Abstract
Internationally, the environmental damage caused by the improper disposal of approximately 100 Mt of plastic waste per annum is of growing concern. Attempts to address this issue have generated many hundreds of scientific studies announcing the discovery of novel plastic-degrading microorganisms and their respective enzymes. On closer inspection, however, evidence remains sparse for the microbial degradation of most of the plastic polymers produced globally. We systematically surveyed the international literature to confirm how many microorganisms proposed to degrade plastics (n = 664) cause substantial (i.e. ⩾20% mass) losses of virgin polymer, rather than losses of plastic additives, filler, and/or shedding of polymer micro-fragments. We noted where degradation was only demonstrated for artificially aged polymer since physicochemical ageing procedures increase the abundance of monomers and oligomers such that they may be degraded by microbial activity. Additionally, artificial ageing may introduce functional groups to the polymer backbone, creating more locations susceptible to microbial degradation than would otherwise occur in the environment. We identified multiple studies demonstrating the effective microbial degradation of heterochain plastic polymers such as polylactic acid, polycaprolactone and polyethylene terephthalate (i.e. polymers containing elements other than carbon in the backbone structure). However, in the literature, we find no evidence for the substantial degradation of unadulterated polyethylene, polypropylene, polystyrene or polyvinyl chloride, homochain polymers which represent the overwhelming majority of global plastics production. Current research demonstrates that the pre-treatment of plastics with elevated temperature or UV-light may speed physicochemical plastic degradation, with valuable applications for downstream microbial processing. However, evidence for the microbial degradation of most plastic polymers in current circulation is lacking. We outline simple criteria that should be met before announcing the microbial degradation of plastic polymers. We hope this may help to address largely unsubstantiated expectations that microorganisms can degrade many plastic polymers in situ.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Background
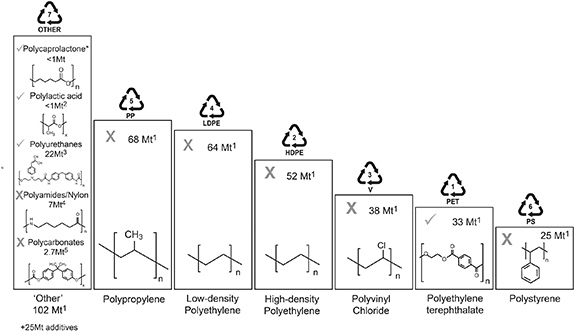
Global plastics production has increased remarkably since their large-scale manufacture began in the 1940s, reaching over 360 million tonnes (Mt) in 2021 (Plastics Europe 2021, figure 1). Today, as much as 99 Mt of plastic waste is estimated to be improperly disposed of per annum (Lebreton and Andrady 2019), causing significant environmental damage. For this reason, considerable efforts have been placed towards isolating novel microorganisms and enzymes with traits for plastic degradation. However, evidence for the microbial degradation of a majority of even the globally most abundant plastic types remains very limited. Biodegradable plastics are defined as those which can be degraded by biological, and principally microbial processes. Reliable reports of microbial plastic degradation are restricted mainly to polycaprolactone (PCL), polylactic acid (PLA) and polyurethane (PU), with a smaller number of studies on polyethylene terephthalate (PET; figure 1). These plastics, which contain C=O and C–O–C bonds within and along their polymer backbone, comprise only a minority of global plastics production (estimated as <1%, <1%, 5% and 7%, respectively (Austin and Hicks 2017, Geyer et al 2017, Gama et al 2018, Narancic et al 2020); figure 1). Microbial degraders of the most common plastics in production (figure 1), which in their pure form contain only C atoms in the polymer backbone, such as polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC) and polystyrene (PS), are also widely reported (Gambarini et al 2021). However, in many cases, strong evidence confirming biological degradation of these polymers remains lacking.
