Abstract
Here we demonstrate a high rate capability of all-solid-state lithium batteries using quasi-solid-state electrolytes containing an ionic liquid. We fabricated solid-state electrolyte using an ionic liquid: 1 mol l−1 lithium bis(fluorosulfonyl) imide dissolved 1-Ethyl-3-methylimidazolium bis(fluorosulfonyl)imide (LiFSI/EMI-FSI) and fumed silica nanoparticles with a variety of volume fractions. The fabricated freestanding film with 85% volume fraction of LiFSI/EMI-FSI exhibited an ionic conductivity and self-diffusion coefficient of lithium-containing species; 10.2 mS cm−1 and 3.3 × 10−11 m2 s−1 at 35 °C. We revealed that the increase in the volume fraction of the LiFSI/EMI-FSI led to the decrease in concentration polarization resistance, leading to an enhanced rate capability in Li∣LiFePO4 batteries. The fabricated Li∣LiFePO4 batteries using freestanding electrolyte films with 85 vol% LiFSI/EMI-FSI exhibited a high capacity (>150 mAh g−1) at 1 C (0.6 mA cm−2) based on that at 0.1 C. Further, we fabricated bipolar-type all-solid-state lithium batteries assembled by stacking of Li∣LiFePO4 cell components in a single package. The bipolar-type lithium batteries exhibited the increased packing energy density, depending on the number of stacked cells. These results open opportunities of designing all-solid-state lithium batteries for high energy and power density using quasi-solid-state electrolytes.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Lithium secondary batteries have been promising candidates as energy storage devices for large-scale applications in electric vehicles and smart grids because of their high potential in high energy density.1 On the other hand, the increased battery size makes safety issues more severe due to the use of flammable organic electrolytes. Accordingly, significant efforts are devoted to resolving safety issues such as fire and explosion accidents involving lithium-ion batteries. All-solid-state lithium batteries are now expected to be a fundamental solution to the safety issue because of the use of non-flammable inorganic solid electrolytes. Although the use of inorganic solid electrolytes provide excellent battery performances in safety, and long cycle life owing to no side reactions involving decomposition of electrolytes,2 all-solid-state lithium batteries have suffered from a low power density issue caused by high electrode/electrolyte interface resistances3 and grain boundary resistances.4 Moreover, all-solid-state lithium batteries using inorganic solid electrolytes face a formidable challenge to their practical application in mechanical flexibility and manufacturing processability.5,6
One approach to overcoming the issues above is to develop quasi-solid-state electrolytes consisting of ionic liquids and oxide nanoparticles.7 Ionic liquids can be solidified by an electrostatic interaction with the surface of oxide nanoparticles.8 The solidification of ionic liquid electrolytes should provide the combined benefits of high ionic conductivity, non-volatility, high flame resistance,9 good flexibility, and mechanical properties.10 In addition, the use of solid electrolytes enables stacking multiple electric cells in series within a single package, leading to a weight reduction of the package and increase in an energy density compared to a single unit cell. We have reported a long cyclability in all-solid-state bipolar-type lithium batteries using quasi-solid-sate electrolytes containing a variety of ionic liquid electrolytes and oxide nanoparticles.11,12 In previous studies, however, charging and discharging with a high capacity (∼150 mAh g−1 in Li∣LiFePO4 cell) has been limited to 0.1 C, where the 0.1 C corresponds to 60 μA cm−2, despite the use of quasi-solid-state electrolytes with a relatively high ionic conductivity (∼10−3 S cm−1).8
In this study, we developed quasi-solid-state electrolytes with the order of 10−2 S cm−1 in ionic conductivity in order to improve rate capability. Quasi-solid-state electrolytes were fabricated using the ionic liquid: 1 mol l−1 lithium bis(fluorosulfonyl)imide dissolved 1-Ethyl-3-methylimidazolium bis(fluorosulfonyl)imide, denoted as LiFSI/EMI-FSI. We succeeded in a high rate capability, which showed a high capacity (>150 mAh g−1) even at 1 C (0.6 mA cm−2) in a Li∣LiFePO4 cell using a fabricated quasi-solid-state electrolyte. Moreover, we revealed that the rate determining step in the rate capability was originated from a concentration polarization resistance in the quasi-solid-state electrolytes when fabricating quasi-solid-state electrolytes with a low volume fraction of an ionic liquid. Such a high concentration resistance could be suppressed by increasing the volume fraction of ionic liquid in the quasi-solid-state electrolytes. For further developments, we demonstrate high rate capabilities in bipolar-type all-solid-state lithium batteries assembled by stacking the cell components up to five layers in a single package. The packing energy density increased monotonically without capacity loss, as increasing the number of the stack of Li∣LiFePO4 cells. These results open opportunities for designing all-solid-state lithium batteries with a high power density and energy density using quasi-solid-state electrolytes containing an ionic liquid.
