Abstract
Amino acids, sugars, and nucleobases are considered as the so-called molecular bricks of life, the major subunits of proteins and genetic materials. All three chemical families have been previously detected in meteorites. In dense molecular cloud ice analogs, the formation of a large set of amino acids and sugars (+derivatives) has been observed. In this contribution, we demonstrate that similar ices (H2O:13CH3OH:NH3 ices, 2:1:1) can also lead to the formation of nucleobases. Using combined UPLC-Orbitrap mass spectrometric and UPLC-SRM-triple quadrupole mass spectrometric analyses, we have unambiguously detected cytosine in these primitive, realistic astrophysical ice analogs. Additionally, a huge variety of nucleobase isomers was observed. These results indicate that all central subunits of biochemical materials may have already been present at early stages of chemical evolution of the protosolar nebula, before accretion toward planetesimals. Consequently, the formation of amino acids, sugars, and nucleobases does not necessarily require secondary alteration processes inside meteoritic parent bodies. They might have been supplied from dense molecular cloud ices toward post-accretional objects, such as nonaqueously modified comets, and subsequently delivered onto the early Earth's surface, potentially triggering the emergence of prebiotic chemistry leading to the first living systems.
Export citation and abstract BibTeX RIS
1. Introduction
Amino acids, sugars, and nucleobases represent the building blocks of biomolecules, namely proteins and RNA/DNA (Ruiz-Mirazo et al. 2013). Understanding their origins might give insights on the processes by which life's ingredients found their way to our planet (Gilbert 1986). Among possible scenarios on how life emerged, the exogenous delivery of organic materials represents a promising one for having participated in the decisive prebiotic steps that might have triggered life on Earth (Chyba et al. 1990; Chyba & Sagan 1992; Bada 2004; Pearce et al. 2017; Ruf et al. 2018). For instance, meteorites, as an observable of the type of organic matter being present on early Earth, contain a large organic molecular diversity (Schmitt-Kopplin et al. 2010; Ruf et al. 2017, 2019a). These celestial objects include amino acids (Elsila et al. 2016), nucleobases (Martins et al. 2008; Callahan et al. 2011), sugars (Furukawa et al. 2019), and sugar derivatives (Cooper et al. 2001) reflecting the essential building blocks of current biochemical systems on Earth (Figure 1). To practically consider one aspect of the fundamental question of habitability on any telluric planet in general, we have to understand the evolution of these central organic species on broad astronomical timescales (from dense molecular clouds toward newborn planets). Understanding their origin might provide important clues on the capability to transport prebiotically relevant molecules onto the primitive Earth or onto any telluric planet.
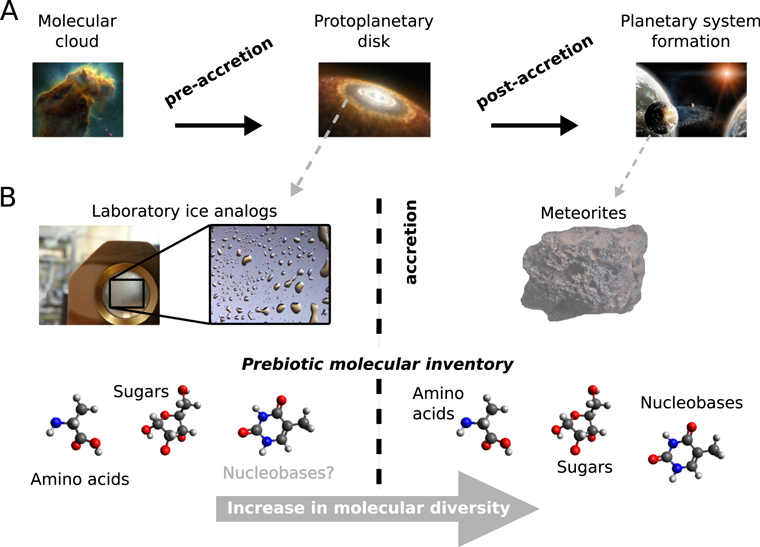
Figure 1. Schematic overview of chemical evolution during planetary system formation. (A) Three major stages from dense molecular clouds via protoplanetary disks toward planetary system formation. (B) Studying the prebiotic molecular contents of pre-accretional astrophysical ice analogs and post-accretional objects (e.g., meteorites) in the laboratory. An increase of chemical diversity is suggested along with accretion by secondary alteration processes (e.g., hydrothermal alteration).
