Abstract
Increasing the compaction density of electrodes is an effective way to increase the volumetric energy density of the full battery. To avoid the problem of low compaction density, material breakage, and porosity decrease during the process of calendering, which greatly deteriorate the electrochemical performance of the battery, in this paper, for the first time, we use ultrasonic vibration-assisted densification technology combining with thermal drying technology to prepare the electrodes. Ultrasonic vibration reduces the friction among the powder particles during the drying process, so that the particles of active materials could aggregate more closely. And then, there are smaller displacements and sliding during the calendering process in ultrasonic vibration-assisted (UV–A) electrode than ordinary ones, which not only avoid the failure of the binder and the shedding of the carbon layer, also increase the number of connection points between material particles. The results show that the UV–A densification technology leads to improved volumetric energy density (increased by 5.3%, 6.6%, 7.6%, 11.6%, 13.4%, and 13.0% compared to the ordinary battery at 0.2C, 0.5C, 1C, 2C, 5C, and 10C, respectively), cycle performance and ion conductivity of full battery.
Export citation and abstract BibTeX RIS
The mass application of Li-ion batteries (LIBs) in digital products and electric vehicles (EVs), requests overcoming the bottlenecks of current LIBs, e.g., safety, lifetime, fast-charging, cost, and optimized volumetric energy density.1–3 Since these devices leave a limited volume for the LIBs, improving the volumetric energy density of LIBs is the fundamental way to improve the endurance time.4–6 To achieve this goal, the electrodes, as the staple power storage components in a battery, have been studied in various aspects, such as changing the ratio of raw material, particle sizes, morphologies, and the thicknesses of electrodes.7–13 For example, Zheng10 et al. reported a cathode electrode consisting of LiNi0.8Co0.15Al0.05O2 particles, polyvinylidene difluoride, and acetylene black, wherein the volumetric energy density of the electrode was enhanced by using a higher percentage of active material. Xiao11 et al. studied the influence of particle sizes and morphologies on the electrochemical performances of the LiMn2O4 cathode, and revealed that the volumetric energy density of LiMn2O4 sample with the middle size spherical particles was higher than the cubic particles. Thick electrodes, which could increase the volumetric energy density and reduce the cost of the battery, have attracted extensive attention from researchers. For instance, Singh12 prepared a series of graphite negative electrodes and NMC positive electrodes with thicknesses from 70 μm to 305 μm to explore the relationship between the electrode thickness and energy density, it was found that the batteries with thicker electrodes have a higher volumetric energy density, Danner13 et al. used a detailed 3D micro-structure resolved model finding that the use of thick electrodes in LIBs given the possibility of reducing the production cost and improving the volumetric energy density. Although the above methods improved the volumetric energy density of LIBs, they reduced the electrochemical performance of the battery, such as the rate performance, cycle performance, and thermal stability, etc.7–13
Ultrasonic vibration-assisted (UV–A) densification processes are usually used in the metal and ceramic powders processing to enhance the powder compact density.14–19 A lot of scholars have explored the densification mechanism of ultrasonic compaction. For instance, Abedini20 founded that the applying of UV–A densification manufacturing processes resulting in a higher relative density and an improved homogeneity of AA1100 aluminum powders under constant applied stress. Hwang21 revealed that in the process of powder compaction, ultrasonic vibration reduce the internal friction between the powders and the compaction die, as well as the internal friction between the powders, resulting in the powders slipping and rearranging, thus improving the powder density. Cha22 proved the three stages of ultrasonic vibration-assisted densification. At the first stage, the powders were compressed in the mold and subjected to vertically applied pressure. The pressure was applied to overcome the obstacle friction between the mold and particles as well as the friction between the powders, at the second stage, the ultrasonic vibration was applied to the mold, the contact time between the powders and mold decreasing, leading to the reduction of friction between the mold and the powders, besides, the friction of powders could be reduced by the particle resonance, which was induced by ultrasonic vibration, finally, at the last stage, it can be expected that the reduction of friction would increase the powder compact density.
