Abstract
Trivalent lanthanide ions (Ln3+) have been used as active centers for fluorescence mainly in inorganic crystalline or glassy solids. Ln3+-containing systems allow easier thermal emission with narrower energy gaps under near-infrared light excitation because the Ln3+ electron–phonon interaction is a weak coupling. The multiphonon relaxation theory helps to quantitatively deal with heat release in the ionic inorganic solids. Recent advances in producing Ln3+-doped nanocrystals, however, are demanding alternative design concepts for Ln3+-based nanomaterials that exhibit luminescence in organic or organic/inorganic hybrid systems. In this paper we discuss the approach for dealing with the effect of organic molecules that surround the Ln3+-doped nanocrystals with the quantitative treatment of electron–phonon coupling. We conclude that this effect is substantially explicable by the chemical polarity of surrounding molecules. This paper reviews applications using thermal absorption and emission including laser cooling and thermometry, as well as biophotonic applications involving the thermal interaction of the Ln3+ such as nanothermometry and photothermal therapy.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Thermal Phenomena at 4f Electrons of Trivalent Lanthanide Ions
Intense luminescence is unquestionably the most important priority in fluorescent material design. An excitation energy for a phosphor ends up with forms of light or heat (Fig. 1). Therefore, a basic approach for the design of bright phosphors is to consider "how not to generate heat." In electronic transitions of trivalent lanthanide ions (Ln3+), which are mainly used for fluorescence, thermal effects have been described primarily by separating the motions of electron and nuclei. When designing phosphors using electronic transitions of Ln3+, the interaction between electrons and heat, which is the main cause of quenching, has been mainly described for the case of inorganic crystalline solids. However, a vast number of applications derived from such fluorescence of Ln3+ have been developed, not only in glass but also in nanocrystals or complex systems that involve organic molecules or polymers. 1–4 Ln3+ have been studied and commercialized as luminescent materials because light emissions ranging from visible to near-infrared (NIR) wavelengths can be generated by the electronic transition of 4f electrons. Unique electron configurations of Ln3+ are stem from shielding of the 4f electrons by electrons in external 5s and 5p orbitals. The combination of Ln3+ and inorganic crystalline host is especially good for applications towards NIR fluorescence or upconversion visible fluorescence with excitation via NIR light. The NIR light is attracting attention for the application in biological photonics with transparency. 5 Since the excitation energy level for the NIR light is narrower than the ultraviolet or visible excitation, the control of the thermal emission is severely required.
Figure 1. Two fates of the excitation energy after coming into trivalent lanthanide electronic system.
Download figure:
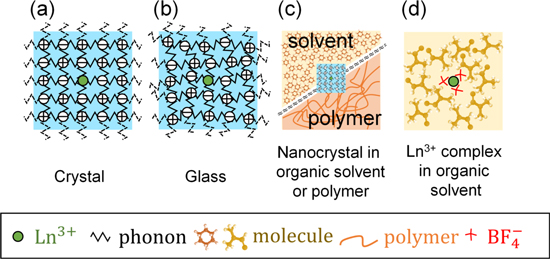
Standard image High-resolution imageAs shown in Fig. 2, Ln3+ in ionic crystals and glass, Ln3+-doped nanocrystals surrounded by organic solvents or polymers,
2,3
and molecular complexes  surrounded by organic solvent molecules
4
have all different thermal surrounding environment. The state of vibration of surrounding charged atoms and ions is different. Especially in Ln3+ in ionic crystals, the basic physics of the interaction between phonons and electrons had been highly developed.
1,6
However, in a system containing organic molecules, a novel approach for understanding the interaction between phonons and electrons is required in its application, especially in a system containing hydrophobic organic molecules.
surrounded by organic solvent molecules
4
have all different thermal surrounding environment. The state of vibration of surrounding charged atoms and ions is different. Especially in Ln3+ in ionic crystals, the basic physics of the interaction between phonons and electrons had been highly developed.
1,6
However, in a system containing organic molecules, a novel approach for understanding the interaction between phonons and electrons is required in its application, especially in a system containing hydrophobic organic molecules.
Figure 2. Schematic of the trivalent lanthanide ions (Ln3+) in a wide variety of environments such as inorganic (a), (b) and organic/inorganic hybrid systems (c), (d).
Download figure:
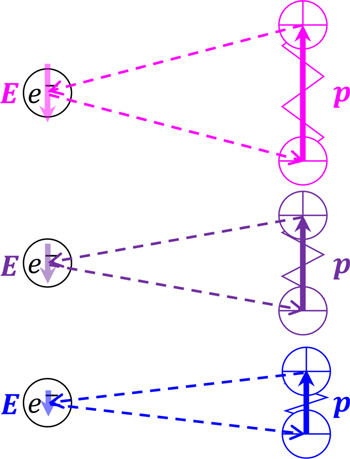
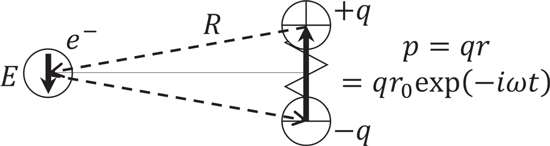
Standard image High-resolution imageThe vibration that an electron basically feels is the vibration of an electric field applied from the surrounding atoms. Once the nucleus of the surrounding atom vibrates, the electric charge that belongs to the atom vibrates accordingly, generating an electric field that reaches the electron of interest to vibrate. As shown in Fig. 3, its intensity is proportional to the magnitude of polarization, and when the polarization becomes zero, it becomes zero. In the electric dipole transition of electrons, the Hamiltonian can be described with the electric dipole moment of the electron system of Ln3+,  and the electric field of light as
and the electric field of light as  as
as

Instead of the electric field of light,  the electric field
E
of the oscillating surrounding electric dipole moment in Fig. 3 gives a quantity proportional to the electron–phonon coupling constant in multiphonon relaxation,
the electric field
E
of the oscillating surrounding electric dipole moment in Fig. 3 gives a quantity proportional to the electron–phonon coupling constant in multiphonon relaxation,

Figure 3. Vibrations of electric fields, E , applied from the surrounding atoms. The effects of atoms are replaced by electric dipole moment, p .
