferential binding of fresh and marine organic matter with FeR. Thus, FeR not only protects organic matter from degradation in marine sediments over millennial time scales (Faust et al 2021), FeR also sequestered contemporary carbon across the Arctic Shelf which further highlights the potential efficiency of this 'rusty carbon sink'.
Figure 4. Difference between δ13C-FeR and δ13Corg bulk (Δδ13Corg ‰) versus fraction modern ratio between OC-FeR and bulk organic carbon (F14COC-FeR/F14Cbulk-Org). The comparison of the results from this study (yellow marks) with findings from the Laptev Sea, West- and East-Siberian Sea (grey filled/open squares and triangles; (Salvadó et al 2015)) shows that, apart from the Barents Sea samples B13: 11.5 cm and B16: 6.5 cm and samples taken close to the Lena River delta (Laptev Sea), the majority of these Arctic shelf regions iron associated organic carbon is radiocarbon enriched and has a higher δ13Corg value compared to the bulk sedimentary organic matter.
Download figure:
Standard image High-resolution image4. Implications and concluding remarks
To better understand the sources and overall fate of organic carbon in the marine realm, both the composition and mode of binding of organic carbon that accumulates in sediments must be determined. The previous findings of the occurrence of large fractions of total organic carbon bound to FeR (>10%) at the sediment-water interface (above the iron redox zone) in the Barents Sea (Faust et al 2021), as well as possibly in the surface sediments from the Eurasian Arctic and Chinese shelf seas, suggests the important role of allochthonous OC-FeR source. Based on the new data of this study and in concert with the findings from the Eurasian Arctic and the Chinese shelves, we propose that areas dominated by a marine carbon pool see a coupling of FeR to relatively fresh and young organic carbon Furthermore, these investigations combined indicate that the origin of OC-FeR varies distinctly with proximity to land, indicating that FeR tends to associate with the pervasive type of organic carbon available. Nevertheless, enriched 13C-FeR and 14C-FeR signatures compared to the bulk organic carbon content indicate a rapid and preferential binding of fresh and marine organic matter with FeR even in Arctic shelf areas containing larger fractions of terrigenous organic carbon. Hence, labile organic matter prone to decomposition seems to be protected and stabilised, underlining the potential of OC-FeR as an efficient carbon burial mechanism. Additionally, young and marine Δ14Corg-FeR and δ13Corg-FeR signatures imply a binding process in the water column, for example, during the formation of particulate iron-oxyhydroxides formed by oxidation of dissolved Fe(II) in the euphotic zone (Gelting et al 2010). To better understand the formation and source of OC-FeR in the marine environment further investigations of the organic carbon type and source as well as a possible allochthonous OC-FeR binding process, prior organic carbon and FeR sedimentation, needs to be investigated. This is crucial for a better estimation of the efficiency of the 'rusty carbon sink', its contribution to the global cycles of carbon and oxygen and its carbon burial function in warming Arctic Ocean.
Acknowledgments
We thank the crew of the RRS James Clark Ross for their professional support during our expedition. Further, we would like to express our gratitude to Sonia Papadaki and Timothy Knowles for their help with the laboratory work at BRAMS. This work resulted from the ChAOS project (NE/P006493/1), part of the Changing Arctic Ocean programme, jointly funded by the UKRI Natural Environment Research Council (NERC) and the German Federal Ministry of Education and Research (BMBF). Furthermore, this work was supported by the NERC Environmental Isotope Facility, NEIF, under Grant NE/S011587/1 (allocation number 2095.0218) and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through Germany's Excellence Strategy to the Cluster of Excellence 'The Ocean Floor—Earth's Uncharted Interface' (EXC-2077 (Grant No. 390741603).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare no competing interests.
Supplementary data (<0.1 MB XLSX)
Supplementary data (0.1 MB DOCX)