Increasing the compaction density of the electrodes is a feasible method to increase the volumetric energy density of the LIBs.23,24 However, in the process of calendaring, blindly increasing the pressure from the roller press or the number of rolling will reduce the pole piece porosity and arouse fragmentation of active particles. Low porosity will cause the insufficient wetting of the electrolyte, which will affect the cycle performance of the LIBs, and the fragmentation of active particles will also lead to the poor electrochemical performance of LIBs.25–29 In this study, for the first time, we applied ultrasonic vibration-assisted densification technology in the process of electrodes preparation to increase the degree of electrodes densification, thereby increasing the volumetric energy density of the battery. Compared with the traditional ways (by overly increasing the rolling pressure and the number of rolling times) to increase the compaction density of electrodes, the ultrasonic vibration-assisted densification technology could greatly improve the compaction density of the electrodes without compromising the battery cycle life. The ultrasonic vibration assist densification technology not only increases the life of the battery by 13.0% than ordinary electrodes, but increases its volumetric energy density by 13.0% at 10C.
Experimental
Preparation of electrodes
Cathode slurry was prepared by dispersing the LiFePO4 powders (LFP, Litao China, 94 wt%), Polyvinylidene Fluoride (PVDF, Solvay 5130, 3 wt%), and Super P (Junyi, Shanghai, 3 wt%) in N-Methyl-2-pyrrolidone (NMP) with a planetary mixer (DYG-170-100l, Da Li, China), the solid content of the slurry was 48% and the viscosity was around 7500 mPa s. After high-speed stirring (4,000 rpm) for 8 h, the slurry was spread onto aluminum foil current collector (16 μm thickness) by coating machine (M12-650A-4C-DZ, Beijing, China). After that, transferring the electrodes to an ultrasonic vibration oven (DYG-132PJ, Da Li, China) with the ultrasonic vibration frequency of 40 kHz and the temperature of 120 degrees Celsius, 10 min later, turning off the ultrasonic vibration and continuing baking the electrodes at 120 degrees Celsius for 6 h to remove the NMP solvent, and these electrodes were named UVE. In contrast, the electrodes without ultrasonic vibration during the baking process were named WVE.
Assembly of cells
14500-type cells were assembled with LiFePO4 cathode as the positive electrode and graphite anode (Xinli, Shanghai, China) as the negative electrode to study the impact of UVE on the full battery, Celgard2400 (Xinli, Shanghai, China) was used as the separator, all cells were assembled with a solvent mixture of 1 M LiPF6/ethyl carbonate (EC) + dimethyl carbonate (DMC) (1:1, v/v) as the electrolyte (AR, Jinniu, Tianjin) under an argon-filled glove box.
CR2032 coin cells were assembled to study the difference between UVE and WUE after cycle tests. 14500-type cells were disassembled after 2,000 cycles in an argon-filled glove box, then, 8 mm ∅ discs were harvested from the positive electrode and washed with DMC. Drying the discs and assembled to CR2032 coin cells with the lithium foil as the counter electrode. The assembly environment and the materials used were the same as the 14500-type cells.
Electrochemical measurements
The electrochemical tests were performed via a battery test system (CT-3008W, Shenzhen, China). Prior to the electrochemical performance study of 14500-type cells, the following formation technique was employed: 0.05C constant current charging for 240 min, 0.1C constant current charging for 120 min, and 0.2C constant current charging for 180 min. Before testing the rate and cycle performance, the cells were aged about 48 h after the formation process.
Five cells were prepared for each test to ensure the reproducibility of the results. For the full cells, the rate tests were charged at various C rates (0.2C, 0.5C, 1C, 2C, 5C, and 10C) to 3.65 V, kept at 3.65 V until the current decreases to 0.05C (CC-CV protocol), and discharged at various C rates (0.2C, 0.5C, 1C, 2C, 5C, and 10C) to 2.50 V. The cycling tests were conducted at 1C for both charge and discharge between 3.65 V and 2.50 V at 25 °C, and using the internal resistance tester (GDBT-8610P, Xigao, China) to measure the internal resistance of the cells every 100 cycles. For the coin cells, after two charge and discharge cycles at 0.2C and 25 °C, the cells were charged to 50% SOC for electrochemical impedance spectroscopy (EIS) tests over the frequency range 0.1 ∼ 1 × 105 Hz at 25 °C, and the cyclic voltammetry (CV) tests were performed at a scanning rate of 0.1 mV s−1 between 2.3 and 4.2 V on an electrochemical workstation (CHI660E, Shanghai, China) at 25 °C.
