Abstract
Rechargeable lithium–sulfur (Li–S) batteries are recognized as one of the most promising candidates for next-generation energy storage devices due to their high energy density and high theoretical capacity, along with abundant natural resources, low cost, and the non-toxicity of sulfur. However, applications of Li–S battery have been hindered by limited specific capacity due to the poor conductivity and low content of sulfur, as well as fast capacity fading caused by polysulfide shuttling problems. Here, silver iodide is introduced as a host material for fabricating sulfur cathodes for Li–S batteries. Silver metal and lithium iodide, as the discharge products of silver iodide, can improve the redox environment, increase the ionic and electronic conductivity, and inhibit the shuttle of polysulfides during the charge and discharge processes. With a high sulfur content of 90.4%, the S/AgI composite delivers enhanced cycle performance with a decay rate of 0.092% per cycle within 500 cycles at 0.5 C, thanks to the synergistic effect of silver and lithium iodide. This opens the door to the rational design of halogen-containing transition-metal compounds as a class of cathode materials for Li–S battery.
Export citation and abstract BibTeX RIS
Rechargeable lithium-ion battery (LIB) has been widely used in portable electronic equipment and electric vehicles over the past decades. 1–3 Nevertheless, LIB has almost approached the upper limit of energy density, mainly due to the mechanism of insertion/extraction reaction, which is difficult to satisfy the ever-increasing demands for energy storage applications. 4,5 Recently, rechargeable lithium–sulfur (Li–S) battery has attracted increasing attention on accounts of its high theoretical energy density (2600 Wh kg−1), as well as high theoretical capacity (1675 mAh g−1), abundant natural resources, and environmentally friendliness of elemental sulfur. 6,7 However, the development of Li–S battery is still suffered from a multitude of challenges. 8,9 The insulating nature of sulfur and its final discharge products are unfavorable for achieving complete utilization and high discharge capacity. Moreover, the undesirable "shuttle effect" of polysulfides in organic electrolytes causes the loss of active material during cycling, resulting in irreversible capacity fading and poor Coulombic efficiency of Li–S battery.
A great deal of strategies has been employed to enhance the conductivity of cathode as well as tackle the shuttle effect of polysulfides. Especially, incorporating sulfur into carbon materials has been investigated thoroughly. 4,10–13 Porous carbon materials are particularly attractive due to their ability to provide electron-conducting substrate for sulfur redox reactions and minimize the shuttling of polysulfides by physical confinement. Unfortunately, the adsorption of nonpolar carbon toward polar polysulfides is not sufficient, leading to the slow release of active materials from porous carbon into the electrolyte, and the obvious capacity decay during the long cycling. 14,15 Therefore, it is challenging to confine soluble polysulfides efficiently on the cathode side only by porous carbon materials. At the same time, because of the large pore volume of carbon materials, a large amount of electrolyte is required to infiltrate porous S/C cathodes, which is contrary to improving the overall energy density of the Li–S battery. 16,17 In light of these problems, metal-based compounds (MX) with high tap density, including metallic oxides, 18–20 sulfides, 21–24 carbides, 25 nitrides, 26 boride, 27 and phosphides, 28 i.e., MX, M = Co, Mn, Mo, Fe, V, Ti, Ru, Ag, Mg; X = O, S, C, N, B, P, have been explored to immobilize the polysulfides by the strong chemical interaction and electrocatalysis process. Besides, it has been reported that electrodes of some transition-metal compounds can react with lithium in reversible conversion reaction to produce conductive metal element during the discharge process, 24,29,30 then improving the conductivity of the electrode, accelerating the transmission of electrons and decreasing the electrochemical impedance. Thus, a transition metal compound that can react reversibly during redox process is expected to improve the electrochemical performance of Li–S battery.
Silver compounds are potential electrodes due to good electrical conductivity in electrochemical systems. 24,31–34 Silver iodide (AgI), known as a semiconductor with a direct bandgap and great detection sensitivity in the visible range, 35–37 is widely studied in the fields of catalytic, 38–40 solar cells, 36 and all-solid-state battery. 41 However, there is little work has been conducted to apply AgI for Li–S battery in our knowledge. It is worth noting that AgI can produce lithium iodide (LiI) and highly conductive silver metal during the discharge process under theoretical conditions. Previous work has suggested proper amount of LiI is beneficial to the electrochemical performance because LiI with ionic conductivity of 10−7 s cm−1 can improve the ionic conductivity of the electrode and induce the formation of a protective coating. 42–45 Such protective coating can inhibit the release of polysulfides into the electrolyte on the cathode side and prevent the reduction of polysulfides on the anode side, minimizing the shuttle effect and improving the Coulombic efficiency as well as the utilization of active materials. Furthermore, metallic Ag is thought to increase the electronic conductivity in the electrode and reduce the electrochemical resistance. Therefore, it is valuable to investigate the application of AgI in sulfur-based composites as cathode materials for Li–S battery.