Download figure:
Standard image High-resolution imageTo better understand the chemical evolution of astrophysical ices, laboratory astrochemistry strategies have been developed next to observations (Gerlich & Smith 2005; Herbst & Van Dishoeck 2009; Smith 2011; Van Dishoeck 2014; Gudipati et al. 2015; Schlemmer et al. 2015; Öberg 2016; Fresneau et al. 2017; see our Figure 1). These kinds of experiments start with the formation of ice analogs (e.g., including H2O, CH3OH, NH3, CO, or CO2) as observed in dense molecular clouds (Gibb et al. 2004). Subsequently, or simultaneously, these analogs are submitted to energetic environments, e.g., UV photons (Muñoz Caro et al. 2002; Meinert et al. 2016), X-ray radiation (Muñoz Caro et al. 2019), low energy electrons (Abdulgalil et al. 2013), and/or ions (Gerakines et al. 2000; Baratta et al. 2002; Hudson & Moore 2002; Moore et al. 2004; Loeffler et al. 2005; Islam et al. 2014; Muñoz Caro et al. 2014; Ruf et al. 2019b) and thermal processing (Theulé et al. 2013), to simulate interstellar ice evolution up to the protoplanetary disk phase (Henning & Semenov 2013) or even up to the meteoritic stage (Vinogradoff et al. 2018).
The analytical monitoring of experiments simulating refractory residues resulting from astrophysical ices has revealed a huge organic molecular diversity. Part of these compounds can be released into the gas phase (Abou Mrad et al. 2016, 2017) but most of the organics remain in the refractory part on grains' surfaces (Danger et al. 2016; Fresneau et al. 2017). The presence of a large molecular diversity in refractory residues suggests that dense molecular clouds/circumstellar ices are a crucial source for the organic matter found in planetary systems, particularly in non or weakly processed minor bodies of our solar system. Thus, this rich molecular inventory might be already present before the accretion phase of matter during planetary system formation. Among this molecular diversity found in astrophysical ice analogs, two key families of prebiotic building blocks, amino acids (Muñoz Caro et al. 2002), and sugars (including ribose, Meinert et al. 2016; and recently deoxyribose, Nuevo et al. 2018), have been detected (Figure 1). Peptides have been indirectly supposed to be present, as hydrolysis of residues leads to a significant increase of amino acids (putatively released from peptide precursors; Meinert et al. 2012). Nucleobases have been detected in laboratory-processed materials, for ices doped with their molecular skeletons, e.g., pyrimidine (Nuevo et al. 2009, 2012, 2014; Materese et al. 2013; Nuevo & Sandford 2014) or purine (Materese et al. 2017, 2018), as well as for more primitive ices originating from H2O:CO:CH3OH:NH3 (Oba et al. 2019). In the latter case, ultra high performance liquid chromatography coupled to high resolution mass spectrometry (UPLC-Orbitrap MS) was used to identify nucleobases. However, while this technique allows us to highlight the high molecular diversity present inside such samples (Ruf et al. 2019a), it is not sufficient to unambiguously identify particular molecules.
Thus, based on astrophysically realistic ices (originating from H2O:13CH3OH:NH3, 2:1:1), we have used a more specific analytical technique to perform selective, targeted analysis to search for the unambiguous presence of five canonical nucleobases (adenine, cytosine, guanine, thymine, and uracil). With these experiments, we further aim to test the hypothesis if the formation of nucleobases requires secondary alteration processes (e.g., aqueous alteration) or whether these complex organic molecules can directly result from the processing of dense molecular cloud ices during the evolution of the protosolar nebula, before the accretion of meteorites' parent bodies.
2. Methods
2.1. Formation of Ice Analogs (Residues)
A gas mixture of H2O:13CH3OH:NH3 (2:1:1) was first deposited on an inert MgF2 window at 77 K, forming an astrophysical ice analog. 77 K simulates the position of an icy grain on the edge of a protoplanetary disk where it can receive a sufficient dose of Lyman α photons (Ciesla & Sandford 2012). Irradiation of deposited gas/ice was performed simultaneously during 72 hr at 77 K using microwave plasma with a constant molecular hydrogen flow providing vacuum ultraviolet photons (at Lyman α (121 nm) with a flux of 2E14 photons cm−2 s−1). After 72 hr of deposition and irradiation, the UV dose in the experiments presented here was 5.2E19 photons cm−2. This roughly corresponds to a grain submitted to 1.7E6 photons cm−2 s−1 (Danger et al. 2013) during a life time of the Solar nebula of 1E6 yr (Ciesla & Sandford 2012). The photoprocessed ice was then slowly warmed up until 300 K with 0.1 K per minute. Roughly 100 μg of residue was obtained and kept under argon atmosphere in a stainless steel vessel, to minimize oxidation prior to analysis (de Marcellus et al. 2015). The residue was then solubilized in 150 μL pure methanol and directly analyzed (Danger et al. 2013).