Electrode characterization
The surface microstructure and morphology of electrodes were observed using Transmission Electron Microscope (TEM, JEOL, Japan) and Scanning Electron Microscope (SEM, FEI, Hong Kong). The 3 M tape was used to test the adhesion of LFP particles on the electrodes. To quantify adhesion, the peel strength of the electrodes was measured by 180° peel strength tests using a tension machine with a 10 N load cell (MX-0580, Jiangsu, China) based on the ISO 2409:2007 standard. The calendered electrodes were cut into strips 19 mm * 70 mm and then attached to the sample stage by a double-sided adhesive tape (Scotch 410 M, 3 M). The peel speed was set to 10 mm per minute. For each electrode, five samples were tested. Both cathodes were calendered by Roller Press (DYG-703BH, Dali, China), four-probe resistivity tester (RTS-4, Shenzhen, China) was used to test the electrode resistance before and after calendering.
Results and Discussion
Sample characterization
The surface morphology and microstructure of electrodes were observed by SEM. As shown in Fig. 1a, for UVE sample, the secondary particles of LFP with different diameters are evenly distributed on the electrode. The particles with small diameters are filling up the gaps between the large particles, and there are more contact area between the particles and fewer pores, under the action of the conductive agent and the binder, the adhesion and conductivity between LFP particles may be stronger. In Fig. 1c, the small particles are not completely filling up the gaps between the large particles, resulting in lots of pores appear in the WVE sample, and there aren't close contact between the particles, which may lead to the poor adhesion and electrical conductivity in WVE sample. It can be seen from Figs. 1b and 1d that fewer pores appear in UVE sample than WVE sample after calendaring, meaning that the compaction density of UVE is higher than WVE. Due to more pores existing in itself, the secondary particles in WVE sample show more displacement and sliding during the rolling process, which may exceed the bonding limitation of the binder, resulting in the failure of the binder.
Figure 1. SEM images of LiFePO4/C electrodes before (a) UVE, (c) WVE and after (b) UVE, (d) WVE calendaring.
Download figure:
Standard image High-resolution imageCalendering is the common compaction process for battery electrodes, which has a substantial impact on the electrodes structure and the electrochemical performance of the lithium-ion batteries. The loading mass of both electrodes is around 0.0266 g cm−1. As shown in Fig. 2a, the average thickness (without collector) before the calendering of the UVE sample (185 μm) is 10.6% thinner than that of the WVE sample (207 μm), and 10.4% after calendering, the thinner thickness means higher electrode density and volumetric energy density in UVE battery. The average density before calendering of the UVE sample (1.65 g cm−3) is 11.5%, which is thinner than that of the WVE sample (1.48 g cm−3), and the value is 11.9% after calendering. The most obvious change is the electrode resistance, as shown in Fig. 2c, the average resistance before the calendering of the UVE sample (98 Ω) is 29% smaller than that of the WVE sample (138 Ω), and 42% after calendering, which shows the corresponding results to the SEM tests and the electrode adhesion tests. According to the above results, it can be inferred that the ultrasonic vibration could make the distribution of the LFP particles more uniform during the drying process, the small secondary particles are filled in the gaps between the large particles, thus greatly improves the space utilization efficiency, meanwhile, there are more contact points and closer distance between LFP particles, which greatly improve the conductivity of the electrodes.
Figure 2. (a) Thickness, (b) density and (c) resistance of both electrodes before and after calendering.
Download figure:
Standard image High-resolution imageSuperior adhesion maintains the structural stability of the electrode, which plays a key factor in achieving excellent electrochemical performance. As shown in Figs. 3a and 3b, the adhesion of the electrodes with different drying methods were measured by 3 M tape peeling tests with calendered electrodes. It can be seen that the active materials can be detached from the collector easily from WVE sample but difficult from UVE sample. The specific adhesion values were given by the peel strength tests, as shown in Figs. 3c and 3d, the average peel strength of the UVE sample (231.9 N m−1) is 25.5% higher than that of the WVE sample (184.8 N m−1 ), illustrating that the addition of ultrasonic vibration technology can greatly improve the adhesion between the LFP particles and the current collector. PVDF behaves more like an elastic-viscoplastic material in battery,30 when the displacement and sliding levels of LFP particles reach critical values, the binder will undergo permanently plastic deformation followed by fracture failure. The larger displacement during the calendering process will cause PVDF plastic deformation, thereby reducing the adhesion of the electrode. For the UVE sample, due to the reduction of pores, the LFP particles will move less during the calendering process, which increased the bonding effect of the adhesive. For the WVE sample, the larger displacement and sliding of LFP particles appear due to the existence of more pores, which will deteriorate the bonding effect of PVDF.