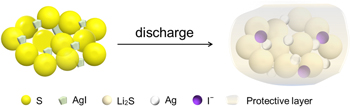
In this work, we used a facile double replacement reaction to prepare AgI microspheres, and furtherly fabricated the S/AgI composite with sulfur content up to 90.4% as a cathode material. A schematic diagram of the role of AgI in the S/AgI composites during the discharge process is shown in Fig. 1. The produced Ag and LiI, as the reduction products of AgI, can improve conductivity and promote sulfur redox reactions during cycling. And I− can induce the formation of a protective layer to reduce the shuttle of polysulfides. When AgI is used as a host material of sulfur, the S/AgI composite cathode exhibited good electrochemical performance, with a lower capacity decay of 0.092% per cycle and higher Coulombic efficiency of 96.2% during 500 cycles at 0.5 C, while the pure S cathode is 0.153% per cycle and 91.9%, respectively. Consequently, this work indicates that AgI-based sulfur composites are potential electrode candidates for Li–S battery.
Figure 1. Schematic diagram of the role of AgI in the S/AgI composites.
Download figure:
Standard image High-resolution imageExperimental
Preparation of sulfur nanoparticle
Nanosized sulfur was prepared by a liquid method. Firstly, Na2S2O3 (3.16 g) and polyvinylpyrrolidone (PVP, K30, 2 g) were dissolved in deionized water (250 ml). Then, concentrated hydrochloric acid (2 ml) was added drop by drop into the mixture and stirred for 2 h at room temperature. The sulfur nanoparticles were collected by centrifugation and washed repeatedly with deionized water and ethanol, and then dried overnight at 70 °C.
Preparation of the S/AgI composite
After the formation of the S nanoparticles in solution as mentioned above, 0.044 g of AgNO3 dissolved in 10 ml deionized water was added dropwise into the pristine colloidal solution of the S nanoparticles, followed by adding KI solution (0.045 g in 5 ml H2O). After being stirred magnetically for 3 h at room temperature, the mixture precipitates were collected, washed and dried at 70 °C overnight. The obtained composite was denoted as S/AgI-90, indicating the sulfur content of about 90% in the composite. Moreover, AgI and S/AgI-85 were synthesized according to the weight content by a similar process.
Materials characterizations
The obtained samples were characterized using powder X-ray diffraction (XRD, Rigaku mini Flex II) with the range of 10°−70° at a scan rate of 4° min−1. The morphology and microstructure were identified by scanning electron microscopy (SEM, JSM-7800). Thermogravimetric analyzer (TGA, Mettler Toledo, TGA/DSC) was taken under argon atmosphere at the rate of 10 °C min−1 to confirm the sulfur content. The conductivity was measured by the ST-2722 semiconductor resistivity of the powder tester.
Electrochemical testing
The working electrodes were prepared from a slurry consisting of 80% sample, 10% super P and 10% polyvinyldifluoride (PVDF) in N-methyl-2-pyrrolidone (NMP). The slurry was coated onto a carbon-coated Al foil, then dried overnight at 60 °C and cut into round disks as cathodes with a diameter of 10 mm. The 2032-type coin cell was assembled using lithium metal as anode and Celgard 2300 membrane as separator in a glovebox filled with Ar gas. The electrolyte was consisted of 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and 1 wt% lithium nitrate (LiNO3) in the mixture of 1,2-dimethoxyethance (DME) and 1,3-dioxolane (DOL) (v/v, 1:1). The loading mass was 1.1–1.3 mg cm−2, and the electrolyte/sulfur ratio is ∼12 μl mg−1. The galvanostatic charge and discharge tests were carried out with LAND-CT2001A instruments (Wuhan Jinnuo, China). Cyclic voltammograms (CV) were measured on an electrochemical workstation (LK2005, China) with a potential window of 1.7–2.8 V at a scan rate of 0.1 mV s−1. Electrochemical impedance spectra (EIS) were tested on a Zahner IM6ex electrochemical workstation from 10 mHz to 100 kHz with a voltage amplitude of 5 mV.