Download figure:
Standard image High-resolution imageSince  in Fig. 3 is shown to be proportional to the magnitude
in Fig. 3 is shown to be proportional to the magnitude  of the surrounding polarization, the factors related to heat release and heat absorption, excluding the frequency factor, is proportional to the strength of polarization of the surrounding atoms, molecules, or ions. It has been argued that the "electron–phonon coupling constant" is inversely proportional to the covalent bonding nature. In the covalent bond, the distance between the positive charge of the nucleus and the negative charge of the electron is much closer in comparison with the case of ionic bond where all the charges are located only in the vicinity of nucleus. Covalent bond allows the bonding electrons to locate in a space in between nuclei of the atoms with the benefit of quantum mechanical effects. Accordingly, the electric dipole moments from the two nuclei to the electrons located in the middle of them cancel each other out.
of the surrounding polarization, the factors related to heat release and heat absorption, excluding the frequency factor, is proportional to the strength of polarization of the surrounding atoms, molecules, or ions. It has been argued that the "electron–phonon coupling constant" is inversely proportional to the covalent bonding nature. In the covalent bond, the distance between the positive charge of the nucleus and the negative charge of the electron is much closer in comparison with the case of ionic bond where all the charges are located only in the vicinity of nucleus. Covalent bond allows the bonding electrons to locate in a space in between nuclei of the atoms with the benefit of quantum mechanical effects. Accordingly, the electric dipole moments from the two nuclei to the electrons located in the middle of them cancel each other out.
Let us return to the old treatment in ionic crystals. Phosphors with Ln3+ as active centers are widely used in ceramics for lasers and displays, among others, and the theory related to them developed rapidly in the 1960s.
7,8
Again, the basis of phosphor design for strong luminescence is not to generate heat. Therefore, the theory of multiphonon relaxation which relates to heat release is the most essential theory as a guideline of material design. This theory, developed in the 1960s, is based on the number of phonons,  required to make a transition with a certain energy separation between energy levels,
required to make a transition with a certain energy separation between energy levels,  focusing on the energy of phonons,
focusing on the energy of phonons,  which is the thermal quantum of the host crystal. The transition rate of multiphonon relaxation is given as,
which is the thermal quantum of the host crystal. The transition rate of multiphonon relaxation is given as,
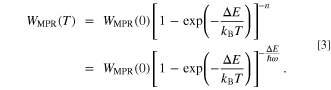
Simple and practical expression of Eq. 3 is well known for a constant temperature as, 9
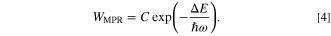
On the other hand, in the nanotechnology boom that originated in the nanotechnology initiative by Bill Clinton in 2000, research on ceramic phosphors centered on lanthanide ions has begun to progress as nanoparticles studies. In particular, the establishment of a method for synthesizing NaYF4 nanoparticles, which can synthesize nanocrystals with low phonon energy relatively easily and stably, has had a great influence on this research field.
10,11
Fig. 4 shows a comparison of the upconversion fluorescence intensity when the fluorescence intensity of fluoride nanocrystals is dispersed either in water  or heavy water
or heavy water  respectively.
12
The understanding of thermal phenomena that have been interpreted using crystalline phonons for many years forced to reflect. The influence of surrounding atoms, ions and molecules on the electronic transition of Ln3+ ion has an interaction length of several tens of nm.
13
For nanoparticles with a particle size of several tens of nm or less, the effect of thermal relaxation of the electron system extends not only to the crystalline host but also to the interaction with the molecules of the dispersion medium. The interpretation of the phenomenon shown in Fig. 4 is simple. The stretching mode wavenumber of OH is 3600 cm–1, whereas that of OD is 2600 cm–1, so vibration is an extension of the multiphonon relaxation shown in Eqs. 3 and 4. It can be interpreted that Ln3+ ions are less likely to release heat and are more likely to illuminate in a solvent that exhibits vibrations with small wavenumbers.
respectively.
12
The understanding of thermal phenomena that have been interpreted using crystalline phonons for many years forced to reflect. The influence of surrounding atoms, ions and molecules on the electronic transition of Ln3+ ion has an interaction length of several tens of nm.
13
For nanoparticles with a particle size of several tens of nm or less, the effect of thermal relaxation of the electron system extends not only to the crystalline host but also to the interaction with the molecules of the dispersion medium. The interpretation of the phenomenon shown in Fig. 4 is simple. The stretching mode wavenumber of OH is 3600 cm–1, whereas that of OD is 2600 cm–1, so vibration is an extension of the multiphonon relaxation shown in Eqs. 3 and 4. It can be interpreted that Ln3+ ions are less likely to release heat and are more likely to illuminate in a solvent that exhibits vibrations with small wavenumbers.
Figure 4. Pictures of water (H2O) and heavy water (D2O) colloidal dispersion of Tm3+/Yb3+ codoped fluoride nanocrystals in sunlight and with laser excitation at 980 nm. The smaller vibration energy of D2O (2600 cm–1) can suppress the thermal relaxation in comparison with the energy of H2O (3600 cm–1). Adapted from Ref. 12 with permission. Copyright 2013 American Chemical Society.
