Abstract
In this study we systematically investigated the impact of aprotic and protic ionic liquids on the electrochemical behavior of poly(2,2,6,6-tetramethylpiperidinyloxy methacrylate) (PTMA) based electrodes. We showed that the use of aprotic ILs based on the FSI− anion enables the realization of electrodes able to display high capacity, good capacity retention at high current density (42% at 50 C), low self-discharge and high stability during float tests. We also reported, for the first time, that it is possible to successfully use PTMA-based electrodes in combination with PILs. We showed that the PTMA electrodes display good capacity but a limited cycling stability in these ionic liquids. This investigation confirms that ionic liquids are very interesting candidates for the realization of high performance PTMA-based electrodes. A deeper understanding of the interactions taking place between ionic liquids and organic active materials will be required to finely tune the properties of these promising electrolytes for an application in organic radical polymer-based energy storage systems.
Export citation and abstract BibTeX RIS
In the last years, the interest on the use of organic radical polymers (ORPs) in electrochemical storage devices increased constantly. ORPs hold a stable radical in their redox active centers, which provides a fast electron exchange reaction with fast kinetics and high power densities.1–4 The first and most investigated ORP for the application in energy storage is poly(2,2,6,6-tetramethylpiperidinyloxy methacrylate) (PTMA) introduced by Nakahara et al. in 2002.5 PTMA bears a (2,2,6,6-tetramethylpiperidin-1-oxyl)-(TEMPO-) group attached to the polymer chain. During the charging process, this redox active TEMPO-moiety alters from the electroneutral state (free stable radical) to the positive state (oxoammonium cation).1 Therewith PTMA belongs to the p-type polymers, and during the charging process anions need to be inserted in its structure in order to compensate the generated charge.6 Taking this point into account, it is evident that the nature of the salt present in the electrolyte, and in particular of the anion, is significantly affecting the dynamics of the charge process and, consequently, the electrochemical behavior of these materials.7
To date, PTMA-based electrodes have been mostly investigated in combination with electrolytes for lithium-ion batteries (LIBs) and the state-of-the-art electrolyte of these devices, 1 M lithium hexafluorophosphate in ethylene carbonate/dimethyl carbonate (1 M LiPF6 in EC/DMC), can also be considered the state-of-the-art electrolyte for PTMA-based systems. It has been shown that the use of these electrolytes allows the realization of PTMA-based electrodes displaying a stable voltage plateau (3.5 V vs Li/Li+), a specific capacity close the theoretic value (111 mAh g−1) and high rate capability.8–14 However, LIB electrolytes are not designed for the specific needs of organic radical materials, and their use is causing some serious drawbacks like high self-discharge12,15,16 and dissolution of the active material into the electrolyte, which are negatively affecting the cycling stability of these materials.17,18 In order to overcome these limitations, "alternative" electrolytes for organic active materials,19,20 e.g. high concentrated organic electrolytes,21–23 water-in-salt electrolytes,24–26 highly viscous electrolytes,27,28 ionic liquids (ILs),29 polymer electrolytes30–36 or solid state electrolytes,37,38 have been proposed in the last years. These studies indicate that the use of highly viscous electrolytes might improve the cycling stability32,39,40 and reduces the self-discharge41 of PTMA-based electrodes. Recently, it has been also shown that ILs, due to the occurrence of dispersion force, are stabilizing the free electron of TEMPO.42
Considering these points, ILs can therefore be considered as promising electrolytes for PTMA, but more in general for ORP-based systems. Nonetheless, it is important to notice that a systematic investigation about the influence of the nature of the cation, anion (and their combination) of ILs of the electrochemical behavior of PTMA-based electrodes has not been carried out so far. Furthermore, to the best of our knowledge, until now only aprotic ILs (AILs) have been considered, while no studies have been dedicated to the use of protic ILs (PILs) in combination with PTMA-based electrodes. PIL-based electrolytes display transport and thermal properties similar to those of AILs but, due to the presence of an additional proton in their cation structure, have markedly different ion-ion interactions compared to the latter.43 In the last years it has been shown that PILs are interesting electrolytes for supercapacitors and LIBs.44–51 For this reason, the investigation of PIL-based electrolytes in combination with PTMA-based electrodes appears important in order to understand whether this class of ILs can also be introduced in organic batteries.