Results and Discussion
As depicted in Fig. 2a, the sulfur nanoparticles were synthesized firstly by combined a mixture of sodium thiosulfate and hydrochloric acid in the presence of PVP, and then the S/AgI composites were prepared based on the double replacement reaction at room temperature. The morphology of the as-prepared AgI and S/AgI particles were observed by using SEM as shown in Figs. 2b and 2c, respectively. The AgI appears rough surface morphology formed by stacked spheres with inhomogeneous sizes. There is a difference in morphology after integration with sulfur as compared with the AgI, and the S/AgI composite exhibits a closely connected structure. EDS mappings of S/AgI-90 composite is further provided in Fig. 2d, showing the distribution of sulfur, silver and iodine elements. Obviously, silver and iodine elements are randomly distributed, indicating AgI particles are randomly precipitated throughout the sulfur nanoparticles.
Figure 2. (a) The illustration of the preparation of S/AgI composite. SEM images of the as-prepared (b) AgI and (c) S/AgI-90. (d) EDS mappings of the S/AgI-90. (e) XRD pattern of the AgI and S/AgI-90. (f) TG curves of the S, AgI and S/AgI-90 composite.
Download figure:
Standard image High-resolution imageThe XRD patterns were used to investigate the crystal structure and composition of the as-synthesized samples and the results are shown in Figs. 2e and S1 (available online at stacks.iop.org/JES/168/060536/mmedia). For the pure AgI, the diffraction peaks can be easily indexed to the hybrid crystal including hexagonal β-AgI (PDF # 09–0374) and cubic γ-AgI (PDF # 09–0399). 46 The XRD result of the S/AgI-90 (Fig. 2e) and S/AgI-85 (Fig. S1) proves that sulfur exists in a crystalline state, and the characteristic peak of (002) at 23.7° for AgI can be observed. Notably, no additional peak is observed, indicating the high purity of the S/AgI-90 sample. The conductivity of pure AgI and S/AgI composite was measured to facilitate the analysis of electrode performance, and the conductivity of S/AgI-90 is significantly higher than that of sulfur (Table SI).
Thermogravimetric (TG) analysis was conducted for the S, AgI and S/AgI composites, as shown in Figs. 2f and S2. It can be seen that AgI is thermally stable within the entire heating range. A dramatic weight loss is observed within the temperature range from 200 °C to 350 °C, which is due to the thermal evaporation of sulfur. To probe whether silver sulfides are formed during thermal treatment, the final product of S/AgI in TG experiment is characterized using XRD (Fig. S3). The final product is the mixture of β-AgI and γ-AgI, and no signals from Ag2S, Ag2S2, or Ag are detected. Thus, the sulfur contents in the S/AgI-90 and S/AgI-85 composites are determined to be 90.4 wt% and 84.7 wt%, respectively. This result proves a high sulfur content in the S/AgI-90 composite.
In order to identify the products of AgI and S/AgI composite at the different charge state, XRD was employed and relevant patterns are shown in Fig. 3. It is clear that the peak of about 65° corresponds to the contribution of the Al foil. 21 The XRD pattern of the AgI electrode (Fig. 3a) in the origin state almost consistent with the standard pattern. After discharge to 1.7 V, the diffraction peaks of AgI almost disappear, two new peaks appear at 38.1° and 44.4°, which can be assigned to Ag metal (PDF # 04–0783). When recharged to 2.8 V, the disappearing peaks of AgI reappear, but the peak intensity weakens. Meanwhile, the peak of Ag metal can still be observed, indicating that Ag was not fully oxidized to AgI. However, the reversible oxidation and reduction of AgI via lithiation can be identified from the XRD patterns observed after the charge/discharge processes, and the reaction of AgI electrode in the range of 1.7 V to 2.8 V are as follows:
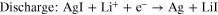

Figure 3. XRD patterns of the (a) AgI and (b) S/AgI-90 electrodes with different states.
Download figure:
Standard image High-resolution imageThe XRD patterns for the S/AgI-90 at different states are shown in Fig. 3b. The uncycled electrode exhibits peaks from crystalline sulfur. After discharge to 1.7 V, the diffraction peak of sulfur can still be observed, meaning that sulfur was not fully reduced, possibly due to the high content. However, a new peak appears at 38.2°, which can be assigned to Ag metal combined with the XRD patterns of AgI. The reappearance of the disappearing peaks of sulfur can clearly be observed after charging to 2.8 V, indicating the formation of sulfur again. In addition, it also can be inferred that the final oxidation product of Ag metal in the S/AgI composites could be a mixture of AgI, Ag2S and Ag2S2 by comparing with the standard card and considering the thermal stability. 47 From the above results, we can consider that Ag and LiI are produced during the discharge process, which can improve the conductivity of the electrode. In addition, it is believed that the proper amount of LiI can inhibit the shuttle effect by inducing the formation of protective coating, combined with literature reports and our previous work. 42,44 This is the reason why the electrochemical performance of the cells with S/AgI as the electrode can been improved.