2.2. UPLC-Orbitrap MS Analysis
Analyses were performed with two quaternary Accela LC pumps (pump 1: Accela 600 pump; pump 2: Accela 1250 pump) working together and interfaced with a Q-Exactive (Hybrid quadrupole-OrbitrapTM) mass spectrometer equipped with an HESI (Heated Electrospray ionization) source (Thermo Fisher Scientific, Waltham, MA, USA). Peak integration and MS spectra acquisition were performed with Thermo XcaliburTM Qualitative Browser (Thermo Xcalibur 2.2 SP1.48, Thermo Fisher Scientific Inc.). A mass tolerance of 10 ppm was applied for the extraction of targeted ions. Single Ion Monitoring (SIM) chromatograms were traced with Origin (Origin 9.0.0, OriginLab Corporation, Northampton, USA). In order to certify the identification of the targeted compounds, a stock solution of 12C nucleobases (cytosine, uracil, thymine, adenine, guanine, lyophilized powder, and Sigma-Aldrich) all at 1E−4 mol l−1 was prepared in methanol.
2.3. UPLC-triple Quadrupole MS (UPLC-SRM-TQ MS) Analysis
Triple quadrupole MS analyses were performed with a Shimadzu Nexera X2 UHPLC system coupled to an MS 8050 triple quadrupole mass spectrometer (TQ MS) equipped with an ESI (Electrospray ionization) source (Shimadzu, Marlborough, MA, USA). In order to certify the identification of the targeted compounds, a standard solution containing 12C-nucleobases (adenine, cytosine, guanine, thymine, uracil, lyophilized powder, and Sigma-Aldrich) was prepared at 1E−4 mol l−1 in methanol. It was diluted in order to prepare a calibration curve ranging from 5E−8 mol l−1 to 5E−7 mol l−1 (five point-concentrations, each injected in triplicate). Limits of detection (LODs) were calculated from the equation of a linear regression, as three times standard deviation of noise signal/slope ratio. The determined LODs were 1.63E−8 mol l−1 for adenine, 3.34E−9 mol l−1 for guanine, 1.37E−8 mol l−1 for thymine, 1.45E−7 mol l−1 for cytosine, and 1.27E−9 mol l−1 for uracil. Linearity, sensitivity, and reproducibility were ensured.
3. Results and Discussion
It is known that carbonaceous chondrites have undergone some degree of secondary alteration in their parent bodies (Brearley 1997) that could have led to the formation of nucleobases. Furthermore, a relation between the abundance of nucleobases and the degree of aqueous alteration within meteorites (or their parent bodies) has been proposed (Burton et al. 2012).
However, since we do not have any direct information on astrophysical organics before the parent body accretion stage, it is challenging to test this hypothesis directly on "natural" samples. On the other hand, the synthesis and subsequent analysis of laboratory analogus can help in understanding the chemical reactions that could have triggered the formation of the nucleobases. In this context, we experimentally simulate the primary processes that led to the formation of complex organic molecules within the solid phase of photo- and thermal processed ices before accretion and parent body formation. In the resulting refractory residues, we searched for the presence of the five canonical 13C-nucleobases ((adenine, cytosine, guanine, thymine, and uracil, Figure 2). Residues were formed from initial ices containing H2O:13CH3OH:NH3 in ratio 2:1:1.
Figure 2. Search for nucleobases within primitive, realistic astrophysical ice analogs. Refractory residues were probed for all five canonical nucleobases (adenine, cytosine, guanine, thymine, and uracil) while only cytosine got detected in a residue formed by photo- and thermal processing of an interstellar ice analog containing H2O:13CH3OH:NH3 in ratio 2:1:1. m/zSTANDARD refers to [12C-M+H]+ ions while m/zRESIDUE represents 13C-isotopically labeled [13C-M+H]+.