Download figure:
Standard image High-resolution imageThose who have tried to synthesize the NaYF4 nanoparticles in an organic solvent and to disperse them in water have experience as follows. The NaYF4 nanoparticles are luminescent well when dispersed in hexane but are quenched in water. How should we understand this? In many conventional material developments,  of Eq. 3 or C in Eq. 4. are left out. In other words, the question of how to interpret the electron–phonon coupling constant rather than the phonon energy dependence of the host has hardly been discussed. As discussed above, the electron–phonon coupling constant is proportional to the polarization of the system by surrounding atoms, ions, and molecules. Regarding the transition of Ln3+ ions in the 4f electron shell, the forced-electric dipole moment transition, magnetic dipole transition, and electric quadrapole transition compete with the oscillator strength of about 10–5 to 10–6, respectively. Here, for the sake of simplicity, let us consider the electric dipole transition.
of Eq. 3 or C in Eq. 4. are left out. In other words, the question of how to interpret the electron–phonon coupling constant rather than the phonon energy dependence of the host has hardly been discussed. As discussed above, the electron–phonon coupling constant is proportional to the polarization of the system by surrounding atoms, ions, and molecules. Regarding the transition of Ln3+ ions in the 4f electron shell, the forced-electric dipole moment transition, magnetic dipole transition, and electric quadrapole transition compete with the oscillator strength of about 10–5 to 10–6, respectively. Here, for the sake of simplicity, let us consider the electric dipole transition.
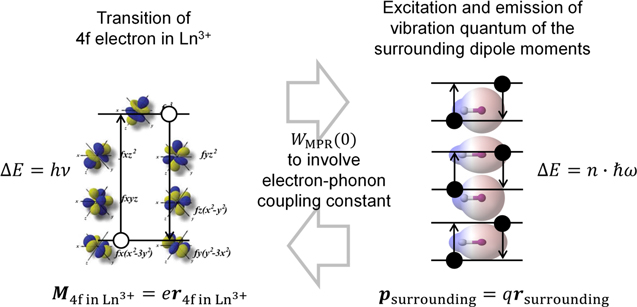
What should be considered are the "electric dipole moment  " when the electronic system undergoes an electric dipole transition, and the electric dipole moment
" when the electronic system undergoes an electric dipole transition, and the electric dipole moment  created by the charges of surrounding atoms, ions, and molecules as shown in Fig. 5. Here, in estimating the effect of the solvent described above, the electric dipole formed by surrounding atoms, ions and molecules can be understood as so-called "polarization,"
created by the charges of surrounding atoms, ions, and molecules as shown in Fig. 5. Here, in estimating the effect of the solvent described above, the electric dipole formed by surrounding atoms, ions and molecules can be understood as so-called "polarization,"  That is, the thermal relaxation of Ln3+ is more likely to occur as the polarization created by surrounding atoms, ions, and molecules is larger, excluding the influence of the vibration energy (
That is, the thermal relaxation of Ln3+ is more likely to occur as the polarization created by surrounding atoms, ions, and molecules is larger, excluding the influence of the vibration energy ( ) itself. This gives an interpretation to the fact that the nanoparticles dispersed in water do not exhibit luminescence while they do when dispersed in hexane. The development of a novel fluorescent nanostructure applying this will be described later. Therefore, the essence of the electron–phonon coupling constant that is involved in
) itself. This gives an interpretation to the fact that the nanoparticles dispersed in water do not exhibit luminescence while they do when dispersed in hexane. The development of a novel fluorescent nanostructure applying this will be described later. Therefore, the essence of the electron–phonon coupling constant that is involved in  term is the polarity of the surrounding,
term is the polarity of the surrounding, 
Figure 5. Thermal interaction between 4f electrons and the surrounding charge in the surrounding atoms, ions and molecules resembles to the dipole-dipole interaction in Forster resonance energy transfer.
Download figure:
Standard image High-resolution imageInterpretation from Phonon in Crystal to Vibration in Molecular System
The thermal effect in crystalline solids was sufficiently described in the previous section, except for the electron–phonon coupling constant. Here we will explain the essence of this constant by extending the interpretation of the multiphonon relaxation theory to the organic molecular system. Let us start from a discussion on the absorption and emission rates of phonons by the electronic transition within 4f states in Ln3+ ions.
1
In the discussion, at first the optical absorption and emission due to the transition of 4f electrons are solved assuming the Hamiltonian,  to describe
to describe  state.
14,15
On the other hand, the vibration effects of the surrounding ions for describing phonons are involved in
state.