Experimental
Fabrication of freestanding films using quasi-solid-state electrolytes
We selected the LiFSI dissolved at the amount of 1 mol l−1 in EMI-FSI (KISHIDA CHEMICAL Co., Ltd.) as the ionic liquid electrolyte, as the EMI-FSI possess a high ionic conductivity (>10 mS cm−1 at 25 °C) among a variety of ionic liquids.13 The LiFSI/EMI-FSI liquid electrolyte and fumed silica nanoparticles (Sigma-Aldrich Co., specific surface area: 390 m2 g−1, average particle size: 7 nm) were mixed at a variety of volume fractions in the ranges from 75 to 85% of LiFSI/EMI-FSI and stirred in methanol for three hours. The resulting mixtures were dried on a hot plate at 60 °C for two hours to obtain a quasi-solid-state electrolyte powder. The quasi-solid-state electrolyte powder and polytetrafluoroethylene (PTFE, Teflon-J, DuPont-Mitsui Fluorochemicals Co., Ltd.) were mixed with 95:5 in the weight ratio. Subsequently, the quasi-solid-state electrolytes composites were formed by a roll press to freestanding films with 200 μm thickness within an error of ±10 μm and 12 mm diameter. The thickness was measured using a micro caliper by hand. We utilized the measured QSE film thickness to estimate ionic conductivity. The photographs of each material and process are shown in Supplemental material (available online at stacks.iop.org/JES/167/040511/mmedia).
Fabrication of Li∣LiFePO4 cells
We investigated the dependence of the LiFSI/EMI-FSI volume fraction in freestanding electrolyte films on battery performances. Cathode composites with the weight of 4 mg were prepared by mixing LiFePO4 powder (Tatung Fine Chemicals Co., theoretical capacity: 170 mAh g−1), acetylene black (AB), the quasi-solid-state electrolyte powder, and PTFE (35:10:45:10 by weight), which were ground in a mortar for a few minutes. The cathode composites were prepared to form a film with 7 mm diameter. Photographs of the cathode composite are shown in Supplemental material. Single-layered all-solid-state lithium batteries were fabricated by directly stacking the cathode composite, the freestanding electrolyte film (12 mm diameter and 200 μm thickness), and a lithium metal anode (10 mm diameter and 100 μm thickness). The interface of cathode composite/QSE freestanding film possesses a good contact without noticeable voids, which was confirmed through scanning electron microscopy/energy-dispersive X-ray spectrometry measurements (See Supplemental material). The stacked cell components were sandwiched between stainless steel current collectors to make the 2032-type of Li∣ LiFePO4 coin cells.
For bipolar-type cells, the double-, triple-, fourfold-, and quintuple-layered cell components were sandwiched between stainless steel and stacked in series, respectively. Each stacked bipolar-type cell was assembled within a 2032-type coin cell. The whole preparation process was carried out in a dried argon atmosphere glove box.