In this work we present an investigation about the impact of four different ILs, namely 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (Pyr14TFSI, AIL), 1-butyl-1-methylpyrrolidinium bis(fluorosulfonyl)imide (Pyr14FSI, AIL), 1-butyl-pyrrolidinium bis(trifluoromethane-sulfonyl)imide (PyrH4TFSI, PIL) and 1-butyl-pyrrolidinium bis(fluorosulfonyl)imide (PyrH4FSI, PIL), on the electrochemical behavior of PTMA-based electrodes. These ILs have been selected because their use enables a systematic investigation of the influence of the anion and cation nature on the electrochemical behavior of PTMA. At the same time, a direct comparison of the impact of AILs and PILs on the performance of PTMA-based electrodes is possible.
Experimental
The synthesis of PTMA has been carried out as described in Ref. 52, leading to a polymer with a radical content of 90%, as determined with electron spin resonance (ESR, Bruker). The electrode composition resulted to 60% of active material (crosslinked PTMA) with 35% of conducting agent (SuperP®, Alfa Aesar) and 5% of binder (polyvinylidene fluoride (PVdF, Sigma Aldrich). All components were mixed in N-methyl-2-pyrrolidone (NMP, TCI) with a dissolver (Dispermat®, VMA-GETZMANN). The slurry was casted with a doctor blade (250 μm wet film thickness) on an aluminum current collector and dried in a drying oven with air circulation overnight at 80 °C. The electrode area was equal to 1.13 cm2 and the mass loading was on average 0.7 mg cm−2. As counter electrodes, oversized free-standing activated carbon-based electrodes have been prepared using a procedure identical to that reported in Ref. 53. The aprotic ionic liquids utilized in this investigation were purchased from IoLiTec (Ionic Liquids Technology, Germany). Protic ILs were synthesized similar as described in Ref. 49. All ILs were used neat. The water content of all ionic liquids was lower than 20 ppm, as measured by Karl Fischer titration. The ILs were stored in a glove box (LabMaster, MBRAUN GmbH) under argon atmosphere with a water and oxygen content below 0.1 ppm. The viscosity of the electrolytes was measured with a rheometer (Anton Paar, MCR 102) using a shear rate of 1000 1/s, while their conductivity was determined by impedance spectroscopy using a Modu-Lab XM ECS Electrochemical Test System (AMETEK Scientific Instruments). Both investigations have been carried out at 20 °C. All electrochemical tests reported in this study have been carried out utilizing a Swagelok-cell type with a three electrodes configuration, in which an Ag wire was used as a quasi-reference and a glass fiber sheet (Whatmann), drenched with 160 μl of electrolyte, was used as separator. Cyclic voltammetry (CV) was performed using a scan rate of 2 mV s−1. Galvanostatic charge-discharge cycling (CC) was carried out between 0.3 V and 1.4 V vs Ag (which corresponds to 3.3 V and 4.4 V vs Li/Li+) using current densities ranging from 0.2 C to 100 C (1 C has been defined considering the theoretical capacity of PTMA, 111 mAh g−1). The self-discharge of PTMA-based electrodes was recorded by fully charging (to 1.4 V vs Ag) the electrodes at 1 C and afterwards monitoring the residual stored charge of the active material by galvanostatic discharge for seven days at room temperature. Float tests were performed by charging the PTMA-based electrodes to 1.1 V vs Ag and keeping this voltage for a total of 70 d. In order to monitor the changes over the time of the experiment, every 10 h charge/discharge cycles with a current density of 5 C were carried out.
All electrochemical measurements were carried out using a VMP multichannel potentiostatic-galvanostatic workstation (Biologic Science Instruments, VMP 3) or an Arbin potentiostat-galvanostat workstation (Arbin instruments, LBT21084) at room temperature (if not indicated otherwise). Before starting the actual measurements, 3 h of open circuit voltage (OCV) were recorded to set the systems into an equilibrium.
