Introduction
Published November 2015
•
Copyright © 2015 Morgan & Claypool Publishers
Pages 1-1 to 1-6
You need an eReader or compatible software to experience the benefits of the ePub3 file format.
Download complete PDF book or the ePub book
Abstract
In recent decades, nanotechnology has emerged as a highly innovative field showing great potential in various areas of science and technology. Nanotechnology is influential in pure science (e.g. chemistry, physics, etc), materials science, energy science, biotechnology, biomedicine and pharmaceutics.
In recent decades, nanotechnology has emerged as a highly innovative field showing great potential in various areas of science and technology. Nanotechnology is influential in pure science (e.g. chemistry, physics, etc), materials science, energy science, biotechnology, biomedicine and pharmaceutics. Due to the widespread and increasing burden of perilous diseases, such as drug-resistant infections, malignancies like cancer, Alzheimer's disease, diabetes, hepatitis, cardiovascular disease, systemic inflammatory disorders and so on, more efficacious therapies are urgently required with a focus on the targeted and individualized treatment of the diseased site. Furthermore, the diagnostic and imaging aspects of therapy have become of interest, especially in the diagnosis and treatment of various cancers. In this respect, important breakthroughs have been accomplished in diagnosis and therapy, particularly in the combination form called theranostics. There is an increasing requirement for clinical trials in nanomedicine, which has resulted in many successes, and more nanoparticles (NP) are receiving approval from the US Food and Drug Administration (FDA) [1–6].
Micro/nanosystems have been applied for drug delivery using various materials and approaches, such as nanostructured particles and surfaces and diffusion-controlled delivery systems, and these are enabling novel therapies. Other new applications in biosensing and implantable devices, such as drug-eluting/bioresorbable stents, can be improved by nanotechnology [7–11]. The administration of different nano/microparticle-based drug/gene delivery systems (DGDS) has been proposed as a way to effect targeted delivery of therapeutic agents towards specific disease locations inside the body, with substantial advantages such as reduced toxicity and lessened damage to normal tissues and cells, enhanced solubility, effective treatment of diseases, minimal/controllable side-effects for drugs or the therapeutic method, etc [12–14]. In this respect, significant improvements in therapeutics and pharmaceutics can be achieved. Furthermore, macromolecules are increasingly used as therapeutic agents and their targeted delivery is an important challenge [15]. The delivery of such macromolecules should be both time-controlled and site-specific [16]. For DGDS, smart targeting/delivery approaches are highly desirable and in this area stimuli-responsive systems are important. Therefore, the design of intelligent systems with controllable and accurate feedback to multifarious stimulation has been considered extensively. Newly developed smart nano/microparticles have shown great potential in various fields, particularly for the targeted delivery of drugs/genes [17]. In such smart systems, triggered delivery and the release of therapeutic agents in a targeted and controlled way can be achieved through the application of a wide variety of external or internal stimulations [18]. This is due to the high sensitivity of specific NP to triggering by various stimuli and the resulting far-reaching physicochemical alterations [19, 20].
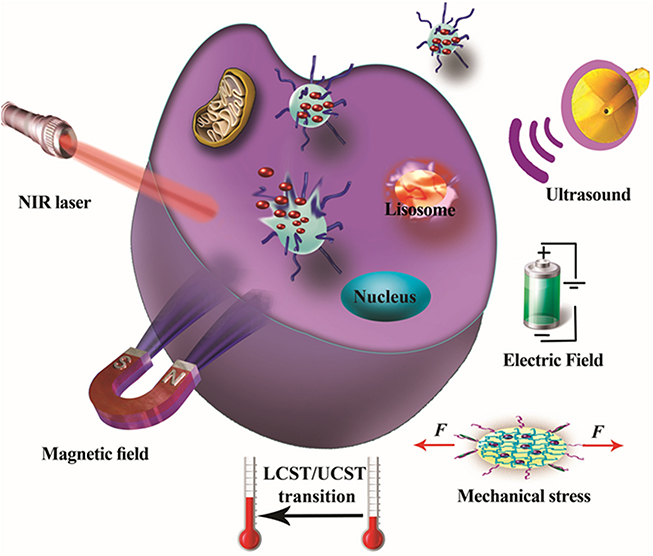
Different external physical stimuli can take the form of changes in magnetic and electric fields, light irradiation, the application of ultrasound and heating sources, and the use of mechanical force. Figure 1.1 shows a schematic depiction of various external stimulations that can be applied in smart DGDS.
Figure 1.1. Schematic of different classes of stimuli, including the external (e.g. electric and magnetic field, light irradiation and ultrasound), that can act as triggers for the design of smart stimuli-responsive targeted DGDS.