Figure 1. Global primary plastics production (in million metric tons). Group 7 plastics contain plastics that do not fall into groups 1–6. A tick indicates clear evidence for microbial degradation of the polymer, according to our analyses of the contemporary literature (see supplementary material). The category 'other' contains several plastic polymers, including the biopolymers polyhydroxyalkanoate (PHA; Tokiwa and Calabia (2004)) and polybutylene succinate (PBS; Jung et al (2018)) and fossil fuel-based polymers such as poly(butylene adipate-co-terephthalate) (PBAT; Muroi et al (2017)). They are each biodegradable but only produced in relatively minor quantities globally and so are not highlighted individually in this plot (i.e. ⩽0.1 Mt; Aeschelmann and Carus (2015)). Note that the chemical structure of several polymers (e.g. polyurethane, polyamide, polycarbonate) are shown using a representative example and are not the only possible structure to represent these diverse polymer groups. 1Geyer et al (2017), 2Narancic et al (2020), 3Austin and Hicks (2017), 4Weslowski and Plachta (2016), 5Jones et al (2016). *Authors' estimate of the global production of polycaprolactone.
Download figure:
Standard image High-resolution imageBefore critiquing the extent to which various plastics may be degraded, it makes sense to provide a clear definition of the term 'plastics' as used in this study. Plastics are polymers that may be shaped when soft and then hardened to retain their rigid or elastic form. They may broadly be categorized as either homo- or heterochain polymers, with the former having a backbone solely of carbon atoms and the latter containing additional heteroatoms within their backbone chains. Whereas the backbone of pure homochain polymers lack functional groups that are susceptible to degradation, for example by microbial enzymes, the presence of oxygen, nitrogen and other heteroatoms increases the susceptibility of heterochain polymers to degradation by both biotic and abiotic hydrolysis reactions (Gewert et al 2015). A diversity of chemical additives (∼25 Mt per annum; figure 1) are incorporated into many plastic polymers, for reasons including to stabilize the polymer and make it more resistant to degradation, as flame retardants, antioxidants, colourants, mineral fillers, as reinforcing agents or to make the materials softer and more flexible. These additives can comprise more than 30% of the mass of some plastics (Bridson et al 2021) and since most are not covalently bound to the polymer, these additives may leach readily, causing weight losses of the plastic material. Additionally, additives such as salts of manganese and iron may be added to 'oxo-degradable' plastics, acting as a catalyst for fragmentation yet polymer may not biodegrade, except over a very long time (Xochitl et al 2021). In this review, our interests lie in confirming the biological degradation of high molecular weight plastic polymers rather than losses of additives or fillers or from the shedding of micro-debris from compromised plastic surfaces. Evidence of polymer degradation is important since, in many cases, negative environmental consequences of environmental plastics are caused by physical blockage of digestive systems and reduced feeding rates, following accidental polymer ingestion (Lear et al 2021). Detailed knowledge of plastic composition and surface morphology is therefore essential to confirm microbial degradation of the polymer, as opposed to mass losses of plastic additives due to leaching or microbial degradation or losses of microplastic debris via surficial weathering. This is particularly important when the percentage mass loss of the plastic is small (e.g. <3% as reported by Aravinthan et al (2016), Tian et al (2017), Kim et al (2021)). With this in mind, we question, have microbial abilities to degrade the most widely produced plastic polymers globally been overstated? Despite numerous industry standards being in place, for example to determine which plastics may be defined as 'compostable' (e.g. ISO 14855 [International Organization for Standardization], ASTM D5338 [American Society for Testing and Materials]), these are rarely applied in academic studies exploring microbial plastic degradation. Here, we outline fundamental criteria which should be met as a first step, before microbial degradation of plastic polymers is reported.
2. Methods
We used the database of Gambarini et al (2021; http://plasticdb.org/) to collate research published on the theme of microbial plastic degradation. The database is updated monthly and we assessed all publications released up to 1st December 2021. Briefly, Gambarini et al (2021) used two approaches to mine the available literature for evidence of microbial plastic degradation: (a) acquiring all publications released through the Web of Science platform with the search terms [plastic* AND *degradation AND (bacter* OR fung* OR archaea*)] and (b) capturing all other information that we knew to exist, for example, reports that were already summarized by plastic degradation reviews and all microbes reported to biodegrade plastic that were present in the PMBD database (Gan and Zhang 2019). Some taxa, plastics, and enzymes will inevitably have been missed by these search terms. However, similar searches using terms including *eukaryot* and diatom*, yield no results.