Figure 3. The adhesion strength measurement of the calendered electrode made using UVE and WVE. (a) and (b) are the surfaces of the electrodes after 3 M tape peeling test corresponding to UVE and WVE, respectively. (c) Plots of peel strength of the electrode strips. (d) Two typical curves of the peel strength-extension.
Download figure:
Standard image High-resolution imageMechanism Analysis of UV–A densification manufacturing during the production of electrodes
In order to verify the improvement of particle accumulation in electrode, which was caused by UV–A densification manufacturing, the structural diagrams of UVE sample (Fig. 4a) and WVE sample (Fig. 4b) are exhibited. During the drying process, the LFP powders are subjected to the common effects of their own gravity, the frictional force, the bonding force from adhesive, and strain stress to achieve a force equilibrium state. The relationship between them is given in the formula below:
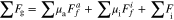
Where,
Figure 4. Schematic diagram of UVE and WVE, and particle movement (c) before and (d) after calendering process in electrode.
Download figure:
Standard image High-resolution image
 gravity of LFP secondary particles.
gravity of LFP secondary particles.
 adhesive force between PVDF and LFP secondary particles.
adhesive force between PVDF and LFP secondary particles.
 friction between LFP secondary particles.
friction between LFP secondary particles.
 formation force result from strain stress.
formation force result from strain stress.
μa: adhesive coefficient between PVDF and LFP secondary particles.
μi: friction coefficient between LFP secondary particles.
As the solvent slowly evaporates during the drying process, many pores will be formed in the electrode, as shown in Fig. 4b. When the ultrasonic vibration is applied to the electrode preparation, high-frequency vibration could reduce the contact time between particles, thereby reducing the friction between particles,21,22,31 at the same time, the equilibrium state of force is broken, the particles move toward the direction of smallest potential energy under the action of gravity, when the solvent is completely evaporated, active particles with different size are interlaced and bonded together, so there will be fewer pores and more contact points in UVA sample (as shown in Fig. 4a), which enhance the adhesion of LFP secondary particles and density in the UVE sample.32 Due to the use of lithium iron phosphate secondary spherical particles, this tight accumulation will not affect the diffusion rate of lithium ions, which has been proved in the EIS tests. The calendaring principle of the electrode in the rolling process is shown in Fig. 4c, the applied force from the roller press occurs to progress the densification of electrode compact. Under the pressure of the roller press, the LFP particles will fill up the pores.33 Owing to the fewer pores, the particles show less displacement and sliding during the calendering process in UVE than WVE sample, consequently, there is more adhesive failure followed by particles shedding in WVE sample.
Electrochemical performance tests of both electrodes
To demonstrate the excellent electrochemical performance of the UVE sample, the electrochemical tests of UVE sample were compared with those of WVE sample. Figure 5a shows the discharge curves of both samples at different C-rates: 0.2C, 0.5C, 1C, 2C, 5C, and 10C. As the discharge current density increased, the specific discharge capacity of the LFP particles decreased, as shown in Fig. 4a, the discharge capacity of WVE sample decreased from 150.6 mAh g−1 at 0.2C to 102.5 mAh g−1 at 10C, which may be attributed to the low conductivity due to the loose bonding between LFP particles. However, UVW sample shows superb rate capability with the discharge capacities of 151.1, 145.9, 139.5, 132.7, 129.6, and 113.1 mAh g −1 at 0.2C, 0.5C, 1C, 2C, 5C, and 10C, respectively. Furthermore, as shown in Fig. 5b, UVE sample provides much higher volumetric energy density than WVE sample (increased by 5.3%, 6.6%, 7.6%, 11.6%, 13.4%, and 13% at 0.2C, 0.5C, 1C, 2C, 5C, and 10C, respectively), which may be attributed to the reasons that the particles are more tightly bound and the charge transfer is easier in UVE sample. Moreover, the rate capabilities of the two samples are exhibited in Fig. 5c. UVE sample delivers higher specific discharge capacity at different current rates. 75.8% capacity retention of 0.2C is maintained at 10C for UVE sample, compared to 68% for that of WVE sample, which means that the UVE sample has a better rate capability than WVE sample.