14,15
On the other hand, the vibration effects of the surrounding ions for describing phonons are involved in  separately as the motion of the surrounding nuclei. Finally, the perturbation is applied to the
separately as the motion of the surrounding nuclei. Finally, the perturbation is applied to the  by adding the interaction between the electron and phonon as
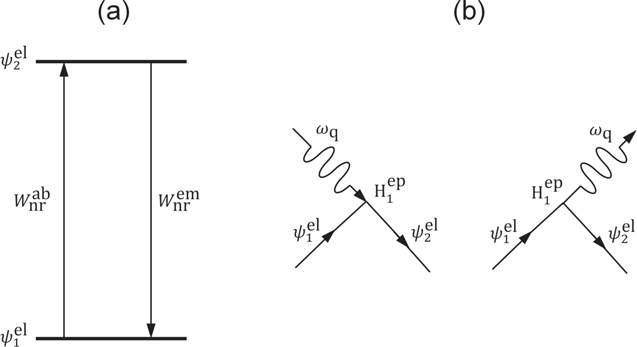
by adding the interaction between the electron and phonon as  The concept of the electron–phonon interaction is illustrated in the Feynman diagrams, as shown in Fig. 6. When considering a single phonon is absorbed or emitted, along with the transition from an initial state
The concept of the electron–phonon interaction is illustrated in the Feynman diagrams, as shown in Fig. 6. When considering a single phonon is absorbed or emitted, along with the transition from an initial state  to a final state
to a final state  the non-radiative transition rates,
the non-radiative transition rates,  for direct phonon absorption and emission are given by,
for direct phonon absorption and emission are given by,
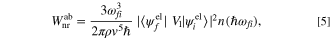
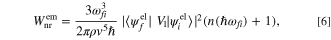
respectively, where  is the frequency that corresponds to the energy separation between
is the frequency that corresponds to the energy separation between  and
and 
 is the phonon occupation number of
is the phonon occupation number of 
 is the density of the system, and
is the density of the system, and  is the velocity of phonons. The weak-coupling system considered here can be expressed by product-state functions including a part that describes the nuclei and the others that describe the phonon field,
is the velocity of phonons. The weak-coupling system considered here can be expressed by product-state functions including a part that describes the nuclei and the others that describe the phonon field,
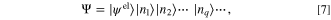
where the index q refers to a phonon with a specific wave vector, polarization, and branch. As can be seen by the Eqs. 5 and 6, the absorption and emission rates of a phonon are dominated by the  that has temperature dependence and the matrix element
that has temperature dependence and the matrix element  including V1, which comes out with the first order perturbation by the electron–phonon interaction. Here, we will find an exact form of the V1 in this system by applying a first-order perturbation. The Hamiltonian for the system is written as,
including V1, which comes out with the first order perturbation by the electron–phonon interaction. Here, we will find an exact form of the V1 in this system by applying a first-order perturbation. The Hamiltonian for the system is written as,
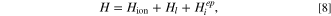
using the Hamiltonians described above. Since the electron–phonon interaction occurs through the change in the crystal field due to the change in the relative positions of the nuclei of the ions or surrounding ligands, the dynamic crystal field, Vc
, can be written by the expansion in terms of strain  with the static crystal field, V0,
with the static crystal field, V0,
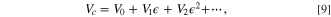
where  is the local strain caused by thermal vibration in the crystal. Detailed definition of the strain can be found in the textbook.
1
For extending this discussion from crystal or glass to general systems such as nanocrystals in organic solvent or polymers and Ln3+ ions in organic solvent, we should go back to the scheme in Fig. 3. For simplicity, we assume the simplest case that the direction of the electric dipole moment is perpendicular to the line between the electron and the dipole moment as illustrated in Fig. 7. The strain along the direction of the polarization of dipole can be expressed as
is the local strain caused by thermal vibration in the crystal. Detailed definition of the strain can be found in the textbook.
1
For extending this discussion from crystal or glass to general systems such as nanocrystals in organic solvent or polymers and Ln3+ ions in organic solvent, we should go back to the scheme in Fig. 3. For simplicity, we assume the simplest case that the direction of the electric dipole moment is perpendicular to the line between the electron and the dipole moment as illustrated in Fig. 7. The strain along the direction of the polarization of dipole can be expressed as  where r is the length of the electric dipole created by the surrounding atoms, ions, and molecules, and r0 is the length of the electric dipole at the equilibrium position. Then, the Hamiltonian for the electron–phonon interaction is represented as follows,
where r is the length of the electric dipole created by the surrounding atoms, ions, and molecules, and r0 is the length of the electric dipole at the equilibrium position. Then, the Hamiltonian for the electron–phonon interaction is represented as follows,

The Hamiltonian for the linear interaction  is proportional to the strain,
is proportional to the strain,  On the other hand, as shown in Eq. 2, the
On the other hand, as shown in Eq. 2, the  is proportional to the dot product of the electric dipole moment of electronic states of Ln3+,
M
, and the electric field,
E
, that is applied from the surrounding electric dipole moment,
p
, as shown in the Eq. 2. Thus, as obvious from Fig. 7, the
is proportional to the dot product of the electric dipole moment of electronic states of Ln3+,
M
, and the electric field,
E
, that is applied from the surrounding electric dipole moment,
p
, as shown in the Eq. 2. Thus, as obvious from Fig. 7, the  can be rewritten by,
can be rewritten by,
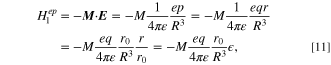
where ε is the dielectric constant of the vicinity of the system. By comparing the Eqs. 10 and 11, the V1 is expressed as,
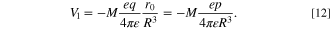
Inserting Eq. 12 into Eqs. 5 and 6, we obtain the following expressions for the nonradiative transition rate for direct phonon absorption and emission,
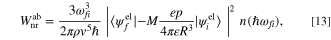
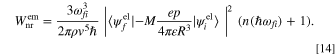
The importance of the results is that the thermal absorption and emission rate by an electronic system corresponds to the second powers of the dipole moment, p2. This gives the interpretation that the more polar the surrounding vibration system is, the more thermal absorption and emission occur. This p2 factor has a major impact on  in Eq. 3 or C in Eq. 4 as the strength of the interaction between electron and molecular vibration. Now we can apply the system also to the organic system with molecules and/or polymers.
in Eq. 3 or C in Eq. 4 as the strength of the interaction between electron and molecular vibration. Now we can apply the system also to the organic system with molecules and/or polymers.