Results and Discussion
Figure 1 compares the viscosity and the conductivity of the 4 ILs investigated in this work: The aprotic ILs Pyr14TFSI and Pyr14FSI and the protic ILs PyrH4TFSI and PyrH4FSI. In the past years these ILs have been utilized as electrolytes for supercapacitors and LIBs.24,47,49,51,54–57 However, to the best of our knowledge, Pyr14TFSI is the only one that until now has been used in combination with organic redox active materials.29,32,40 The values reported in Fig. 1 are given for 20 °C, except for PyrH4TFSI, which is solid at room temperature. For this reason, the values relative to this PIL are referring to 40 °C. This latter temperature has been also utilized for all electrochemical tests carried out with this PIL. As shown, Pyr14TFSI displays the highest viscosity and the lowest conductivities of all investigated ionic liquids (90 mPa s 2.2 mS cm−1, respectively, at 20 °C).47 The substitution of the TFSI− anion with the FSI− is considerably decreasing the viscosity (58 mPa s) and increasing the conductivity (5.32 mS cm−1 ), making Pyr14FSI an IL with promising transport properties.47 The trend observed in the AILs is also observed in the PILs. At 40 °C PyrH4TFSI displays a viscosity of 60 mPa s and a conductivity of 2.7 mS cm−1.51 On the other hand, PyrH4FSI displays at 20 °C a viscosity of 35 mPa s and a conductivity of 5.3 mS cm−1.49 Taking these values into account, it appears that the viscosity and the conductivities of the investigated ILs are mainly determined by the nature of the anion, which is in good agreement with literature.58–60 Furthermore, the use of FSI− enables the realization of ILs with improved transport properties compared to TFSI−. In the meantime, the use of the cations Pyr14+ and PyrH4+ is leading to ILs with very comparable properties. The overall electrochemical stability windows of the aprotic ILs are more than 5 V, while the windows for the protic ones are ca. 3 V44,47,51 (the ESW of PyrH4FSI is reported in the Supplementary Information S1 is available online at stacks.iop.org/JES/167/120546/mmedia). Due the presence of the proton, the cathodic stability of the investigated PIL is lower compared to their aprotic counterparts (ca. −1 V vs Ag for the PIL). Nevertheless, all ILs show comparable anodic stability (up to 2.5 V vs Ag). In the presented study, all experiments have been carried out at a potential of maximum 1.5 V vs Ag, which is within the stability of all ILs.
Figure 1. (a) viscosity and (b) conductivity of Pyr14TFSI, Pyr14FSI, PyrH4TFSI (40 °C), PyrH4FSI at 20 °C.
Download figure:
Standard image High-resolution imageFigure 2 shows the electrochemical behavior of PTMA-based electrodes used in combination with Pyr14TFSI and Pyr14FSI. Figure 2a compares the CVs (scan rate 2 mV s−1) of PTMA-based electrodes in these two AILs. As shown, in both of them the electrodes reveal a reversible single step redox peak. However, the nature of the used AIL strongly affects the potential at which the storage process is occurring as well as its according peak intensity. In Pyr14TFSI the cathodic peak is located at 0.75 V vs Ag, while the anodic peak can be found at a potential of 0.51 V vs Ag. This broad peak shift for oxidation and reduction is typical for highly viscous electrolytes. When Pyr14FSI is used, the cathodic and anodic peaks associated to the storage process are much closer (0.89 V vs Ag and 0.7 V vs Ag, respectively). Furthermore, the intensity of the peak is significantly increased, almost doubling. These latter results are clearly related to the low viscosity and higher conductivity of Pyr14FSI compared to that of Pyr14TFSI. It must be noted, nonetheless, that the use of both AILs guarantees a high coulombic efficiency. Figure 2b reports the voltage profile of PTMA-based electrodes in the two AILs as obtained by applying a current density corresponding to 1 C. As shown, also in the case of these experiments the nature of the used ILs has a strong influence on the potential at which the plateaus associated to the charge-discharge are located as well as on the specific capacity delivered by the electrodes. As a matter of fact, the electrode used in combination with Pyr14TFSI displays a charge plateau at 0.69 V vs Ag and delivers a specific capacity of 58 mAh g−1, while those used in combination with Pyr14FSI display a charge plateau at 0.8 V vs Ag, and deliver a capacity of 74 mAh g−1. Figure 2c compares the capacity retention of the PTMA-based electrodes utilizing current densities ranging from 0.2 C to 100 C. At 