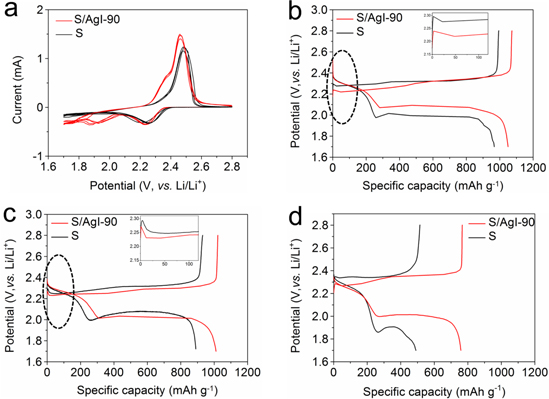
The Li–S coin cells were fabricated to evaluate the electrochemical performance of various cathodes. The cyclic voltammetry (CV) curves of the S/AgI-90 and S electrodes carried at a scan rate of 0.1 mV s−1 are presented in Fig. 4a. During the cathodic scan, the S/AgI cathode shows two discernible reduction peaks located at 2.23 and 1.91 V, which are associated with the conversion of sulfur to long-chain lithium polysulfides and finally to Li2S. Actually, the reduction of AgI to Ag and LiI also occurs during this process. However, the S cathode without AgI only exhibits one peak, this phenomenon is caused by the low conductivity and incomplete reaction of the electrode due to high sulfur content. Additionally, deformed and widened peaks are also associated with high content of sulfur. In the subsequent anodic scan, there is merely one oxidation peak of the sulfur electrode at about 2.48 V. In contrast, the S/AgI-90 electrode exhibits two distinct oxidation peaks at about 2.36 V and 2.46 V, indicating the effective reversible conversion of sulfur species. In fact, there is also the oxidation of Ag into AgI, Ag2S or Ag2S2 in the anodic process. However, these processes cannot be distinguished because the redox potentials are close to each other and overlapped. For example, CV curve of the pure AgI electrode was investigated, as shown in Fig. S4. The cathodic peak and anodic peak are broadened, at about 2.45 V and 2.47 V, corresponding to the reduction of AgI to Ag, and the oxidation of Ag to AgI, respectively. According to the previous report, 24 Ag2S or Ag2S2 should be formed and involved in the redox process from the thermodynamical aspect, although the identification is difficult. In general, such a comparative result between S/AgI and S electrodes indicates that the presence of AgI improves the redox environment within the electrode to some extent, the S/AgI-90 electrode has better ionic and electronic conductivity than the S electrode, which benefit from the formation of Ag and LiI.
Figure 4. (a) Cyclic voltammetry curves of the S/AgI-90 and S electrodes at a scan rate of 0.1 mV s−1. Galvanostatic discharge/charge curves of the S/AgI-90 and S for the (b) 1st at 0.1 C rate, (b) 1st at 0.2 C rate (insets are the enlarged view of the circled areas), and (c) 100th at 0.2 C rate, respectively.