To assess if microbial taxa met fundamental requirements demonstrating substantial degradation of plastic polymers, we asked five different questions of the putative plastic degrading microorganisms identified in each study (table 1). A list of all references and our interpretation of how these match the criteria outlined are provided in supplementary data S1 (available online at stacks.iop.org/ERL/17/043002/mmedia).
Table 1. Criteria required to meet author-defined standards for the confirmation of plastic polymer degradation of a microbial taxon.
| Question | Criteria | |
|---|---|---|
| (a) | Does the study identify a microorganism (or associated enzyme) with putative plastic degradation potential? | Meets the criteria for inclusion into plasticdb.org, as described by Gambarini et al (2021). |
| (b) | Is the composition of the plastic confirmed, including the concentration of any additives or fillers? | Plastic is described as containing no additives, is sourced as analytical grade polymer, or the concentration of any additives is provided. |
| (c) | Can substantial degradation of the polymer be confirmed? | Evidence is presented of either: (a) a defined 'clear zone' around the organism when grown on plastic-infused media or (b) mass losses of ⩾20% of the plastic are reported, in addition to any losses that may be caused by losses of additives known to be present. |
| (d) | Is the identity of the putative plastic-degrading microorganism confirmed? | The organism is from a national culture collection (e.g. ATCC, JCM, DSMZ a ) or is identified by DNA sequencing of an appropriate marker gene (e.g. 16S rRNA gene) as a minimum b . |
| (e) | Was the plastic 'artificially-aged'? | Studies were excluded if exposed to prolonged elevated temperatures or UV light. We excluded studies where samples were exposed to temperatures of 60 °C or greater for hours (Pramila et al 2012) to months (Manzur et al 2004) since temperatures over 60 °C are regularly used in the literature to 'age' plastics. For similar reasons, we excluded studies in which samples were exposed to UV light for a period of more than 60 mins, noting that in many cases, exposure was for many weeks (Zahra et al 2010, Auta et al 2017). |
a ATCC = American Type Culture Collection; JCM = Japan Collection of Microorganisms; DSMZ = Deutsche Sammlung von Mikroorganismen und Zellkulturen. b Note that where a microbial enzyme was the main subject of study, either the enzyme should be fully described, or the microbe from which the enzyme is isolated or the associated gene must be described as already outlined.
2.1. Synthesis
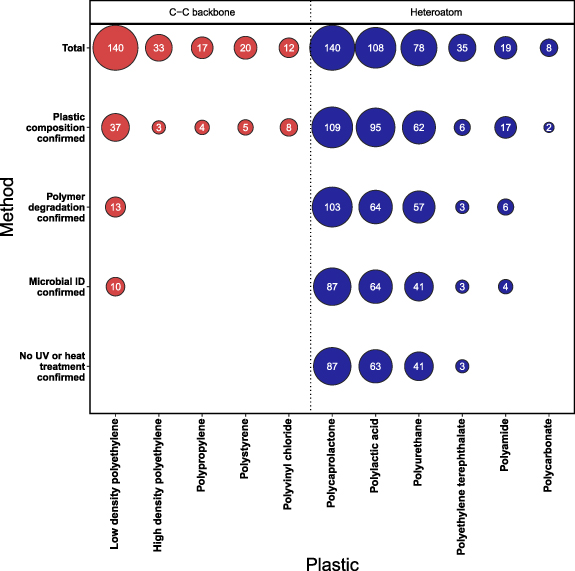
With additives and fillers contributing a significant mass component of many plastics, before assessing polymer degradation, studies should first quantify or confirm the absence of any additional substance in the base polymer, as outlined in table 1. Since many polymer manufacturers and vendors are unwilling to confirm the full compositional content of commercial plastics and in only a minority of cases is the purity of purchased plastic described (Jeon and Kim 2016, Montazer et al 2018, Kumari et al 2019), some researchers choose to synthesize their own plastics from polymer starting materials (Nakajimakambe et al 1995, Akutsu et al 1998, Nomura et al 1998). In other cases, gas or liquid chromatography/mass spectrometry (GC/MS or MS) and Fourier-transform infrared spectroscopy methods are employed to verify the composition of the plastics (Oceguera-Cervantes et al 2007, Savoldelli et al 2017, Skariyachan et al 2018). In recent years, pyrolysis GC/MS has become a favoured method to analyse the presence of organic additives from their thermal degradation products (Akoueson et al 2021) and can simultaneously be used for polymer identification (Matsui et al 2020). Despite the importance of defining the composition of the original plastic material, our analysis of the literature revealed just 20% of studies on plastics with a C–C backbone clearly defined if their plastic was free from additives and fillers (figure 2); multiple studies instead use undefined consumer products such as single-use plastic bags and bottles (Harshvardhan and Jha 2013, Gajendiran et al 2016) and with no apparent attempt to identify and quantify the additives present. Without defining the exact composition of the plastic, it is impossible to quantify the extent to which weight loss is caused merely by the leaching or degradation of chemical additives (Danso et al 2019).