0.2 C the PTMA-based electrode cycled in Pyr14TFSI exhibits a specific capacity of 66 mAh g−1 (which has been set as the 100%. The absolute values of capacitance are reported the Supplementary Information S2). When the C-rate is increased to 1 C the electrode loses 15% of its initial capacity, and 63.5% at 20 C. At 50 C the electrode can still deliver some capacity (14% compared to the initial value), while at 100 C no capacity is accessible anymore. As expected, the use of Pyr14FSI improves the capacity retention of the PTMA-based electrodes. As observed above, the electrode capacity at 0.2 C is higher compared to that achievable with Pyr14TFSI and is equal to 76 mAh g−1 (which has been set as the 100%. The absolute values of capacitance are reported the Supplementary Information S2). At 1 C and 20 C the electrode displays a capacity of 67 mAh g−1 (88%) and 39 mAh g−1 (51%), respectively. At 100 C the PTMA-based electrode displays a capacity of 22 mAh g−1, which corresponds to ca. 29% of the initial capacity. From these results it is evident that Pyr14FSI is a very promising electrolyte in view of high-power applications. Nevertheless, it is important to notice that in both AILs, which have a very high viscosity compared to state-of-the-art organic electrolytes, the PTMA-based electrodes are able to deliver high capacity also at very high C-rates. After the rate capability tests, also the cycling stability at 1 C of PTMA-based electrodes has been investigated. As shown in Fig. 2d, the PTMA-based electrodes display an initial capacity of 54 mAh g−1 in Pyr14TFSI, which decreases to 50 mAh g−1 after 100 cycles. In Pyr14FSI the electrodes have an initial capacity of 63 mAh g−1, which is fully maintained after 100 cycles at 1 C. Therewith in both ILs the electrodes display high cycling stability. However, it has to be noticed that during the cycling process the coulombic efficiency of the charge-discharge process appears lower compared to the observed in the previous experiments, most likely due to the occurrence of some side reactions.
Figure 2. (a) Cyclic voltammetry at a scan rate of 2 mV s−1, (b) voltage profile at 1 C, (c) rate capability from 0.2 C to 100 C and (d) cycling stability at 1 C of PTMA-based electrodes in combination with Pyr14TFSI and Pyr14FSI as electrolytes (filled dots refer to specific capacity, empty to coulombic efficiency).
Download figure:
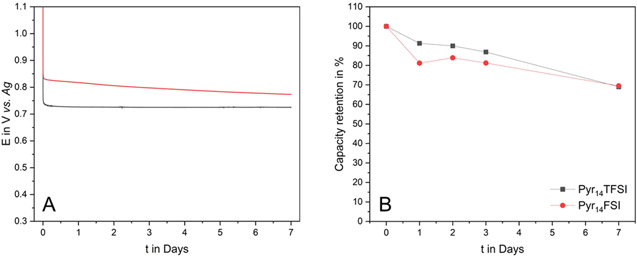
Standard image High-resolution imageAs mentioned in the introduction, the self-discharge of PTMA-based electrodes, and in general of ORPs is one of the drawbacks of these active materials. This process can be caused by the dissolution of active material into the electrolyte12 and in a recent work we showed that the nature of the electrolytes has a strong influence on the self-discharge.40,41 Therefore, also the impact of Pyr14TFSI and Pyr14FSI on this process has been evaluated. Figure 3a compares the self-discharge curves of the PTMA-based electrodes in the two electrolytes. From these curves it is possible to qualitatively evaluate the capacity retention of the electrodes over the time. If the potential of the electrodes remains at the redox potential of PTMA (0.7 to 0.8 V vs Ag), charge is kept inside the active material of the electrode. As soon as the voltage drops below this value the electrodes are completely discharged. Furthermore, the slope of the voltage curve indicates how fast the active polymer is discharged. The smaller the slope, the lower the self-discharge, the higher the capacity retention. In Fig. 3a a very stable potential is observed for PTMA in Pyr14TFSI at ca. 0.73 V vs Ag, which indicates an excellent capacity retention. Compared to this graph the potential of PTMA in Pyr14FSI is set to a higher value (0.8 V vs Ag). However, the slope of the voltage loss is higher than for Pyr14TFSI, which suggests a higher self-discharge for PTMA in this electrolyte. This is verified in Fig. 3b, which displays the capacity retention with respect to the time. Within the first three days PTMA shows a high capacity retention of ca. 90% when used in combination with Pyr14TFSI. In the meantime, ca. 81% are kept with Pyr14FSI as electrolyte. However, in both ILs the PTMA-based electrodes were able to keep ca. 70% of their initial capacity after seven days of self-discharge. This value is significantly higher than that observed in electrolytes containing organic solvents, indicating the use of ILs might significantly reduce the self-discharge of PTMA-based electrodes.