Figure 5. (a) Discharge curves, (b) volumetric energy density and (c) rate capabilities of both electrodes at different current rates, (d) cyclic performance and (g) internal resistance of both electrodes in cyclic test. DQ/dV curves of (e) UVE and (f) WVE in cyclic test.
Download figure:
Standard image High-resolution imageAdditionally, the cyclic performance of both samples was tested at 1C/1C and room temperature (25 °C), as shown in Fig. 5d. It can be seen that the cyclic stability of UVE sample (91% ) is 13% higher than that of the WVE sample (78%) after 2,000 cycles, implying it's structure is more stable during the cyclic tests. Methods for characterizing and optimizing the internal resistance of electrodes are crucial for achieving the simultaneous goals of high energy density and long cyclic life in lithium–ion batteries, as shown in Fig. 5g, the UVE sample (increased by 51.7 mΩ) show smaller internal resistance increase than WVE sample (increased by 103.8 mΩ) during the test, indicating that the application of ultrasonic vibration electrode could reduce the internal resistance and improve the cycle performance. In order to understand the difference in cyclic life of both type of cells, the differential capacity dQ/dV was plotted against cell voltage over various cycles and shown in Figs. 5e and 5f. Differential capacity measurements vs potential and cyclic number offer greater sensitivity to probe cell degradation over a cyclic life test. Figure 5f shows the differential capacity vs cell voltage at various cyclic numbers for WVE cell, as the test proceeds, the dQ/dV peaks for Li-ion delithiation shift to higher voltages, while the dQ/dV peaks for Li-ion intercalation shift to lower voltages. In addition, the dQ/dV peak magnitude decreases with cycles. The decay of dQ/dV peak indicate the loss of lithium in LFP particles.34 The shift of dQ/dV peaks may resulted from the struction destruction of electrode in cyclic test. However, UVE cell shows less dQ/dV peak decay and less dQ/dV peak shift (Fig. 5e) compared to WVE cell (Fig. 5f), which may result from the less loss of lithium in LFP powders and less structure destruction of electrode in cyclic test.
The 14500 type batteries were disassembled in a glove box filled with argon to analyze the difference of samples undergoing the cyclic test, the positive electrode was rinsed with DMC solution, and then, it's cross-sectional shape was observed with SEM and TEM, as shown in Fig. 6. It can be seen from Figs. 6a and 6b that the LFP particles on the UVE sample are still tightly bonded to the current collector, but there are a number of transverse cracks and longitudinal cracks on the WVE electrode, in addition, the LFP particles on WVE sample have an obvious tendency to fall off, which may be the root reasons of the worse cyclic life. Furthermore, it can be seen from Figs. 6c and 6d that the more amorphous carbon layer adheres to the surface of LFP particles in UVE sample, but less in WVE sample. The lack of the amorphous carbon layer may be owing to the lack of tight adhesion between the carbon layer and the LFP particles during the sliding process, and the greater amount of displacement and sliding during the rolling process in WVE could make the carbon layer easier to fall off, which will greatly affect the electrochemical performance of the WVE battery.
Figure 6. SEM images of (a) UVE and (b) WVE after cyclic test. TEM images of (a) UVE and (b) WVE after cyclic test.
Download figure:
Standard image High-resolution imageCoin cells were prepared by cycled electrodes to explore the diffusion kinetics of both electrodes. 8 mm ∅ discs were harvested from cycled UVE and WVE samples, respectively, and one knife was used to scrape off all the attachments on one side of the discs, and then assembling them with lithium chip to form CR2032 coin cells. The EIS tests were carried out to analyze the diffusion dynamics of both samples, and the corresponding Li-ion diffusion coefficient was calculated by software fitting. The results are demonstrated in Figs. 7a, 7b, and Table I. Figure 7a shows the Nyquist plots for both samples and the inset is an equivalent circuit, the Nyquist plot is composed of a semicircle in the high-frequency region and a straight line in the low-frequency region. The ohmic resistance (Re) of the cell is represented by the intercept of the curve and the real axis Z', which mainly attributes to the resistance of the cell module and electrolyte. Moreover, the semicircle in the high-frequency region corresponds to the charge transfer impedance (Rct) result from the impedance caused by the migration of charge between the LFP particles and the electrolyte interface. The Warburg impedance (W) is represented by the straight line in low frequency, which is due to the diffusion of Li-ion in the electrodes.35,36 Z/view software was used to play the curve fitting based on the equivalent circuit. It can be seen from Fig. 7a that the charge transfer resistances of UVE and WVE cells are 27.37 and 46.31 Ω. It is clear that UVE cell exhibits lower charge transfer resistance, indicating that the ultrasonic vibration process can greatly reduce the interfacial resistance. Besides, the Li-ion diffusion coefficient is closely related to the Warburg coefficient (σ), which can be calculated according to the formula:
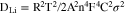
Where,
Figure 7. (a) Nyquist plots of both electrodes after cyclic test and (b) Variation and fittings between Z' and the reciprocal square root of the angular frequency in the low frequency region. (c) Cyclic voltammograms of UVE and WVE after cyclic test.