Download figure:
Standard image High-resolution image3.1. Search for Nucleobases: Unambiguous Identification of Cytosine
UPLC-Orbitrap MS was first used to target for the presence of nucleobases similarly as performed by Oba et al. (2019). Thanks to high resolution and high mass accuracy analysis, exact masses of 13C nucleobases in residue samples were targeted at the same retention time of the 12C nucleobase standards. With this technique three pyrimidine-based nucleobases (cytosine, uracil, and thymine) match in retention time and exact m/z values to their chemical standards. Purine-based nucleobases were not observed. It could be explained by the fact that CO was only formed by photoprocessing of CH3OH in the here studied ices and is therefore present in lower amounts than in the initial ice studied by Oba et al. (2019). However, UPLC-Orbitrap MS analyses also show that numerous isomers are present (Figure 3). Therefore, providing exact masses at the same retention times as chemical standards could not be a fully satisfactory method to unambiguously reveal the presence of nucleobases.
Figure 3. UPLC-Orbitrap MS analysis of five nucleobases in residue from astrophysical ice analogs. Selection ion monitoring (SIM) chromatograms of cytosine, uracil, thymine, adenine, and guanine in residues as compared to chemical standards. The position of nucleobases' retention times in ice mixtures are indicated by a gray star. A large number of isomers of cytosine, uracil, and thymine is indicated by many peaks present in residue analysis. m/zSTANDARD refers to [12C-M+H]+ ions while m/zRESIDUE represents 13C-isotopically labeled [13C-M+H]+.
Download figure:
Standard image High-resolution imageConsequently, UPLC-selected reaction monitoring triple quadrupole mass spectrometry (UPLC-SRM-TQ MS) analysis was performed. In contrast to UPLC-Orbitrap MS, this method specifically uses a fingerprint of a fragmentation pattern of a given molecule. It targets a compound by monitoring simultaneously intact molecules (named the precursor ion) and a particular fragment ion. The pair of a precursor and fragment ion is named "transition." For instance, the fragmentation of the cytosine precursor ion ([cytosine+H]+, m/z = 112) resulted in a fragment ion with a m/z = 95 due to a loss of a NH3 group (m/z = 17), giving evidence for a primary amine group (transition m/z = 112.25  m/z = 95.20). Insights into molecular structures of probed analyte molecules are given. Thus, UPLC-SRM-TQ MS enables better discrimination between isomers compared to the UPLC-Orbitrap MS analysis. Following this strategy, we targeted specific transitions of all five canonical nucleobases (adenine, cytosine, guanine, thymine, and uracil) to give evidence for their presence in refractory residues (Figure 4).
m/z = 95.20). Insights into molecular structures of probed analyte molecules are given. Thus, UPLC-SRM-TQ MS enables better discrimination between isomers compared to the UPLC-Orbitrap MS analysis. Following this strategy, we targeted specific transitions of all five canonical nucleobases (adenine, cytosine, guanine, thymine, and uracil) to give evidence for their presence in refractory residues (Figure 4).
Figure 4. SRM-triple quadrupole MS analysis of five nucleobases in refractory residues from astrophysical ice analogs. For each nucleobase, the selected transition includes the m/z value of the nucleobase precursor ion [M+H]+ and the m/z value of its fragment ion (e.g., release of NH3: m/z = 112  m/z = 95 for cytosine; Nelson & McCloskey 1994; Lu et al. 2006). SRM transitions were used in combination with retention time to identify nucleobases in ice analogs. All analyses were compared to chemical standards. Chemical standards were measured as 12C isotopologues while residues were made of 13C-isotopically labeled compounds (mass shift of precursor ions for chemical standard and residue).
m/z = 95 for cytosine; Nelson & McCloskey 1994; Lu et al. 2006). SRM transitions were used in combination with retention time to identify nucleobases in ice analogs. All analyses were compared to chemical standards. Chemical standards were measured as 12C isotopologues while residues were made of 13C-isotopically labeled compounds (mass shift of precursor ions for chemical standard and residue).
Download figure:
Standard image High-resolution imageOnly cytosine has been positively identified by matching both retention time and selective SRM molecular transitions (fragmentations) with its 12C chemical standard (Nelson & McCloskey 1994; Lu et al. 2006). A limit of detection (LOD) of 1.45E−7 mol l−1 for cytosine was determined, indicating its minimal concentration within the analyzed residue samples.