Figure 2. Summary of studies meeting author-defined standards for confirming plastic polymer degradation by a microbial taxon. A total of 246 studies were assessed to verify: (i) the plastic was free from, or contained negligible (<1%) or quantified concentrations of additives and fillers, (ii) the degradation of at least 20% of the polymer mass, or demonstration of 'clear zone' formation on plastic infused agar, (iii) the microorganisms were identified using appropriate molecular methods (e.g. analysis of the bacterial 16S rRNA gene) or use of characterised library strains, (iv) that no heat or UV-light treatment was applied to artificially age the plastic. Each study was assessed sequentially against parameters (i)–(iv), such that if (i) plastic composition is not confirmed, the study scored zero for all subsequent parameters (ii)–(iv). Since many studies explored the degradation attributes of multiple microorganisms, a total of 664 individual records were assessed. Details of the criteria for our analysis are provided in the supplementary material. An additional 54 studies (data not shown) were defined simply as polyethylene (PE). These broadly follow the data trend shown for LDPE. One exception was the study of Paço et al (2017) which met all of the criteria; however, this study explored the degradation of biomass-associated microplastics <1000 μm, for which mass losses are hard to accurately ascertain using filtration methods alone.
Download figure:
Standard image High-resolution imageIn addition to understanding the potential contribution of additives and fillers to plastic weight losses occurring over time, thorough polymer characterisation is indispensable. Multiple studies indicate faster degradation rates of low molecular weight polymers (Tian et al 2017, Antipova et al 2018). Indeed, the most rapid rates of plastic degradation are reported immediately following plastic immersion into an aqueous environment (Erni-Cassola et al 2020), where they are quickly colonised by putative plastic degrading taxa. These rapid mass losses are presumed to occur due to initial losses of contaminating monomers, oligomers and oxidised short polymer fragments, along with plastic additives that may support the colonising community's growth. Although levels of contaminating styrene, for example, resulting from unreacted residual monomers or degradation of polystyrene, are typically low (Balema et al 2021), styrene monomers, dimers and trimers readily leach from PS into the environment (Kwon et al 2015) where they can be degraded; microbial styrene metabolism is both well described and understood (Lee et al 2006). The leaching and degradation of contaminating plastic monomers and short polymers may help to explain why, despite the perceived mass losses of PS plastics, no enzyme has yet been shown capable of efficiently degrading the high-molecular-weight polymer (Danso et al (2019)). This also explains why mass losses of PS are typically reported as being no more than 20% of the starting weight and often far less (Atiq et al 2010, Sekhar et al 2016, Auta et al 2017, Tian et al 2017, Chauhan et al 2018), noting that Kim et al (2021) recently provided evidence for alkane-1-monooxygenase involvement in PS biodegradation, but reported mass losses of only 1.5%. Where plastic mass losses are low, confirmation of any degradation of high-molecular-weight polymers by microbial activity necessitates that polymer molecular weight distributions are reported, using a method such as gel permeation chromatography (Yang et al 2015, Antipova et al 2018, Novotny et al 2018). In the present study, where recorded, the average additive concentration in plastics was ∼4%, but with some plastics being comprised of more than 10% additive. For this reason, we decided on demonstration of 20% mass losses of polymers as being a suitable criterion for evidence of polymer degradation in most instances, while also acknowledging that demonstration of such losses can be challenging; lower extents of polymer degradation are more frequently demonstrated to occur. Where observed, biotechnological advances may subsequently be used to improve the degradation potential of high weight molecular polymers, as appears to be the case for PET to some degree, following manipulation of the PETase enzyme's structure (Austin et al 2018).