Figure 6. (a) Energy-level diagram and (b) diagrams of quantum-mechanical interaction for single-phonon absorption and emission processes.
Download figure:
Standard image High-resolution imageFigure 7. Schematic to explain the simplest assumption to calculate the exact form of  as the factor of the first perturbation term of the dynamic crystal filed expanded on the strain,
as the factor of the first perturbation term of the dynamic crystal filed expanded on the strain, 
Download figure:
Standard image High-resolution imageLanthanide-Doped Nanocrystals in Organic System
The luminescence intensity of phosphors decreases with an increase in temperature. This effect is best known as thermal quenching. The results obtained from the discussion for phonons in ionic solids is applicable for engineering phonons in the inorganic/organic hybrid systems. The thermal absorption and emission rates by the electronic system are proportional to the square of the dipole moment of surroundings. In the ionic solid systems, for example, NaYF4 has been used as a suitable host lattice for upconversion luminescence due to its low phonon energy (∼360 cm–1). 16 For making the luminescence of Ln3+-doped ceramics nanoparticles brighter in aqueous environment, versatile approaches include surface coating by hydrophobic polymers 17 and ceramics shell formation. 18 It is effective because the Ln3+ ions are shed from stretching vibrations of hydroxyl bonding of water molecules, which is the main cause of thermal quenching in aqueous environment.
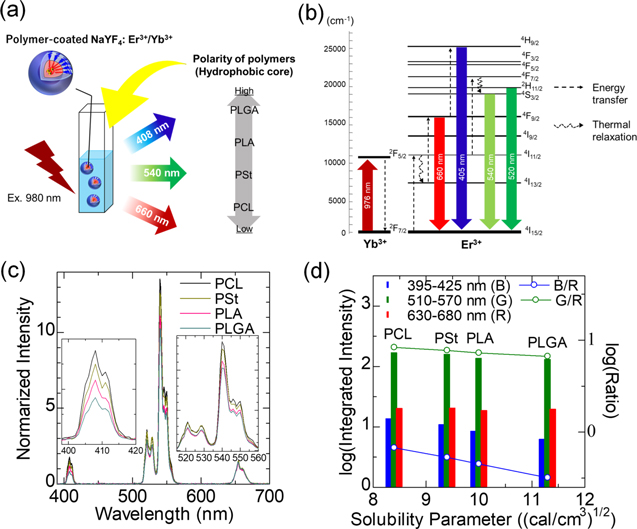
In fact, Ln3+-doped nanoparticles brightly illuminate in hydrophobic solvents as compared to those dispersed in water. Our group investigated the effects of surroundings, such as dispersion solvents and polymers that cover nanoparticle surfaces, on the upconversion luminescence of NaYF4:Yb3+,Er3+ nanoparticles under 980 nm optical excitation, 2 as shown in Fig. 8. Two sets of measurements were performed: (1) NaYF4:Yb3+,Er3+ nanoparticles dispersed in solvents such as dichloromethane, tetrahydrofuran, cyclohexane, and hexane and (2) water dispersion of NaYF4:Yb3+,Er3+ nanoparticles coated with either poly(ε-caprolactone) (PCL), polystyrene (PSt), poly(L-lactide) (PLA), or poly(lactide-coglycolide) (PLGA) as a hydrophobic part of an amphiphilic block copolymer. The study revealed that chemical polarities of the organic solvents and the hydrophobic core polymers had a major impact on the luminescence intensity of the nanoparticles. The thermal relaxation (4I11/2 → 4I13/2) was enhanced with an increase in the polarity, resulting that the emissions at 660 nm (4F9/2 → 4I15/2) increased and the emission at 405 nm (4H9/2 → 4I15/2) decreased. The chemical polarity of molecules is quantified by the Hansen solubility parameter, the most widely accepted one among all. 19,20 It contains three parts such as a dispersion force component, a hydrogen bonding component, and a polar component. A higher solubility parameter of molecules means stronger polarization. The results suggested that if the polymers and solvents that surround the Ln3+ ions in lattices possess the high solubility parameters, the degree of the thermal quenching is likely to increase. With this in mind, we designed and proposed a biodegradable, organic/inorganic hybrid nanostructure for NIR-induced photodynamic therapy using ultrasmall NaYF4:Yb3+,Er3+ nanoparticles (∼9 nm in diameter) and hydrocarbonized rose bengal dyes, both of which were encapsulated at the core of poly(ethylene glycol) (PEG)-block-PCL micelles. 3 By the encapsulation in the hydrophobic PCL core, the green upconversion luminescence was effectively enhanced, and the increased activation of the rose bengal dyes led to larger amount of singlet-oxygen generation. In addition, the hybrid nanostructures developed can be degradable after their use in vivo since the size of the nanoparticles allows them renal excretion and the PEG-block-PCL polymers are biodegradable. The rational design based on the understanding of electron–phonon interaction and chemical polarity provided a novel approach for the development of lanthanide-doped phosphors.
Figure 8. Effects of hydrophobic polymer on upconversion emission of polymer-coated NaYF4:Yb3+,Er3+ nanoparticles dispersed in water. (a) Illustration of the samples and the order of chemical polarities of the polymers. (b) Energy level diagram of NaYF4:Yb3+,Er3+. (c) Upconversion spectra of polymer-coated NaYF4:Yb3+, Er3+ with different hydrophobic core polymers. Each spectrum shown is normalized with a peak height at 660 nm. (d) Integrated intensity of the upconversion emission of each wavelength range and their ratio to the intensity of red (630–680 nm). The relationship between the emission intensities and the ratio with the solubility parameter of the hydrophobic core polymers is shown. Adapted from Ref. 2 with permission. Copyright 2021 Elsevier B.V.