Figure 3. (a) Voltage excursion of PTMA in Pyr14TFSI and Pyr14FSI during 7 d of self-discharge and (b) Self-discharge of PTMA in Pyr14TFSI and Pyr14FSI at 1 C after 1, 2, 3 and 7 d.
Download figure:
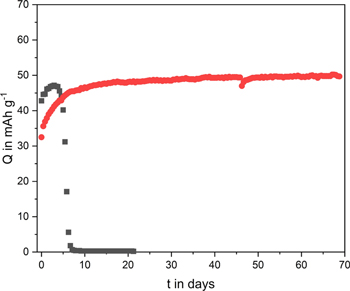
Standard image High-resolution imageAfter the self-discharge, also float tests have been carried out. These aging tests are widely used to determine the stability of supercapacitors (electrodes and/or devices) at a certain potential. Although they are not often used for the investigation of ORP-based electrodes, they represent valuable experiments to evaluate the stability over time also of these types of polymeric electrodes. Figure 4 compares the variation of the capacity of PTMA-based electrodes which have been hold at 1.1 V vs Ag for several days. The details of the experiments are reported in the experimental section. As shown, the PTMA-based electrode used in combination with Pyr14TFSI displays an initial specific capacity of 40 mAh g−1, which is a value comparable to that reported in Fig. 2c. During the first three days of float, the electrode in combination with Pyr14TFSI experienced an increase in capacity (up to 47 mAh g−1). Starting from the fourth day, however, a rapid decrease of capacity was observed and after eight days the electrode was no longer able to deliver any capacity. A very different behavior was observed for the PTMA-based electrodes cycled in Pyr14FSI. In this case, the electrode delivers an initial capacity of 32 mAh g−1, which is 20 mAh less than the values from Fig. 2c. A possible explanation for this is low capacity value could be the occurrence of some degradation process during the self-discharge test. However, very interestingly, the electrode capacity increased over time, and after 20 d a stable value of ca. 50 mAh g−1 is reached, which was then maintained (and even slightly increasing) for 50 d. These tests are clearly indicating that PTMA-based electrodes display an extremely high stability when used in combination with Pyr14FSI (corresponding to over 9300 cycles carried out at 5 C assuming a specific capacity of ca. 50 mAh g−1).
Figure 4. Capacity retention of PTMA in Pyr14TFSI and Pyr14FSI at 5 C after 70 d of stable potential steps at 1.1 V vs Ag.
Download figure:
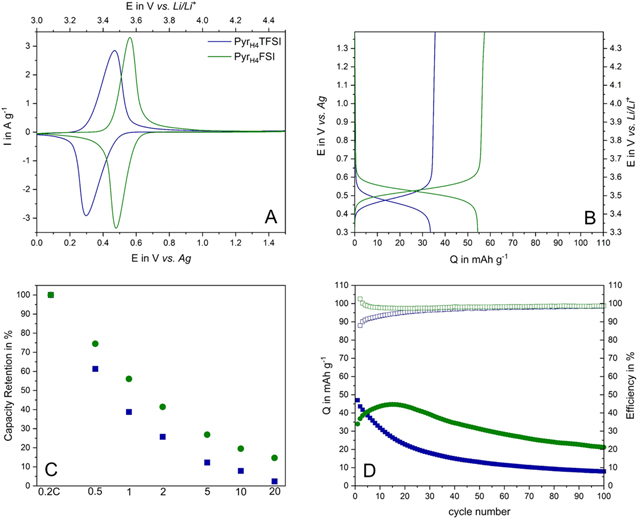
Standard image High-resolution imageAfter the investigation of AIL-based systems, the use of PILs in combination with PTMA-based electrodes has been investigated for the first time. As mentioned above, PIL-based electrolytes have been successfully used in supercapacitors and LIBs. The results of these studies indicate that the proton(s) present in the structure of these ILs can strongly influence the storage process taking place in these systems.43 The use of PILs in combination with PTMA-based electrodes appears therefore interesting to verify whether these electrodes can be cycled in a non-aqueous protic environment. As shown in Fig. 5, this is indeed the case. Figure 5a is comparing the CVs carried out at 2 mV s−1 of PTMA-based electrodes in PyrH4TFSI and PyrH4FSI. As shown, the use of both PILs enables a highly reversible charge-discharge process. However, it is interesting to notice that the cathodic and anodic peaks of these CVs are shifted towards lower potentials compared to the results observed in AILs. Specifically, in PyrH4TFSI the PTMA-based electrode displays a cathodic redox peak at 0.47 V vs Ag and an anodic peak at 0.3 V vs Ag. These values are ca. 0.2 V lower compared to that obtained in Pyr14TFSI. When PyrH4FSI is used, the cathodic and anodic peaks are set to 0.56 V and 0.48 V vs Ag, respectively, which are values ca. 0.3 V lower