Based on these findings, we emphasize that high resolution and high accuracy UPLC-Orbitrap MS alone, as performed by Oba et al. (2019), is not sufficient to unambiguously identify specific compounds (e.g., nucleobases) in such complex mixtures including a large diversity of isomers. It is thus absolutely necessary to selectively target respective analytes of interest to identify their presence (e.g., via SRM-LC-MS strategies).
The presence of cytosine in pre-accretional residue analogs suggests that this nucleobase has been formed in astrophysical environments via radical chemistry. This hypothesis is in agreement with the detection of its putative precursor building blocks (Sadr-Arani et al. 2015) formed by UV processing of astrophysical ice analogs, e.g., HNCO (Fedoseev et al. 2016), OCN− in the presence of NH3, Figure 5 (Hudson et al. 2001), HNC (Quinto-Hernandez et al. 2010), or NH2 radicals (Borget et al. 2015; Fedoseev et al. 2016). In contrast, the mechanism of formation of the other four nucleobases remains unclear. They are either formed by radical chemistry as well but resulting in lower yields, or via an alternative mechanism. Thus, CO in ices (Oba et al. 2019) may be significant reactants in the formation of nucleobases. Alternatively, nucleobases might be formed more efficiently in aqueous environments in parent bodies of meteorites (Robertson & Miller 1995; Saladino et al. 2001) which could explain the higher abundance of guanine observed in meteorites (Callahan et al. 2011).
Figure 5. IR spectrum of the irradiated ice at 77 K (after 24 hr of irradiation). OCN− and NH4+ bands indicate the presence of HNCO (HNCO + NH OCN− + NH4+).
OCN− + NH4+).
Download figure:
Standard image High-resolution image3.2. Astrophysical Implications: Astrochemistry Meets Prebiotic Chemistry
The evolution of extraterrestrial organic matter, starting from dense molecular clouds toward planetary systems can be experimentally simulated in the laboratory (Öberg 2016). Collapsing clouds lead to the progressive formation and evolution of the solar nebula that evolves toward the solar system formation (and more generally to any planetary/exoplanetary system). During this evolution, icy grains constitute the initial solid reservoir in molecular clouds, as dictated by cosmic abundances and CHNOPS-based organic chemistry (d'Hendecourt 2011). This starting solid molecular pool, primarily made out of hydrides (Oort & Van de Hulst et al. 1946), will evolve toward an increasing molecular diversity and molecular complexity (Schmitt-Kopplin et al. 2010; Danger et al. 2013, 2016; Fresneau et al. 2017; Ruf et al. 2018), driven by energetic processes surrounding icy grains. If one wishes to better formalize this hypothesis, we might consider the holistic model of Ciesla & Sandford (2012). This model considers turbulent radial and convective dust grain transport, in and out of plane, that brings icy grains into outer regions of the solar nebula. These outer grains are then directly exposed to UV light, either from the central star or, more importantly, from UV light of surrounding stars (Gounelle & Meynet 2012).
Such energetic processes enrich the organic chemistry that has already been present on individual grains and has got furthermore incorporated into asteroids or comets, the "leftovers" of incomplete accretion processes (Öberg 2016). These interplanetary bodies might have played a decisive role in "shaping" the ecospheres of telluric planets, as this might have been the case for Earth (Catling & Kasting 2017). In these small bodies, organic matter can endure secondary processing, mainly in asteroids (Gounelle 2011). Meteoritic analyses give insight on these secondary processing, such as hydrothermalism or metamorphism, that can lead to a reprocessing of the accreted organic content (Vinogradoff et al. 2018). In comets, much weaker secondary processing is presumed to occur. Comets represent reservoirs of the most primitive organic matter (Cottin et al. 1999; Brearley 2006). Asteroids or comets are then able to deliver their organic content onto the surface of a telluric planet, particularly during the period of the Late Heavy Bombardment where interplanetary dust particles/micrometeorites, asteroids, and comets could have been the main drivers of organic matter at the surface of the early Earth (Chyba et al. 1990; Whittet 1997).