Ionic transport characterization of freestanding electrolyte films
Fabricated freestanding films with a variety of volume fractions of the LiFSI/EMI-FSI were sandwiched between two stainless-steel blocking electrodes to fabricate the 2032-type coin cells. The coin cells were used for evaluating ionic conductivities of the freestanding films, measured by electrochemical impedance spectroscopy with an electrical potential amplitude of 10 mV in the frequency ranges from 1 × 10−1 to 1 × 106 Hz at temperatures from 0 °C to 80 °C using Solatron analytical, CellTest System.
A pulsed gradient spin-echo nuclear magnetic resonance (PGSE-NMR) spectroscopy was performed to evaluate the self-diffusion coefficient of 1H-, 19F-, and 7Li-containing species in the fabricated freestanding films. These measurements were carried out in temperatures ranging from 0 °C to 60 °C. The measurement conditions are described in detail in Supplemental material.
Electrochemical characterization of Li∣LiFePO4 batteries
Electrochemical impedance spectroscopy was performed in the frequency ranges of 1 × 10−2 to 5 × 105 Hz at 35 °C for the single-layered Li∣LiFePO4 cells at the 50% of the density of state of discharge. Rate capability measurements for the single-layered cells were performed using a Hokuto Denko Corp. 8CH charge/discharge unit for the single-layered Li∣LiFePO4 cells, and a Hokuto Denko Corp. HZ-7000 electrochemical measurement system for the bipolar-type lithium batteries. The fabricated lithium batteries were discharged at a variety of C rates in the ranges from 0.1 C to 100 C after charging at 0.1 C at 35 °C, where the 0.1 C rate corresponds to the current density of 60 μA cm−2. Cycle performance was examined by charging and discharging at 1 C and 35 °C for the single-layered cell using a freestanding film with 85 vol% LiFSI/EMI-FSI.
Results and Discussion
Ionic conductivity and self-diffusion coefficient of quasi-solid-state electrolytes
First, we show temperature dependent ionic conductivities of free-standing films with a variety of volume fractions of the LiFSI/EMI-FSI. The ionic conductivity increased monotonically by increasing the volume fraction of the LiFSI/EMI-FSI in the quasi-solid-state electrolyte as shown in Fig. 1a. The similar volume fraction dependence of ionic conductivity has been reported for a variety of quasi-solid-state electrolytes containing ionic liquids.14 An electrostatic interaction between ionic liquids and oxide surfaces leads to an increase in the viscosity of ionic liquids,15 which results in the decrease in the ionic conductivity and self-diffusion coefficient of ionic species in the quasi-solid-state electrolytes.16 As the LiFSI/EMI-FSI volume fraction was decreased, Li-, H-, and F-containing ionic species exhibited a monotonic decrease in the self-diffusion coefficients (Figs. 1c−1e), corresponding to the decrease in the ionic conductivity of the QSE freestanding films. This trend between the ionic conductivity (σ) and ion diffusion coefficient (D) agrees well with a linear proportional relationship ( ).17 The blue squares indicate the reported values of the liquid state in the LiFSI/EMI-FSI.16 The transference numbers of lithium in the freestanding films were evaluated from the values of the self-diffusion coefficient of Li-containing species divided by the sum of the self-diffusion coefficients of Li-, H-, and F-containing species, which are summarized in Table I. This trend in the ionic conductivity and ionic species diffusion agreed well with a nano-scale confined ionic liquid in oxide materials,15 and were good consistent to the Walden rule: the decrease in the ionic conductivity of the ionic liquid due to the increase in the viscosity of the ionic liquid.18 This result suggests that ionic species are strongly interacted electrostatically with fumed silica nanoparticle surfaces. Thus, it is required to prepare QSE freestanding films using LFSI/EMI-FSI with the volume as large as possible while the mixture remains solid-state even with a high volume fraction of the ionic liquid. The 85 vol% LiFSI/EMI-FSI in the quasi-solid-state electrolyte showed a high ionic conductivity and self-diffusion coefficient of lithium-containing species at 10.2 mS cm−1 and 3.3 × 10−11 m2 s−1 at 35 °C, which were very close to those of the liquid state. We stress that the LiFSI/EMI-FSI liquid electrolyte on the fumed silica nanoparticles could be solidified with a high volume fraction achieving at 85 vol%. In previous studies, the volume fraction for the solidification was limited to 80 vol%.10 However, we found that quasi-solid-state electrolytes can be prepared even with a high content over 80 vol% of the ionic liquid in the hybrid composites with oxide nanoparticles. As shown in Fig. 1b, the fabricated freestanding electrolyte film with 85 vol% of LiFSI/EMI-FSI remained a solid state and showed a good flexibility without tears. In addition, we performed an ignition test for a QSE freestanding film with 85 vol% LiFSI/EMI-FSI (See Supplemental material). Although a color of the QSE freestanding film changed, no smoke and fire were generated, indicating that the QSE possess a high flame resistance compared to conventional organic electrolytes.