Download figure:
Standard image High-resolution imageIn some cases, using smart DGDS can eliminate the risks and drawbacks of other carrier systems, such as viral vectors in clinical gene therapy [21]. Although NP-based nanocarriers generally show only low cytotoxicity towards normal cells and in biological environments [22], the various effects NP can have on biological environments have led to the establishment of a new field, 'nanotoxicology', and these must be considered in the design of new nanocarriers. These toxicity issues have been one of the main concerns in the recent literature [23] and have worried the general public; efforts have been made to define and, if necessary, reduce this toxicity [24, 25]. In addition, the interactions of NP with biological molecules and materials, and the occurrence of phenomena such as the coating with proteins known as a 'corona' and the cell-type specific effect known as 'cell vision', can significantly affect the biological fate of NP, their targeting ability [26–28] and their cytotoxicity [29]. Smart NP have demonstrated notable therapeutic potential, particularly in cancer therapy where they have been designed to be triggered in tumor sites [30]. Smart NP can respond to a variety of tumor-specific stimuli [31] and dramatically improve the cytotoxicity of anticancer drugs in respect of malignant cells while reducing their toxicity towards normal cells [32]; large-scale molecular simulations and systems biology approaches can be used to model these effects [33].
In smart DGDS, various mechanisms can be designed to effect the targeted delivery and release of cargos from nanocarriers, which are strongly dependent on the type of stimulus applied. Detailed understanding of these mechanisms is required for the design and development of smart DGDS in order to study their interactions with biological environments, analyze probable side-effects and obtain the desired delivery and release characteristics, such as drug release rate, controlled delivery and release, sensitivity level of nanocarriers to stimuli, etc.
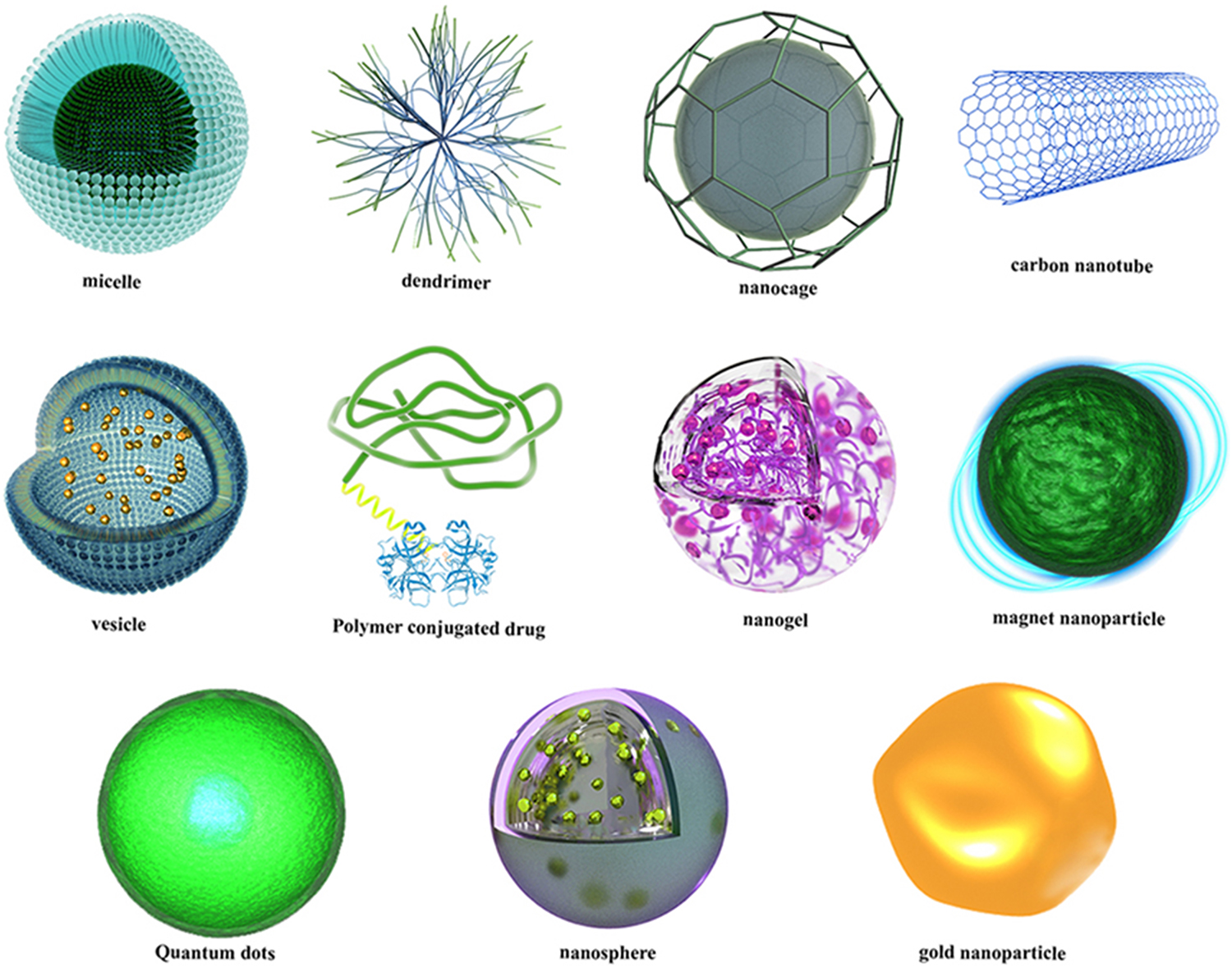
Various NP and nanotechnology methods have been investigated, not only to provide more reliable micro/nanocarriers triggered by one or more stimuli, but also to deliver facile and economical preparation methods for drug-carrier NP with higher loading efficiency and prolonged and sustained release times [34]. In smart DGDS, much effort has been put into the exploration of novel stimuli-responsive nanocarriers [18]. The most studied classes of nanocarriers are: various types of polymer NP (e.g. hydrogels/nanogels, micelles, etc); liposomes; carbon-based nanomaterials (e.g. graphene, carbon nanotubes (CNT), fullerene); ceramic-based NP (magnetic NP, mesoporous silica NP (MSN), etc); metal NP (gold NP, silver NP, etc); and solid lipid NP (SLN). Several different types of micro/nanoparticles (MNP) employed in the design of smart micro/nanocarriers for DGDS are illustrated in figure 1.2.
Figure 1.2. Several classes of NP used for the design of smart micro/nanocarriers, including micelles, dendrimers, nanocages, CNT, polymeric conjugates, nanogels, magnetic NP, quantum dots (QD), nanospheres and gold (Au) NP.