All these evolutionary processes can be systemically simulated by laboratory experiments, as presented in this work. These experiments have shown that individual irradiated icy grains lead to a large molecular diversity, prior to their incorporation in minor solar system bodies (Danger et al. 2013, 2016; Fresneau et al. 2017). Specific molecules have been detected that might have played a key role in emergence of life processes, e.g., amino acids (Muñoz Caro et al. 2002) or sugars (Meinert et al. 2016). Here, we demonstrate that at least one nucleobase (cytosine) is formed during the same ice processing, without any aqueous alteration effects (e.g., catalytic reactions of minerals).
Consequently, all major prebiotic building blocks (amino acids, sugars, and nucleobases) might have already been present in the solid phase (icy grains) of forming protoplanetary disks, before accreting toward planetary systems. So, is there a direct pathway in chemical evolution from astrochemistry toward prebiotic chemistry? While in asteroids secondary alteration could occur, comets could have delivered their primitive, unaltered organic content to the surface of telluric planets. Thus, comets could be the direct link between organic matter which has been formed during solar nebula evolution and the surface of telluric planets. Once on a surface of telluric planets, these exogenous prebiotic building blocks could have acted as an organic source toward the emergence of evolved prebiotic systems, as might have been the case in Earth's early environment.
4. Conclusions
In this Letter, we have searched for nucleobases in residues coming from pre-accretional ices. We used two different analytical techniques UPLC-Orbitrap MS and UPLC-TQ MS. Our results demonstrate that UPLC-Orbitrap MS analysis is not selective enough to unambiguously identify nucleobases. While UPLC-Orbitrap MS suggests the presence of cytosine, uracil, and thymine, cytosine is the only nucleobase that was unambiguously detected in soluble organic refractory residues as proved by the targeted UPLC-triple quadrupole mass spectrometric (UPLC-SRM-TQ MS) analysis.
In an astrophysical context, the probed residues may correspond to those present in comets or in very weakly altered meteorites by following dedicated simulations considering a plausible astrophysical scenario. These residues may originate from ice radiation during solar nebula evolution, at a stage where small grains are still present and not yet fully aggregated into asteroids or comets.
These results indicate that some nucleobases may not require secondary alteration processes inside parent bodies to be formed (e.g., via aqueous alteration by catalytic processes involving minerals). Consequently, all molecular building blocks (amino acids, sugars, and nucleobases), needed for the potential emergence of living systems on early Earth's surface and/or on any telluric planet, might have been supplied by comets, the least altered bodies of our solar system.
The authors thank the Agence nationale de la recherche (ANR, ANR-16-CE29-0015), the Centre National d'Etudes Spatiales (CNES, R-S18/SU-0003-072 and R-S18/SU-0003-072), and the Centre National de la Recherche Française (CNRS, "Physique et Chimie du Milieu Interstellaire" (PCMI) and "Programme National de Planétologie" (PNP) programs) for financial support. P.P. and C.G. also acknowledge financial support from the European Union (ERDF) and "Region Nouvelle Aquitaine."
Appendix
A.1. Details on UPLC-Orbitrap MS Analysis
Before injection, samples were stored at 4 °C using a Stack cooler CW (CTC Analytics AG, Zwingen, Switzerland). MS functions and LC solvent gradients were controlled by the Xcalibur data system (Thermo Fisher Scientific). Acetonitrile with  formic acid was used as buffer A and water with
formic acid was used as buffer A and water with  formic acid as buffer B. Instrument calibration in positive mode was done every day via the direct infusion of positive ion calibration solutions. Compounds elution was performed via a 1D-LC configuration composed of three serially coupled columns (Eddhif et al. 2018). It combined a porous graphitic carbon Hypercarb (50 mm × 2.1 mm, 3 μm, 250 Å; Thermo Fisher Scientific), a Pentafluorophenyl reverse-phase Kinetex F5 (100 mm × 2.1 mm, 1.7 μm, 100 Å, Phenomenex) and a C18 reverse-phase Hypersil Gold Q (50 mm × 1 mm, 1.9 μm, 175 Å, Thermo Fisher Scientific). The elution was performed at a constant flow of 100 μl minute−1 