Download figure:
Standard image High-resolution imageTable I. Charge transfer resistance Rct, Warburg factor σ and Li-ion diffusion coefficient DLi.
| Samples | Rct/Ω | σ/Ω s−1/2 | DLi/cm2 s−1 |
|---|---|---|---|
| UVE | 27.37 | 36.78 | 1.6 × 10−13 |
| WVE | 46.31 | 60.32 | 5.9 × 10−14 |
R: ideal gas constant.
T: absolute temperature.
A: active area of the electrode.
n: the number of electrons lost or reduced in each molecule.
F: Faraday constant.
C: molar concentration of Li-ion intercalated in the LFP particles.
σ: Warburg coefficient, which is obtained by fitting a straight line composed of the low frequency regions Z' and ω−1/2.
The Warburg coefficient (σ) is represented by the slope of the fitted straight line, and ω represents the angular frequency of the low-frequency region. The relationship between Z' and ω−1/2 exhibited by the following formula.
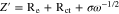
Under the assumption of the dynamic limitations related to electrode's resistance are negligible, as shown in Table I, Li-ion diffusion coefficients of both electrodes can be calculated to be 1.6 × 10−13 and 5.9 × 10−14 cm2 s−1 for UVE and WVE cells, respectively. UVE cell exhibits higher Li-ion diffusion coefficients than WVE cell, which can be attributed to the shedding of the LFP particles from electrode accompanying the shedding of the carbon layer from LFP particles. These results above are well consistent with that the ultrasonic vibration process makes the electrode possess better cyclic stability and rate capability.
CV measurements were conducted to investigate the electrode kinetic parameters of both electrodes reaction, as shown in Fig. 7c. Each electrode shows a set of peaks around 3.4 V, consisting of an oxidation peak (charge) and a reduction peak (discharge), which corresponds to lithium-ion intercalation and deintercalation. The CV profile of UVE presents the more symmetric and the sharpest redox peaks than the WVE, demonstrating that UVE sample shows higher electrochemical reaction activity after 2,000 cycles. In addition, the differences between the redox peaks of both electrodes are 0.373 V and 0.589 V for UVE and WVE samples, respectively. WVE sample exhibits higher polarization, which may result from the blocking of electrons conduction induced by the shedding of LFP particles. The electrode with ultrasonic vibration process added during the drying process exhibited more stable conduction pathway for electronic and lithium-ion.
Conclusions
This comprehensive study demonstrates that ultrasonic vibration-assisted densification technology can yield a significant improvement in the mechanical and electrochemical performance of electrodes. The molecular models proposed (Fig. 4) appear to be corroborated by all physical and electrochemical analyses conducted. During the drying process of the electrodes, the powders are subjected to the common effects of gravity, friction, adhesion forces, and strain stress to achieve a force equilibrium state. Ultrasonic vibration changes this equilibrium state and reduces the friction force among the powders so that the particles move toward the direction of the smallest potential energy under the action of gravity. The contact point of the powders increases and the LFP particles are closely bonded together under the action of the binder. Therefore, compared with the ordinary electrodes, the ultrasonic vibration-assisted electrodes exhibit a stronger bonding force of the electrode and lower resistivity. Due to the fewer pores in the electrode, the particles also perform less displacement and sliding during the calendering process, in contrast, the larger displacement and sliding of the particles in the ordinary electrode causes more binder failure and carbon layer shedding, so the ultrasonic vibration-assisted electrodes show a higher calendaring density and volumetric energy density. This study has a great significance for improving the volume energy density of lithium-ion batteries and increasing their cycle life.
Acknowledgments
The authors are grateful to the Tianjin Technical Expert Project (grant number 19JCTPJC43900) for the financial support to this work.