Download figure:
Standard image High-resolution imageIn this book and its companion (Smart Internal Stimulus-Responsive Nanocarriers for Drug and Gene Delivery), different smart DGDS are discussed according to their stimulus type and have been categorized according to their external or internal stimulation route. The principles and mechanisms of each stimulus type are taken into consideration, and recent progress and the latest achievements in biomedicine and pharmaceutics applications are discussed. The focus is on the use of smart nano/microcarriers to carry out targeted delivery of therapeutic agents to particular cells, tissues or disease states.
In this book, we discuss DGDS triggered via external stimuli (including light irradiation, temperature change, ultrasound irradiation, magnetic and electrical fields, and mechanical stress) in detail. Finally, a conclusion and future perspectives section discusses nanotoxicology briefly and addresses innovative future concepts and new challenges in the smart DGDS field.
References
- [1]Gaheen S, Hinkal G W, Morris S A, Lijowski M, Heiskanen M and Klemm J D 2013 caNanoLab: data sharing to expedite the use of nanotechnology in biomedicine Comput. Sci. Disc. 6 014010
- [2]Weintraub K 2013 Biomedicine: the new gold standard Nature 495 S14–S26
- [3]Mirkin C A, Meade T J, Petrosko S H and Stegh A H 2015 Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer (Berlin: Springer)
- [4]Gallo J and Long N J 2014 Nanoparticulate MRI contrast agents The Chemistry of Molecular Imaging (New York: Wiley) pp 199–224
- [5]Matoba T and Egashira K 2014 Nanoparticle-mediated drug delivery system for cardiovascular disease Int. Heart J. 55 281–6
- [6]Tiwari P 2015 Recent trends in therapeutic approaches for diabetes management: a comprehensive update J. Diabetes Res. 501 340838
- [7]LaVan D A, McGuire T and Langer R 2003 Small-scale systems for in vivo drug delivery Nat. Biotechnol. 21 1184–91
- [8]Son D et al 2015 Bioresorbable electronic stent integrated with therapeutic nanoparticles for endovascular diseases ACS Nano 9 5937–46
- [9]Takahashi H, Letourneur D and Grainger D W 2007 Delivery of large biopharmaceuticals from cardiovascular stents: a review Biomacromolecules 8 3281–93
- [10]Ruedas-Rama M J, Walters J D, Orte A and Hall E A 2012 Fluorescent nanoparticles for intracellular sensing: a review Anal. Chim. Acta 751 1–23
- [11]Karimi M et al 2015 Carbon nanotubes part I: preparation of a novel and versatile drug-delivery vehicle. Expert Opin. Drug Deliv. 2015 1–17
- [12]Torchilin V 2011 Tumor delivery of macromolecular drugs based on the EPR effect Adv. Drug Deliv. Rev. 63 131–5
- [13]Karimi M, Avci P, Ahi M, Gazori T, Hamblin M R and Naderi-Manesh H 2013 Evaluation of chitosan-tripolyphosphate nanoparticles as a p-shRNA delivery vector: formulation, optimization and cellular uptake study J. Nanopharmaceut. Drug Delivery 1 266–78
- [14]Jahromi M A M, Karimi M, Azadmanesh K, Manesh H N, Hassan Z M and Moazzeni S M 2013 The effect of chitosan-tripolyphosphate nanoparticles on maturation and function of dendritic cells Comparative Clin. Path. 23 1421–7