The crystallinity of a plastic polymer, which describes the alignment of the polymer chains as being highly ordered (more crystalline) or less structured (amorphous), is also important when considering the degradation of commercial plastics. Commercial polymers are typically semi-crystalline, consisting of amorphous and crystalline domains, and crystallinity varies across the geometry of stretched polymers, such as plastic bottles (Demirel and Daver 2009). Microbial enzymes are generally capable only of degrading flexible amorphous domains and hence the biodegradation rate of plastics typically declines with increasing crystallinity (Marten et al 2005, Taniguchi et al 2019). Notably then, most studies confirming successful PET degradation have used low-crystallinity films (e.g. crystallinity 1.9% (Taniguchi et al 2019), 3%–5% (Furukawa et al 2019), 15%–17% (Austin et al 2018, Sagong et al 2021)). Since the crystallinity of PET in commercial-grade bottles maybe 30% or greater (Edge et al 1991, Bach et al 2009), it is unsurprising that the extent of reported PET degradation is typically low and it remains unclear the extent to which putative plastic-degrading taxa and enzymes could degrade the majority of post-consumer PET waste. Despite Yoshida et al (2016) reporting 75% mass loss reductions of low crystallinity (1.9%) PET film by I. sakaiensis, Wallace et al (2020) suggest that 52% to 82% of the plastic in PET water bottles is not amenable to microbial degradation without further treatment, due to the crystalline content of the polymer. PETase enzymes are one of the best-studied enzyme groups in reference to plastic polymer hydrolysis and biotechnological advances provide additional scope for enhanced polymer degradation or the production of high-value compounds from waste PET (Danso et al 2019). For example, Austin et al (2018) provide evidence for the degradation of more crystalline PET (∼15%) after modifying the enzyme active site. Nevertheless, they concede that substantial improvements in the performance of the enzyme are required for this PETase to reliably be used to degrade highly crystalline, commercial PET that is poorly managed and accumulates in the environment.
To ensure research is sufficiently transparent to allow others to repeat or expand upon previous experimental work, unless well-characterised strains are used, for example, from national culture collections, it is important that the taxonomic identity of strains be confirmed by sequencing common DNA barcode markers (e.g. of 16S ribosomal RNA (rRNA) genes and internal transcribed spacer regions, for bacterial and fungi, respectively; table 1). In our survey of the literature, most studies completed such analyses, with morphological and biochemical identification (e.g. using Biolog (Auta et al 2017) or Analytical Profile Index, or API assays (Kay et al 1991)) being the primary method of taxonomic identification in just 20% of studies surveyed. Where feasible, evidence of the microbial enzyme, or corresponding gene, presumed capable of plastic degradation should be provided. This has the added advantage that data on these enzymes may be collated (such as in http://plasticdb.org) for purposes including to reconstruct putative pathways for plastic degradation in microbial strains and communities, as detailed in Gambarini et al (2022). Critically, we could find no studies identifying enzymes that have been used to support the substantial degradation of virgin plastic polymer, or the presumed biochemical mechanisms associated with the degradation of PE, PVC, PP or PS (i.e. polymers with a C-C backbone; but see Yoon et al (2012), Bardají et al (2019), Kim et al (2021)). In contrast, multiple enzymes are linked to the degradation of PET (Yoshida et al 2016), PLA (Nakamura et al 2001), PCL (Oda et al 1997) and ester-linked PU (Gautam et al 2007, Russell et al 2011), i.e. polymers with heteroatoms in the carbon backbone that meet our criteria for evidence of microbial polymer degradation (figure 1).