Download figure:
Standard image High-resolution imageApplications of Thermal Absorption
The thermal absorption caused by the electron–phonon interaction has been investigated for applications including thermal control and thermometer. Laser-induced cooling, or simply called as laser cooling, is one of the representative techniques that makes use of the temperature dependence of luminescence mediated by the electron–phonon interactions. In laser cooling of solids, heat is reduced through the annihilation of phonons in the process of anti-Stokes shift luminescence, which converts long-wavelength excitation to short-wavelength emission as shown in Fig. 9. Pringsheim proposed a concept of the laser cooling based on anti-Stokes luminescence in 1929. 21 After the invention of the laser in 1960's, Kushida and Geusic at Bell Labs found a reduction in heating of Nd3+-doped yttrium aluminum garnet (Nd3+:YAG) crystals in 1968. 22 Epstein's research group demonstrated for the first-time net radiation cooling by anti-Stokes fluorescence in Yb3+-doped fluorozirconate (ZrF4-BaF2-LaF3-AlF3-NaF-PbF2; ZBLANP) glass in 1995. 23 Since then, the laser cooling has been performed on a wide variety of glasses and crystals including Yb3+-doped hosts: ZBLANP, 23,24 ZBLAN, 25 and LiF4; 26 Tm3+-doped ZBLANP 27 and Er3+-doped CnBZn. 28 Readers interested in the laser cooling of solid materials are encouraged to see a recent review by Seletskiy. 29
Figure 9. Energy diagram that shows the routes to anti-Stokes cooling.
Download figure:
Standard image High-resolution imageThermalization (thermal excitation) can be realized through the process of anti-Stokes fluorescence. Ohishi and Takahashi demonstrated a temperature sensor using Eu3+-doped ZrF4 glass fibers in which the optical absorption varies as a function of temperature due to 4f–4f transition in the Eu3+ ions. 30 An absorption band at 2.2 μm was monitored while the temperature of the optical fiber was changed in the range of 77–150 K, confirming that the temperature resolution was 0.5 K. Maurice reported a fiber-optic temperature sensor based on the change in the green intensity ratio of Er3+ ions in silica. 31 The different processes, in particular 800 nm excited state absorption in Er3+-doped fibers and 980-nm energy transfer, were investigated to determine the population of 2H11/2 and 4S3/2 energy levels. The sensitivity and measurement range achieved were 0.016 dB/°C and 25 °C–600 °C, respectively. In addition, the fiber-optic temperature sensors have been investigated using materials such as Yb3+-doped silica fiber 32 and Pr3+:ZBLANP. 33 The performance of such fiber-optic temperature sensors that rely on the thermal absorption is affected by the optical path, and thus they are essentially unsuitable for measuring temperature in the space down to ∼1 mm or smaller. Besides the use of the absorption property modulated by temperature, the luminescence properties such as band-shape, intensity, bandwidth, spectral shift, and lifetime are useful for thermometry because these spectral properties vary with an increase of the temperature around the Ln3+-doped phosphors, which will be described in the following section.
Applications of Biothermometry
Based on the principles described in above sections, research has been conducted to apply them to fluorescence thermometry. The principles are very complicated for practical use; thus, it has been important not to clarify all of them but to determine empirically the correlation, so-called calibration curve, between the measuring parameters of interest and the temperature, and to make it usable for temperature measurement. Fluorescence thermometry has been investigated to achieve contactless measurement of intracellular temperature micro-distribution and temperature of deep tissues in biology. Imaging by mapping fluorescence information representing temperature has attracted much attention as temperature imaging techniques, which can measure the temperature with positional (spatial) information. Particularly, phosphors containing Ln3+ with electron energy levels that absorb and emit in the NIR (especially over-thousand nanometer, OTN) enable to measure the temperature of deep tissues in vivo. 5 Fluorescent thermometry using NIR light is advantageous in that it can acquire temperature values of deep tissues where the fluorescent material (thermometer) is placed, not only the temperature of the surface of objects as in a currently-used thermography.
Although deep body temperature correlates in part with the surface temperature that can be measured by thermography, the temperature of the body surface is easily affected by surrounding environment. 34 Therefore, measuring the deep body temperature provides us a more accurate picture of our body's internal condition. Many people may have experienced repeated non-invasive body temperature measurements in public spaces to counter the prolonged spread of SARS-CoV-2 infection since 2020. Of course, it makes sense for screening for fever, while many people may also be wondering, "Why aren't the readings my body temperature as I know it?" "Why are the current readings so low?" and so on. These questions arise because the temperature at the body surface is sensitive to external environment and does not reflect the in vivo deep temperature, which represents more accurately the condition of the cells, tissues, and organs. The imaging technique for measuring local temperature of deep tissues that cannot be monitored via surface temperature is an important tool in biomedical (the field of disease treatment that desires an option of photothermal therapy) and physiological research on a potential regulation of biological functions by temperature change).
Highly accurate thermometry methods for deep tissues have been investigated by utilizing ratiometric and lifetime-based self-referencing principles. 35–37 The performance of nanothermometers is evaluated by the following figure of merits; (1) sensitivity: how the fluorescence characteristics (wavelength ratio or lifetime) changes when the temperature changes by 1 °C, and (2) accuracy: how much the error in the measured fluorescence characteristics that can be converted to temperature is relative to the measured value. 38 The fluorescence intensity and sensitivity of the fluorescent nanothermometer to temperature have been tuned; the obtained results can in principle be mostly interpreted in terms of the phonon energies of the surrounding molecules including host materials as described in previous sections.