than those observed in Pyr14FSI. This significant peak shift indicates that not only the viscosity and conductivity of the electrolyte are affecting the potential and the intensity of the redox reactions taking place in PTMA-based electrode, but that also ion-ion interactions and/or ion-electrode surface interactions are affecting these processes. This is an important aspect, which need to be further investigated in the future in order to understand the impact of the proton of the PIL on the storage process of PTMA-based electrodes. This investigation, however, is out of the scope of this work and it will not be further considered in the following. As shown in Fig. 5b, when the PTMA-based electrodes are cycled in combination with PyrH4FSI they deliver higher capacity (55 mAh g−1) than in combination with PyrH4TFSI (24 mAh g−1). This result is in line with the results observed for the AILs. However, it has to be noticed that the capacities of the PTMA-based electrodes in PILs are overall lower than the ones reached in AILs. Furthermore, the efficiency of the charge-discharge process is lower in PILs than in AILs. This lower efficiency reduces the stability of the electrodes during the cycling process. Figure 5c compares the current rate capability of PTMA in the protic ILs. As shown, in these electrolytes the PTMA-based electrodes do not perform as well as in the AILs, and they display a constant decrease of capacity when the C-rate is increased. At 20 C the electrode cycled in PyrH4FSI retains ca. 20% of its initial capacity, while the one cycled in PyrH4TFSI does not deliver any significant capacity (The absolute values of capacitance are reported the Supplementary Information S2). vAs shown in Fig. 5d, the electrode cycled in PyrH4TFSI is losing more than 80% of its initial capacity after 100 cycles at 1 C. The electrode cycled in PyrH4FSI displays a better stability and retains more than 62% of its maximum capacity (after an initial increase) after 100 cycles. These results indicate that although it is possible to cycle PTMA-based electrodes in PIL, the stability of these electrodes in these protic electrolytes is significantly lower than the one observed in aprotic electrolytes. It is reasonable to suppose that this difference is caused by the presence of the proton of PILs, which might interact with the PTMA structure, reducing its stability.
Figure 5. (a) Cyclic voltammetry at a scan rate of 2 mV s−1, (b) voltage profile at 1 C, (c) rate capability from 0.2 C to 100 C and (d) cycling stability at 1 C of PTMA-based electrodes in combination with PyrH4TFSI and PyrH4FSI as electrolytes (filled dots refer to specific capacity, empty to coulombic efficiency), all measurements for PyrH4TFSI at 40 °C.
Download figure:
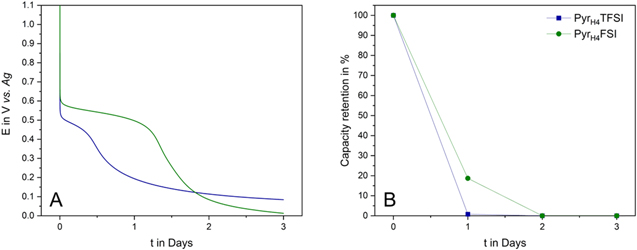
Standard image High-resolution imageTo further investigate the behavior of PTMA-based electrode in PIL, also the impact of these electrolytes on the electrode self-discharge has been investigated. Figure 6a displays the self-discharge curves of the PTMA-based electrodes in PyrH4TFSI and PyrH4FSI. As visible, the electrode self-discharge is significantly higher in these ILs than in their aprotic counterparts. The electrode cycled in combination with PyrH4TFSI loses all its capacity after one day. In the meantime, the electrode cycled in PyrH4FSI is able to deliver ca. 20% of its initial capacity. This high self-discharge could be caused by the dissolution of the active material into the electrolyte, the occurrence of side reactions involving the proton of the PILs and the presence of a high amount of redox shuttles in the ionic liquids provided by dissolved polymer.