High molecular weight polymers must be broken into smaller molecules before they may pass through cellular membranes and be degraded within microbial cells. To enhance biological degradation rates, the weathering of plastic polymers is frequently stimulated by exposure of the polymer to elevated temperatures or UV light to reduce the average molar mass of polymers by macromolecular chain bond scission and promote the generation of oxygen-rich functional groups, which are presumed more amenable to enzymatic degradation. Physicochemical degradation may also cause polymer chains to cross-link, a process which on its own would increase the molar mass of polymers and be expected to slow rates of microbial degradation; however, chain-scission reactions typically dominate (Shyichuk et al 2001, Gewert et al 2015). UV light treatment is commonly applied to pre-treat PE (Zahra et al 2010, Montazer et al 2018) and PP plastics prior to degradation experiments (Jeyakumar et al 2013, Aravinthan et al 2016). The saturated bonds in the C–C backbone of such homochain polymers are broken down through photo-initiated chain-scission and crosslinking reactions, a process that can be accelerated by the presence of chemical impurities and structural abnormalities (Lee and Li 2021). Chemical bonds in the main polymer chain are broken by the energy of light, followed by β-scission propagation and free radical reactions, resulting in polymer fragmentation into a diversity of smaller chain lengths and therefore a reduced molecular weight (Yousif and Haddad 2013). The melting points of plastics such as low-density polyethylene (LDPE) and high-density polyethylene (HDPE) may decrease due to ongoing chain scission. Concomitant declines in tensile strength can occur which, as observed by Ainali et al (2021), caused HDPE and PP to become so brittle after 45 and 20 days, respectively, that mechanical strength testing became unfeasible.
In addition, or instead of UV pre-treatment, many studies, particularly those focused on LDPE, pre-treat plastic using elevated temperatures (e.g. 60 °C–80 °C; Volke-Sepulveda et al (2002), Manzur et al (2004)), but below the polymer's melting point (>105 °C for LDPE). Even for plastics marketed as biodegradable, such as PLA, elevated temperatures (i.e. over 55 °C) are necessary for degradation to proceed at rates which make them amenable for degradation in commercial composting facilities. The impacts of heat treatment are multiple. Firstly, the heating of plastics enhances the migration of low-molecular-weight plastic additives (Izdebska 2016), where present, including additives of LDPE (e.g. Irganox 1076, Beldì et al (2012); diphenylbutadiene, Sanches Silva et al (2007)) and PC (e.g. bisphenol A, Kubwabo et al (2009)). These additive losses have the immediate effect of reducing the total plastic mass without being a consequence of any polymer degradation. The accelerated loss of antioxidants from polymers may also speed subsequent hydrolysis reactions, secondary photochemical reactions, or trace contaminant oxidation, thereby increasing future physicochemical rates of polymer degradation. While losses of such additives may be beneficial in speeding polymer degradation, these accelerated 'ageing' methods do not reflect the conditions and/or rates of chemical transformation to which plastics within the environment are typically exposed. Secondly, elevated temperatures enhance rates of polymer oxidation (requiring O2) and hydrolysis (requiring H2O) (Gijsman 2008), shortening the average chain length of individual polymers. This decreases the polymer's glass transition temperature due to the increased mobility and structural stability of the shorter chains (Chamas et al 2020). Conversely, oxidative chain scission of entanglements in the amorphous part of semicrystalline polymers may rearrange them into a crystalline phase (Pospíśil et al 2003) such that the crystallinity of polymers, for example PE, can increase following thermal oxidation (Pospíśil et al 2003). Using our selection criteria (table 1 and figure 2), significant plastic degradation could not be confirmed in any study in which plastics with a C–C backbone were not first exposed to elevated temperatures or UV radiation. It therefore remains unclear the extent to which presumed microbial homochain polymer degradation can be attributed to purely biological processes, or to biological processes that may only proceed after a physicochemical change to the polymer. Pre-treatment may be necessary to facilitate the biological degradation of some plastic constituents, for example, to encourage the migration of biodegradable monomers such as styrene from the base polymer structure (Tawfik and Huyghebaert 1998) or the introduction of heteroatoms (principally oxygen) and the formation of hydrophilic groups in polymers such as polyethylene. These hydrophilic groups may increase rates of microbial attachment but also provide additional sites for both chemical and biological reactions to occur, thereby accelerating the degradation rates of plastic polymers and their associated additives (Suresh et al 2011). In the case of most plastics with a C–C backbone, the degradation of the pure, non-aged polymer is yet to be demonstrated. Still, the industrial pre-treatment of plastics by heat or UV radiation might be a useful tool to stimulate degradation by inducing physicochemical degradation processes and increasing the surface area available for subsequent microbial interaction.