).17 The blue squares indicate the reported values of the liquid state in the LiFSI/EMI-FSI.16 The transference numbers of lithium in the freestanding films were evaluated from the values of the self-diffusion coefficient of Li-containing species divided by the sum of the self-diffusion coefficients of Li-, H-, and F-containing species, which are summarized in Table I. This trend in the ionic conductivity and ionic species diffusion agreed well with a nano-scale confined ionic liquid in oxide materials,15 and were good consistent to the Walden rule: the decrease in the ionic conductivity of the ionic liquid due to the increase in the viscosity of the ionic liquid.18 This result suggests that ionic species are strongly interacted electrostatically with fumed silica nanoparticle surfaces. Thus, it is required to prepare QSE freestanding films using LFSI/EMI-FSI with the volume as large as possible while the mixture remains solid-state even with a high volume fraction of the ionic liquid. The 85 vol% LiFSI/EMI-FSI in the quasi-solid-state electrolyte showed a high ionic conductivity and self-diffusion coefficient of lithium-containing species at 10.2 mS cm−1 and 3.3 × 10−11 m2 s−1 at 35 °C, which were very close to those of the liquid state. We stress that the LiFSI/EMI-FSI liquid electrolyte on the fumed silica nanoparticles could be solidified with a high volume fraction achieving at 85 vol%. In previous studies, the volume fraction for the solidification was limited to 80 vol%.10 However, we found that quasi-solid-state electrolytes can be prepared even with a high content over 80 vol% of the ionic liquid in the hybrid composites with oxide nanoparticles. As shown in Fig. 1b, the fabricated freestanding electrolyte film with 85 vol% of LiFSI/EMI-FSI remained a solid state and showed a good flexibility without tears. In addition, we performed an ignition test for a QSE freestanding film with 85 vol% LiFSI/EMI-FSI (See Supplemental material). Although a color of the QSE freestanding film changed, no smoke and fire were generated, indicating that the QSE possess a high flame resistance compared to conventional organic electrolytes.
Figure 1. (a) Temperature dependence of the ionic conductivity of freestanding electrolyte films with a variety of volume fractions of the LiFSI/EMI-FSI in the ranges from 75 to 85%. The numbers in the figure indicate the volume fractions. The samples were the 2032-type coin cells consisting of the freestanding electrolyte film (200 μm thickness and 12 mm in diameter) sandwiched between two stainless-steel blocking electrodes. 100 vol% indicates the ionic conductivity of LiFSI/EMI-FSI liquid state. (b) The picture of a pinched and bent freestanding electrolyte film with 85 vol% LiFSI/EMI-FSI (200 μm thickness and 12 mm in diameter).Temperature dependence of the self-diffusion coefficients of (c) Li-, (d) H-, and (e) F-containing species in the freestanding electrolyte films with the 75 (black circles), 80 (green triangles), and 85% (red diamonds) in volume fraction, which were evaluated by pulsed gradient spin echo nuclear magnetic response spectroscopy. The 100 vol% (right pointing blue triangles, and blue squares) in the figure indicates the liquid-state LiFSI/EMI-FSI.