Figure 6. (a) Voltage excursion of PTMA in PyrH4TFSI and PyrH4FSI during 3 d of self-discharge and (b) Self-discharge of PTMA in PyrH4TFSI and Pyr H4FSI at 1 C after 1, 2 and 3 d, respectively.
Download figure:
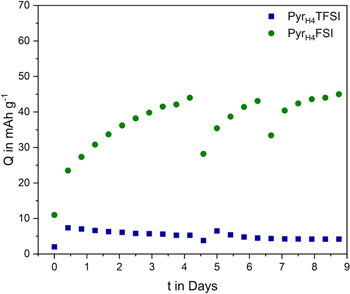
Standard image High-resolution imageTo gain information about the degree of irreversibility of the capacity loss observed during the self-discharge tests, also float tests at 1.1 V vs Ag have been carried out. As shown in Fig. 7, the PTMA electrode cycled in PyrH4TFSI was not able to deliver any significant capacity during this test, indicating that the electrode capacity was practically irreversibly lost. In the case of the electrode cycled in PyrH4FSI the situation was different. At the beginning of the test the electrode cycled in this electrolyte displays 11 mAh g−1. After the application of the constant potential a strong continuous increase of capacity was obtained, and after 4.5 days a capacity of ca. 44 mAh g−1 was delivered by this electrode. It has to be noticed that in between the constant potential periods, when the electrode was charged and discharged (at 5 C), a fade of capacity over the cycling was observed. Nevertheless, it is interesting to notice that during a subsequent float test the capacity could be always recovered. The results of this test are clearly indicating that the presence of the proton of the PIL has a strong influence on the stability and on the electrochemical behavior of PTMA-based electrodes.
Figure 7. Floating behavior of PTMA in PyrH4TFSI and PyrH4FSI at 5 C after 9 d of stable potential at 1.1 V vs Ag.
Download figure:
Standard image High-resolution imageIn order to clarify in which way the presence of the proton causes the inferior performance of PTMA, post mortem analysis (SEM) of the here shown composite electrodes as well as pristine electrodes of the same kind have been carried out, which is reported in the supplementary information (S3 and S4). However, in this analysis no significant differences in the surface or the composition of the cycled electrodes has been found. This indicates that the application of PILs does not cause a structural failure of the electrodes, but more likely interferes with the redox active TEMPO moiety of the used active material. In the future, it will be therefore necessary to investigate and understand the interactions taking place between PILs and organic redox active materials in order to clarify the behavior observed above.
Conclusions
In this work, we reported a systematic investigation about the impact of AILs and PILs on the electrochemical behavior of PTMA-based electrodes. We showed that the use of aprotic ILs based on the FSI− anion, such as Pyr14FSI, enables the realization of electrodes able to display high capacity, good capacity retention at high current density (42% at 50 C) and very low self-discharge. Furthermore, PTMA-based electrodes used in combination with Pyr14FSI display very high stability in float tests and are able to keep all their capacity over 70 d (corresponding to over 9300 cycles at 5 C). We also reported, for the first time, that it is possible to successfully use PTMA-based electrodes in combination with PILs. The use of this protic electrolytes enables the achievement of good capacity. However, the cycling stability of PTMA electrodes in these electrolytes need to be significantly improved.
Overall, this work confirms that ILs represent very interesting candidates for the realization of high performance PTMA-based electrodes. In the future, it will be necessary to intensify the studies dedicated to the interactions taking place between ILs and organic active materials in order to better tune the properties of these promising electrolytes for an application in ORP-based energy storage systems.
Acknowledgments
The authors wish to thank the Deutsche Forschungsgemeinschaft (DFG) within the project "Redox-active ionic liquids in redox-flow-batteries" (BA4956/10-1) for the financial support.