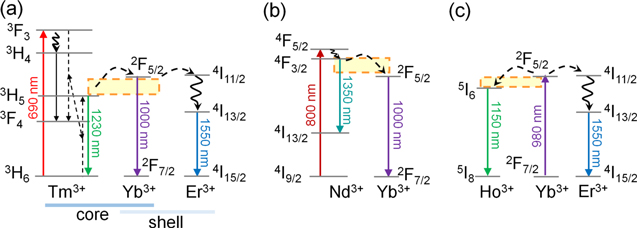
So far, different types of nanothermometers have been demonstrated to measure the temperature of deep biological tissues. In the early stage of the research on temperature imaging of in vivo deep tissues, real-time monitoring of temperature increase by heat treatment followed by thermal relaxation was reported. 39–41 Ceramic nanoparticles doped with Ln3+, which act as photosensitizer and temperature-sensitive emitter, have been reported as nanothermometers. Nd3+ and Tm3+ have been used as photosensitizers that absorb light of wavelength 690 nm and 800 nm, respectively. 34 As an example of using Tm3+, core-shell-structured LaF3 nanoparticles co-doped with Er3+, Tm3+, and Yb3+ (Er-Yb@Yb-Tm LaF3) showed multiple emission peaks in OTN-NIR, of which the ratio of Yb3+ at 1000 nm and Er3+ at 1550 nm responds to temperature change (Fig. 10a). In this core-shell nanostructure, the luminescent intensity ratio of Yb3+ at 1000 nm and Tm3+ at 1230 nm is also available for temperature sensing. The thermal sensitivity of the luminescence ratio of 1000 and 1550 nm is tuned by energy transfer from Tm3+ (excited by 690-nm light) to Yb3+ and Er3+. 39 LaF3 matrix is also available for designing Nd-based ratiometric nanothermometers. Core/shell-structured Yb3+/Nd3+-doped LaF3 nanoparticle showed emission of Nd3+ at 1350 nm and Yb3+ at 1000 nm, whose intensity ratio is sensitive to temperature change (Fig. 10b). 40 In these nanoscale systems, influence of the temperature on phonon-assisted energy transfers from 3H5 (Tm3+) to 2F5/2 (Yb3+) (Fig. 10a) and from 4F3/2 (Nd3+) to 2F5/2 (Yb3+) (Fig. 10b) likely provides the temperature-sensitive changes in the luminescent ratios. We also designed a nanothermometer with Yb3+ as a photosensitizer, synthesized by simple co-doping of Yb3+, Ho3+, and Er3+ into fluoride ceramic β-NaYF4 nanoparticles (Fig. 10c). 42 It demonstrates temperature-dependent luminescence intensity ratio between Ho3+ and Er3+ emissions at 1150 and 1550 nm, respectively, under 980-nm excitation for Yb3+. In this system, the emission intensity of Er3+ at 1550 nm is less sensitive to temperature change because the Yb3+−Er3+ system is dominated by resonant energy transfer that is not temperature dependent. In contrast, the emission intensity of Ho3+ at 1150 nm is largely sensitive to temperature because the energy transfer from 2F5/2 (Yb3+) to 5I6 (Ho3+) is assisted by phonon energy. 42 The use of a silicone composite containing the β-NaYF4 co-doped with Yb3+, Ho3+, and Er3+ enables temperature imaging of deep tissues at the depth of peritoneal cavity level (Fig. 11), 43 which is deeper than the subcutaneous tissues.
Figure 10. Simplified energy schemes of OTN-NIR fluorescent nanothermometers that enable ratiometric temperature measurement. The schemes of (a) Tm3+–Yb3+–Er3+, (b) Nd3+–Yb3+, and (c) Yb3+–Ho3+–Er3+ systems are shown according to the published literatures 39,40,42 with minor modifications. The arrows represent excitation and radiative decays (full lines), nonradiative decays (curved lines), and possible cross-relaxations and ion-ion energy transfer paths (dashed lines). The transfer from 2F5/2 (Yb3+) to 4I11/2 (Er3+) occurs resonant energy transfer without phonon assistance, whereas Tm3+–Yb3+, Nd3+–Yb3+, and Yb3+–Ho3+ systems need phonon assistance as shown by dashed rectangles.
Download figure:
Standard image High-resolution imageFigure 11. Contactless temperature sensing of deep abdominal region in mice by using a ratiometric OTN-NIR luminescence nanothermometer, β-NaYF4 co-doped with Yb3+, Ho3+, and Er3+. (a) Experimental scheme to investigate the availability of the nanothermometer in the peritoneal cavity of mice. The ratiometric temperature images were acquired using bandpass filters at 1150 nm (Ho3+) and 1550 nm (Er3+) under 980-nm laser excitation for temperature measurement. (b) Fluorescence images of the implanted area in the peritoneal cavity acquired using bandpass filters at different temperatures. Scale bars represent 5 mm. (c) Relationship between the temperature in the peritoneal cavity and the luminescence intensity ratio (LIR) of Ho3+ and Er3+. Adapted from Ref. 43 with permission with the terms of the Creative Commons CC BY license.