within the series. The elution gradient started with 5% buffer A and 95% buffer B during 3 minutes. It continued with a first linear gradient to reach 35% buffer A and 65% buffer B in 12 minutes. A second linear gradient was employed to reach 60% buffer A in 5 minutes. A third linear gradient was performed to reach 100% of buffer A in 1 minute. The column was rinsed with 100% buffer A during 2 minutes and then reconditioned for 2 minutes with 5% buffer A and 95% buffer B. Target Selected Ion Monitoring data dependent-MS/MS (t-SIM-ddMS/MS) was used to detect nucleobases in the sample. For chemical standards, the precursor ions selected for cytosine, uracil, thymine, adenine, and guanine were, respectively, [M+H]+ m/z 112.05054, [M+H]+ m/z 113.03512, [M+H]+ m/z 127.05669, [M+H]+ m/z 136.06180, and [M+H]+ m/z 152.05670. Mass detection was performed with an electrospray voltage at 4.0 kV. Capillary and heater temperatures were set to 280 °C and 300 °C. The relative flow rate of the sweep gas (nitrogen) which aids in solvent declustering and adduct reduction was set to 0 (flow in arbitrary units as defined by Thermo Fisher Scientific). To help nebulize sample solution into a fine mist in the ESI nozzle, the relative flow rate of the sheath gas (nitrogen) was set to 40 (flow in arbitrary units as defined by Thermo Fisher Scientific). Finally, the relative flow rate of the auxiliary gas (nitrogen) which assists the sheath gas in dispersing and/or evaporating sample solution was set at 30 (flow in arbitrary units as defined by Thermo Fisher Scientific). Targeted MS parameters were optimized as follows: resolution of 17,500 for precursor ions and 17,500 for fragment ions, AGC target of 5.105 (precursor ion) and 2.104 (fragment ions), microscan 1, max IT of 100 ms (precursor ion) and 200 ms (fragment ions), MSX count 4, loop count 3, and isolation window of 2.0 m/z. The normalized collision energy was set to 35%.
formic acid as buffer B. Instrument calibration in positive mode was done every day via the direct infusion of positive ion calibration solutions. Compounds elution was performed via a 1D-LC configuration composed of three serially coupled columns (Eddhif et al. 2018). It combined a porous graphitic carbon Hypercarb (50 mm × 2.1 mm, 3 μm, 250 Å; Thermo Fisher Scientific), a Pentafluorophenyl reverse-phase Kinetex F5 (100 mm × 2.1 mm, 1.7 μm, 100 Å, Phenomenex) and a C18 reverse-phase Hypersil Gold Q (50 mm × 1 mm, 1.9 μm, 175 Å, Thermo Fisher Scientific). The elution was performed at a constant flow of 100 μl minute−1 within the series. The elution gradient started with 5% buffer A and 95% buffer B during 3 minutes. It continued with a first linear gradient to reach 35% buffer A and 65% buffer B in 12 minutes. A second linear gradient was employed to reach 60% buffer A in 5 minutes. A third linear gradient was performed to reach 100% of buffer A in 1 minute. The column was rinsed with 100% buffer A during 2 minutes and then reconditioned for 2 minutes with 5% buffer A and 95% buffer B. Target Selected Ion Monitoring data dependent-MS/MS (t-SIM-ddMS/MS) was used to detect nucleobases in the sample. For chemical standards, the precursor ions selected for cytosine, uracil, thymine, adenine, and guanine were, respectively, [M+H]+ m/z 112.05054, [M+H]+ m/z 113.03512, [M+H]+ m/z 127.05669, [M+H]+ m/z 136.06180, and [M+H]+ m/z 152.05670. Mass detection was performed with an electrospray voltage at 4.0 kV. Capillary and heater temperatures were set to 280 °C and 300 °C. The relative flow rate of the sweep gas (nitrogen) which aids in solvent declustering and adduct reduction was set to 0 (flow in arbitrary units as defined by Thermo Fisher Scientific). To help nebulize sample solution into a fine mist in the ESI nozzle, the relative flow rate of the sheath gas (nitrogen) was set to 40 (flow in arbitrary units as defined by Thermo Fisher Scientific). Finally, the relative flow rate of the auxiliary gas (nitrogen) which assists the sheath gas in dispersing and/or evaporating sample solution was set at 30 (flow in arbitrary units as defined by Thermo Fisher Scientific). Targeted MS parameters were optimized as follows: resolution of 17,500 for precursor ions and 17,500 for fragment ions, AGC target of 5.105 (precursor ion) and 2.104 (fragment ions), microscan 1, max IT of 100 ms (precursor ion) and 200 ms (fragment ions), MSX count 4, loop count 3, and isolation window of 2.0 m/z. The normalized collision energy was set to 35%.