- [15]Berg K et al 1999 Photochemical internalization: a novel technology for delivery of macromolecules into cytosol Cancer Res. 59 1180–3
- [16]Son S, Shin E and Kim B-S 2014 Light-responsive micelles of spiropyran initiated hyperbranched polyglycerol for smart drug delivery Biomacromolecules 15 628–34
- [17]Motornov M, Roiter Y, Tokarev I and Minko S 2010 Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems Prog. Polym. Sci. 35 174–211
- [18]Tirelli N 2006 (Bio) responsive nanoparticles Curr. Opin. Colloid Interface Sci. 11 210–6
- [19]Mura S, Nicolas J and Couvreur P 2013 Stimuli-responsive nanocarriers for drug delivery Nat. Mater. 12 991–1003
- [20]Cheng R, Meng F, Deng C, Klok H-A and Zhong Z 2013 Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery Biomaterials 34 3647–57
- [21]Nishiyama N et al 2005 Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer Nat. Mater. 4 934–41
- [22]Chang Y T, Liao P Y, Sheu H S, Tseng Y J, Cheng F Y and Yeh C S 2012 Near‐infrared light-responsive intracellular drug and siRNA release using Au nanoensembles with oligonucleotide-capped silica shell Adv. Mater. 24 3309–14
- [23]Shah V et al 2013 Genotoxicity of different nanocarriers: possible modifications for the delivery of nucleic acids Curr. Drug Disc. Technol. 10 8–15
- [24]Luo M et al 2014 Reducing ZnO nanoparticle cytotoxicity by surface modification Nanoscale 6 5791–8
- [25]Hu X, Wang Y and Peng B 2014 Chitosan-capped mesoporous silica nanoparticles as pH-responsive nanocarriers for controlled drug release Chem. Asian J. 9 319–27
- [26]Mahmoudi M et al 2012 Cell 'vision': complementary factor of protein corona in nanotoxicology Nanoscale 4 5461–8
- [27]Mahmoudi M, Lohse S E, Murphy C J, Fathizadeh A, Montazeri A and Suslick K S 2013 Variation of protein corona composition of gold nanoparticles following plasmonic heating Nano Lett. 14 6–12
- [28]Mirshafiee V, Mahmoudi M, Lou K, Cheng J and Kraft M L 2013 Protein corona significantly reduces active targeting yield Chem. Commun. 49 2557–9
- [29]Mortensen N P, Hurst G B, Wang W, Foster C M, Nallathamby P D and Retterer S T 2013 Dynamic development of the protein corona on silica nanoparticles: composition and role in toxicity Nanoscale 5 6372–80
- [30]Karimi M et al 2015 Carbon nanotubes part II: a remarkable carrier for drug and gene delivery Expert Opin. Drug Deliv. 2015 1–17
- [31]Fang Z, Wan L-Y, Chu L-Y, Zhang Y-Q and Wu J-F 2015 'Smart' nanoparticles as drug delivery systems for applications in tumor therapy Expert Opin. Drug Deliv. at press
- [32]Zhao Z, Huang D, Yin Z, Chi X, Wang X and Gao J 2012 Magnetite nanoparticles as smart carriers to manipulate the cytotoxicity of anticancer drugs: magnetic control and pH-responsive release J. Mater. Chem. 22 15717–25
- [33]Jimenez-Cruz C A, Kang Sg and Zhou R 2014 Large scale molecular simulations of nanotoxicity Wiley Interdiscip. Rev.: Syst. Biol. Med. 6 329–43
- [34]Cheng R et al 2011 Reduction and temperature dual-responsive crosslinked polymersomes for targeted intracellular protein delivery J. Mater. Chem. 21 19013–20