Download figure:
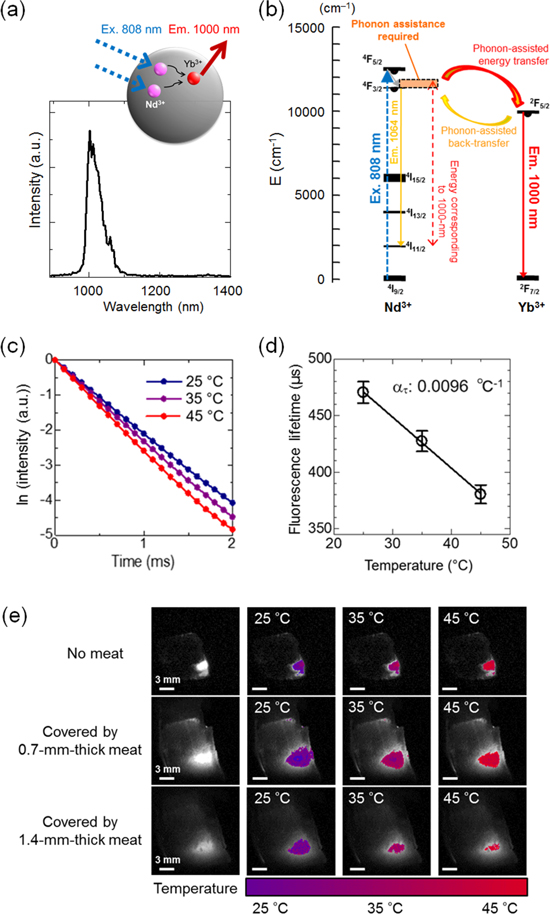
Standard image High-resolution imageThe ratiometric thermometry has the advantage that the temperature can be measured using a conventional optical imaging system with a light source for excitation, a detector, and optical filters. However, the measurement is affected by the temperature-dependent change in optical loss properties of biological tissues between the emitter and the detector. This problem can be resolved in the lifetime-based thermometry. 44 The NIR lifetime-based thermometry was reported using NaYF4 co-doped with Nd3+ as a photosensitizer and Yb3+ as an emitter (Fig. 12a). The Nd3+−Yb3+ system is dominated by phonon-assisted energy transfer as shown in Fig. 10b. Further, the back transfer from 2F5/2 (Yb3+) to 4F3/2 (Nd3+) also requires phonon assistance (Fig. 12b). The fluorescence decay of Yb3+ at 1000 nm is enhanced and thus the fluorescence lifetime decreased by temperature increase (Figs. 12c, 12d). The thermal sensitivity of 0.0096 °C−1 was satisfactory for temperature imaging in biology (Fig. 12e). 44 To realize lifetime imaging of the NIR fluorophores, a time-gated imaging (TGI) system was constructed synchronizing a pulse-laser for excitation and an NIR camera for detection. The TGI system enables acquiring a series of fluorescence decay curves at a pixel level, that can be converted into lifetime-based images. Thus, the intensity ratio and lifetime of fluorescence depend on temperature in a complex mechanism that is influenced by phonon-assisted energy transfer, which has been proposed by Miyakawa and Dexter, 6 in addition to the thermal absorption and emission as described in this paper. Therefore, research is majorly being conducted by simply describing results of temperature-dependent luminescent phenomena rather than strictly revealing the underlying process.
Figure 12. NIR fluorescence lifetime-based temperature imaging. (a) Illustration of NIR luminescence of Nd3+/Yb3+-doped NaYF4 emitting at 1000 nm under 808 nm laser excitation, and the respective emission spectrum. (b) Corresponding energy level diagram and luminescence mechanisms. (c) Fluorescence decay curves measured in optical cuvette at different temperatures. (d) Thermal calibration curve for fluorescence lifetime detected by NIR camera. (e) Temperature images obtained by calculation from the calibration curve of the thermally sensitive phosphor (NaYF4: Nd3+, Yb3+) with and without a sheet of meat as a model of biological tissues. Adapted from Ref. 44 with permission with the terms of the Creative Commons CC BY license.
Download figure:
Standard image High-resolution imageFurthermore, while the luminescence of molecules and materials in response to light irradiation has been used for imaging, heat generation instead of luminescence has been applied to photothermal therapy (PTT), a therapy that removes diseased cells and tissues by damaging them with heating. The purpose of PTT is to reduce and eliminate the lesion by heating the diseased lesion with light in a non-contact manner. During the treatment, it is important to monitor the actual temperature of the lesion tissue in inside of the body for adjusting the intensity of the light irradiation that provides energy for thermal induction and to achieve the targeted therapeutic effect. Until now, OTN-NIR fluorescence single-walled carbon nanotubes has been investigated well for their potential applications to PTT 45,46 including tumor metastasis inhibition by imaging-guided PTT. 47 Future works are expecting better use of Ln3+-based luminescent materials with nanoheaters such as gold nanoparticles for the development of a new type of PTT.
Conclusions
We have discussed thermal interaction with Ln3+ and surrounding molecular systems. The multiphonon relaxation theory traditionally accounts for heat release in ionic solids. The thermal interaction, on the other hand, is strongly controlled by the chemical polarity of surrounding molecules. Therefore, surrounding inorganic systems that contain Ln3+ by hydrophobic molecules or polymers allows strong luminescence from Ln3+ with near-infrared excitation. We have emphasized that the thermal absorption and emission rates in such systems are dominated by the squared dipole moments of the surrounding atoms, ions and molecules, thus suggesting that molecules with less chemical polarity are well suited as surroundings of luminescence systems. With the proper understanding of the electron–phonon interaction, some of our Ln3+-based organic/inorganic material systems exhibited strong luminescence in aqueous environment. We have briefly reviewed applications of thermal absorption, including laser-cooling and absorption-based thermometer, and recent advances in ratiometric or lifetime nanothermometry and photothermal therapy.