A.2. Details on UPLC-triple Quadrupole MS (TQ MS) Analysis
Before injection, samples were stored at 4 °C using a sample cooler (Shimadzu). LC solvent gradients and MS functions were controlled by the LabSolution data system (LabSolution 5.89, Shimadzu). Water with  formic acid was used as buffer A and acetonitrile with
formic acid was used as buffer A and acetonitrile with  formic acid as buffer B. An LC configuration composed of three serially coupled columns was used (Eddhif et al. 2018). It combined a porous graphitic carbon Hypercarb (50 mm × 2.1 mm, 3 μm, 250 Å; Thermo Fisher Scientific), a Pentafluorophenyl reverse-phase Kinetex F5 (100 mm × 2.1 mm, 1.7 μm, 100 Å, Phenomenex) and a C18 reverse-phase Hypersil Gold Q (50 mm × 1 mm, 1.9 μm, 175 Å, Thermo Fisher Scientific). The LC oven temperature was set at 40 °C. The elution was performed at a constant flow of 100 μl minute−1 within the series. The elution gradient started with 5% buffer B and 95% buffer A during 3 minutes. It continued with a first linear gradient to reach 35% buffer B and 65% buffer A in 12 minutes. A second linear gradient was employed to reach 60% buffer B in 5 minutes. A third linear gradient was performed to reach 100% of buffer B in 1 minute. The column was rinsed with 100% buffer B during 2 minutes and then reconditioned for 6 minutes with 5% buffer B and 95% buffer A. The TQ MS instrument operated in SRM mode to detect nucleobases in the sample. The parameters of the mass spectrometer were set as follows: capillary voltage of 4 kV; interface temperature of 400 °C; heat block temperature at 500 °C; and desolvation line temperature at 250 °C. Nebulizing gas flow, heating gas flow, and drying gas flow were set to 180 l hr−1, 600 l hr−1, and 600 l hr−1, respectively. Respective SRM transitions (for 12C compounds), dwell times, and collision energies are summarized as follows: cytosine, 112.25
formic acid as buffer B. An LC configuration composed of three serially coupled columns was used (Eddhif et al. 2018). It combined a porous graphitic carbon Hypercarb (50 mm × 2.1 mm, 3 μm, 250 Å; Thermo Fisher Scientific), a Pentafluorophenyl reverse-phase Kinetex F5 (100 mm × 2.1 mm, 1.7 μm, 100 Å, Phenomenex) and a C18 reverse-phase Hypersil Gold Q (50 mm × 1 mm, 1.9 μm, 175 Å, Thermo Fisher Scientific). The LC oven temperature was set at 40 °C. The elution was performed at a constant flow of 100 μl minute−1 within the series. The elution gradient started with 5% buffer B and 95% buffer A during 3 minutes. It continued with a first linear gradient to reach 35% buffer B and 65% buffer A in 12 minutes. A second linear gradient was employed to reach 60% buffer B in 5 minutes. A third linear gradient was performed to reach 100% of buffer B in 1 minute. The column was rinsed with 100% buffer B during 2 minutes and then reconditioned for 6 minutes with 5% buffer B and 95% buffer A. The TQ MS instrument operated in SRM mode to detect nucleobases in the sample. The parameters of the mass spectrometer were set as follows: capillary voltage of 4 kV; interface temperature of 400 °C; heat block temperature at 500 °C; and desolvation line temperature at 250 °C. Nebulizing gas flow, heating gas flow, and drying gas flow were set to 180 l hr−1, 600 l hr−1, and 600 l hr−1, respectively. Respective SRM transitions (for 12C compounds), dwell times, and collision energies are summarized as follows: cytosine, 112.25  95.20 for 12, 5 ms, 22 V; uracil, 113.25
95.20 for 12, 5 ms, 22 V; uracil, 113.25  96.15, 10 ms, 19 V; thymine, 127.25
96.15, 10 ms, 19 V; thymine, 127.25  110.15, 1 ms, 21 V; adenine, 136.25
110.15, 1 ms, 21 V; adenine, 136.25  119.20, 10 ms, 25 V; and guanine, 152.25
119.20, 10 ms, 25 V; and guanine, 152.25  135.15, 10 ms, 21 V (Nelson & McCloskey 1994; Lu et al. 2006).
135.15, 10 ms, 21 V (Nelson & McCloskey 1994; Lu et al. 2006).