Download figure:
Standard image High-resolution imageThe charge/discharge curves of the S and S/AgI-90 electrodes are presented in Figs. 4b–4d. The 1st charge/discharge curves at 0.1 C (Fig. 4b) and 0.2 C (Fig. 4c) illustrate that the S/AgI-90 electrode exhibits higher capacity and improved Coulombic efficiency as compared with the S electrode. The increased discharge capacity mainly derives from the second plateau, and the improved Coulombic efficiency proves the reduction of the shuttle effect. Besides, the discharge voltage of the S/AgI-90 electrode is more stable and the polarization is smaller than that of S electrode. It was also observed that the oxidation of Li2S/Li2Sx in the S/AgI-90 electrode is improved, as demonstrated by the voltage curves at the start of charge at 0.1 C and 0.2 C. After 100 cycles at 0.2 C, S/AgI-90 electrode delivers the discharge capacity of 758.6 mAh g−1 and the Coulombic efficiency are maintained above 98.9%, while the S electrode shows a capacity of only 490.5 mAh g−1 and Coulombic efficiency of only 95.4% (Fig. 4d). The S/AgI-90 electrode exhibits a smaller voltage gap and stable discharge/charge voltage. The improvement in conversation kinetics and electrochemical performance implies that the conductivity and shuttle effect are optimized by introducing AgI and the formation of Ag and LiI. It's worth noting that when the amount of AgI in S/AgI composites is too much, the electrochemical performance of Li–S battery will be adversely affected. The cathodes with AgI content of 15.3% (S/AgI-85) were investigated, and the charge/discharge curves are shown in Fig. S5a. The obvious phenomenon is the charge voltage is fluctuant and the overpotential is increasing after the following several cycles. It can be seen that the charge voltage suffers continuous fluctuation and it cannot reach the upper cut off limit at the 5th cycle, which is mainly due to the excessive LiI produced by AgI during the discharge process. The dissolution of a large amount of LiI and the shuttle of I−/I3 − between the electrodes, leading to the internal micro-short circuit and severe self-discharge. 48,49 Additionally, the I3 − is possible to oxidize polysulfides while itself reduce to I−, which can also cause the above abnormal phenomena. 50 Furthermore, the high content of AgI (50%) electrode was also investigated, and the charge/discharge curves are presented in Fig. S5b. The charge/discharge process is failed in the case of high AgI content. The possible cause of the cell failure is the formation and growth of Ag dendrites during the discharge process, which results in the short circuit of the cells.
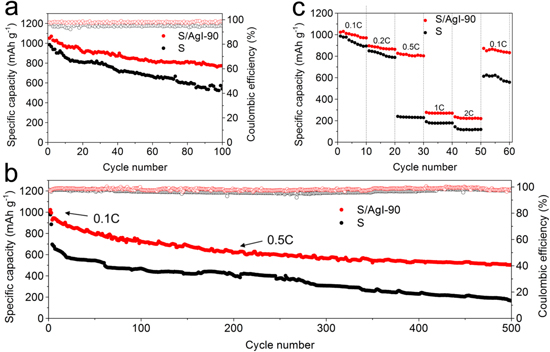
The cycling stability was performed as illustrated in Fig. 5. The S/AgI-90 composite delivers a specific capacity of 1051.2 mAh g−1 in the first cycle, a larger value of 1070.4 mAh g−1 in the second cycle, and retains 773.3 mAh g−1 after 100 cycles at 0.1 C (Fig. 5a). The Coulombic efficiency is maintained above 97.1% during cycles. By contrast, the sulfur electrode exhibits an initial capacity of ∼1000 mAh g−1, which is slightly lower than the S/AgI-90 composite. Since AgI as a semiconductor has little effect on the improvement of conductivity, the S/AgI-90 composite shows no obvious improvement of the initial capacity. However, the formation of Ag and LiI help maintain the capacity during the long cycling. The pure sulfur electrode exhibits more severe capacity fading (only 540.6 mAh g−1 after 100 cycles), and more poor Coulombic efficiency (about 94.8%). When the rate was increased to 0.5 C, as depicted in Fig. 5b, the Coulombic efficiency is above 96.2% for S/AgI-90 and near 91.9% for sulfur electrode during 500 cycles, indicating the shuttle effect owing to polysulfides could be effectively inhibited by applying AgI to the cathode materials. The S/AgI-90 composite still achieves a high discharge capacity of 502.1 mAh g−1 after 500 cycles, representing the capacity decay rate of 0.092% per cycle. As a comparison, the electrode prepared with sulfur exhibited a capacity of only 166.2 mAh g−1, corresponding to the capacity fade rate of 0.153%. Note that, the reversible specific capacity is calculated by the weight of sulfur, while the contribution from AgI is not considered. Actually, AgI is electrochemically active in the voltage range of 1.7–2.8 V, which has been verified in the previous CV curves. The discharge/charge profiles and cycle performances of AgI were presented in Fig. S6. The discharge capacity contribution of AgI is negligible during long cycles as compared with the large capacity of sulfur.
Figure 5. Cycle performance and Coulombic efficiency of the (a) S/AgI-90 and (b) S electrode at 0.1 C and 0.5 C rate, respectively. (c) Rate performance of S/AgI-90 and S at various current density.