In approximately one-third of the studies we reviewed, plastics were pre-treated with solvents, often to recast the polymer from their prior form into a thin film for degradation assays. The impact of using solvents to dissolve and reform polymers remains unclear. Casting methods are considered capable of returning a plastic with broadly the same quality as the virgin materials as judged by properties such as tensile strength (Sherwood 2020). For example, after dissolving LDPE in xylene, Pappa et al (2001) reported no loss of polymer performance. However, increases in crystallinity may be observed (Hadi et al 2014), possibly due to greater losses of low-weight polymers during polymer dissolution, which would be expected to increase the polymer's recalcitrance to degradation. Alternatively, the slower solvent casting process, compared to thermal recrystallisation during industrial polymer processing, may provide more time for polymer chains to realign to form a more crystalline order. Although solvent recasting likely impacts the attributes of some polymers and their associated additives, there is limited evidence that such processing is beneficial for subsequent biological degradation. However, even in the case that solvent recasting has no impact on polymer chemistry, the provision of a thin film provides a greater surface area for microbial attachment and may speed the leaching of any mobile plastic components. For this reason, we suggest reports of the degradation of solvent recast polymers be treated with some caution, but consider the method as appropriate to alter the form of most plastics while seeking to minimise impacts on polymer integrity.
3. Conclusions
We outline a set of fundamental criteria for reporting the microbial degradation of plastic polymers, which must include: (a) clear evidence of the plastic composition, most importantly quantifying its purity and composition of any additives and fillers, and ideally also the contribution of monomers and short-chain polymers, (b) confirmation of substantial polymer degradation (i.e. complete degradation or at least a 20% loss of mass, not including any mass change due to additive losses or shedding of microplastics), (c) confirmation of taxonomic identity and ideally also of the enzyme responsible and/or the encoding gene, and (d) confirmation that the plastic is not artificially aged by UV or heat treatment. According to Gewert et al (2015), when considering the degradation pathways for plastics, it is useful to divide them into two groups: plastics with a carbon-carbon backbone and plastics with heteroatoms in the main chain since the latter are more susceptible to hydrolytic cleavage (e.g. ester or amide bonds). We strongly support this stance since we find no clear evidence for the microbial degradation of unadulterated PE, PP, PS or PVC polymers. Where biological degradation is perceived as a useful endpoint for waste plastics, the use of polymers such as PLA and PET is preferable, particularly where degradation rates and extents may be maximised by also exposing polymers to elevated temperature or UV light. While current systems to degrade plastics by microbial processes remain slow or incomplete, the manipulation of microbial enzymes to enhance plastic degradation rates should be considered a valuable tool for further enhancing the degradation rates of a wide range of plastic polymers (Austin et al 2018, Jem and Tan 2020). The scientific literature contains many hundreds of publications providing support for the microbial degradation of plastics, yet evidence for the degradation of the vast majority of plastic polymers used globally is lacking. Where stronger evidence for plastic degradation is provided, significant degradation is rarely or never demonstrated under ambient environmental conditions or using polymer forms in widespread commercial use. Following a business as usual approach, global outputs of mismanaged plastic waste could triple in the next 40 years (Lebreton and Andrady 2019). Yet, at present, there is no credible microbial approach to deal with the majority of the world's accumulated plastic pollution.
Acknowledgments
This work was conducted as part of the Aotearoa Impacts and Mitigation of Microplastics (AIM2) project, in receipt of funds from a New Zealand Ministry of Business, Innovation and Employment (MBIE) Endeavour Research Programme Grant (CO3X1802).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare no conflict of interest.