Download figure:
Standard image High-resolution imageThe rate performance of the S/AgI-90 and S electrodes were investigated at various current densities, measuring for ten cycles at each current density from 0.1 to 2 C and returning to 0.1 C, as shown in Fig. 5c. The S/AgI-90 composite always exhibits higher discharge capacity under the corresponding current density in general. For the S cathode, a sharp decline of the discharge capacity occurred when the current density was switched from 0.2 to 0.5 C. The discharge capacity of the 10th cycle at 0.2 C was 788.3 mAh g−1, whereas the initial capacity was only 240.2 mAh g−1 at 0.5 C. A similar dramatic decrease in capacity was also observed on the S/AgI-90 cathode, but at a higher current density (i.e., from 0.5 to 1 C). The capacity drops rapidly with the increase of current density might be due to slow kinetics of cathode reactions caused by the high sulfur content, compact electrode structure and continuous passivation layer. 51 But it should be noted, when the current density is decreased to 0.1 C again, the specific capacity of S/AgI-90 cathode can be recovered to ∼89.9% of its final capacity at 0.1 C (872.2 mAh g−1 vs 970.1 mAh g−1). However, the S cathode only restores to 613.1 mAh g−1, which just corresponds to 68.5% of the capacity recovery. The reason for the rapid drop in capacity and poor reversibility may be related to a very high overpotential combined with the observation in Fig. 4d. Generally, the discharge capacity of the S/AgI-90 composite is not satisfactory at a high rate according to the current literature reports, but the results also indicate that the consecutive cycling reliability is improved compared with that of the pure S electrode.
The electrochemical impedance spectroscopy (EIS) was measured for the S and S/AgI-90 electrodes before and after cycling, and the equivalent circuit models are shown in Fig. 6. Before cycling, one semicircle in the high frequency and a sloped line in the low-frequency region can be observed. The former corresponds to the charge transfer resistance (Rct ) and the latter stands for Warburg resistance (Wo ). 52,53 Comparing the Nyquist plots of both electrodes, the S/AgI-90 holds lower charge transfer resistance than the S electrode, indicating the improved conductivity and the sulfur redox reactions in the cathode by the introduction of AgI. This inference has also been confirmed by the conductivity measurement data and previous electrochemical results. After cycling, both Nyquist plots are consisted of two semicircles in the high-medium frequency region and a sloped line in the low-frequency region, corresponding to the formation of interfacial films, charge transfer, and ion diffusion process, respectively. The interfacial films include solid electrolyte interface, insulating Li2S2/Li2S layers, and protective coating by the reaction of DME and I−. 50 The S/Ag-90 electrode has lower interfacial resistance (R1 ) and charge transfer resistance (Rct ) than the S electrode, suggesting that the S/AgI cathode possesses more favorable interfacial properties and higher activity of charge transfer reaction, which is inseparable from the active role of the discharge products Ag and LiI. The Ag metal increases the conductivity of the electrode based on its inherent properties, and LiI can improve the interface properties of electrodes by dissolving into the organic electrolyte and increasing the contact between the electrolyte and the electrodes, as well as inducing the formation of protective coating. For the S cathode, however, there is no such advantage. After cycling, as the side products passivate the electrode interfaces, the effective cathode surface is decreased and Li+ transport pathway is hindered, thus the S cathode processes larger R1 and Rct . Based on this point, the introduction of AgI and the presence of Ag and AgI help improve the electrochemical performance.
Figure 6. Nyquist plots and corresponding equivalent circuit model of the (a) S/AgI-90 and (b) S electrodes before and after cycling.
Download figure:
Standard image High-resolution imageConclusion
In summary, we have fabricated S/AgI composites as cathode materials by a facile chemical process for Li–S battery. With high sulfur content of up to 90.4%, the electrochemical performance of the S/AgI electrode has been effectively improved due to the presence of Ag and LiI. In particular, the Coulombic efficiency and cycling stability are improved by optimizing the conductivity and inhibiting the shuttle effect of polysulfides. The S/AgI-90 cathode exhibited a discharge capacity of 502.1 mAh g−1 after 500 cycles, accounting for a capacity decay rate of only 0.092% per cycle, and the Coulombic efficiency remained above 96.2% during the whole cycles at 0.5 C. These electrochemical performances are significantly better than the pure sulfur cathode without AgI, suggesting the S/AgI composite would be a promising candidate for the Li–S battery. This work proposes a reactive metal halide as a host material for the cathode of Li–S battery. It is necessary to further optimize the electrode structure and investigate the effect of halides for the better electrochemical performance of the Li–S system.
Acknowledgments
Financial support from the National Natural Science Foundation (22075151, 21373118) and Fundamental Research Funds for the Central Universities of China are gratefully acknowledged.