Download figure:
Standard image High-resolution imageTable I. The self-diffusion coefficient of H-, F-, and Li-containing species and transference number of lithium denoted as tLi for the freestanding electrolyte films with a variety of volume fractions of LiFSI/EMI-FSI at 35 °C.
| 75 vol% | 80 vol% | 85 vol% | 100 vol% | |
|---|---|---|---|---|
| H- | 2.4 × 10−11 (m2 · s−1) | 3.8 × 10−11 (m2 · s−1) | 4.4 × 10−11 (m2 · s−1) | 7.2 × 10−11 (m2 · s−1) |
| F- | 1.4 × 10−11 (m2 · s−1) | 3.1 × 10−11 (m2 · s−1) | 3.6 × 10−11 (m2 · s−1) | 5.8 × 10−11 (m2 · s−1) |
| Li | 1.7 × 10−11 (m2 · s−1) | 2.6 × 10−11 (m2 · s−1) | 3.3 × 10−11 (m2 · s−1) | 4.8 × 10−11 (m2 · s−1) |
| tLi | 0.31 | 0.27 | 0.29 | 0.27 |
LiFSI/EMI-FSI volume fraction dependence on the rate capability of all-solid-state lithium batteries
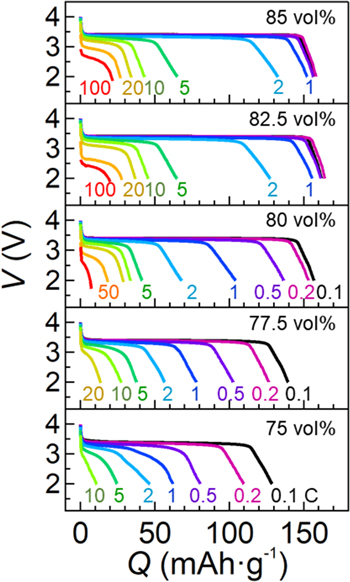
Next, we show battery performances of single-layered lithium batteries fabricated using freestanding electrolyte films with a variety of LiFSI/EMI-FSI volume fractions. Figure 2 shows the discharging curves of Li∣LiFePO4 cells at varied C rates under 35 °C after charging at 0.1 C. Below 80 vol%, the capacity decreased monotonically as the C rate increased. For 75 vol%, the discharging capacity could not achieve at 150 mAh g−1 even at 0.1 C. In contrast, as increasing the volume fraction, the rate capability was improved. We obtained a capacity retaining over 95% at 1 C based on that at 0.1 C when using freestanding electrolyte films with over 80 vol% LiFSI/EMI-FSI. The best performance was obtained for the battery sample using a 85 vol% LiFSI/EMI-FSI freestanding electrolyte film, which exhibited the 84% of the discharging capacity (133 mAh g−1) retaining at 2 C (1.2 mA cm−2) based on that at 0.1 C. This value was 2.89 times higher than that at 2 C (46 mAh g−1) for the battery sample using a freestanding electrolyte film with 75 vol% LiFSI/EMI-FSI. Moreover, although the retained discharging capacity was low (22 mAh g−1), the battery sample with the 85 vol% LiFSI/EMI-FSI freestanding electrolyte film discharged even at 100 C rate (60 mA cm−2), which was very fast discharging comparable to reported lithium-ion batteries using conventional organic liquid electrolytes.19,20
Figure 2. Rate capabilities of the single-layered Li∣LiFePO4 batteries using the freestanding electrolyte films with a variety of LiFSI/EMI-FSI volume fractions ranging from 75 to 85 vol%. Samples were discharged at varied C rates in the ranges from 0.1 to 100 C after charging at 0.1 C (60 μA cm−2) under 35 °C.
Download figure:
Standard image High-resolution imageFor a battery sample using a freestanding electrolyte film with 85 vol% LiFSI/EMI-FSI, we tested cycle durability. Figure 3 shows a cycle performance of charging and discharging at 1 C within the cutoff voltages ranging from 2.0 to 3.7 V. Charging and discharging capacities exhibited high capacities (approximately 150 mAh g−1) and good coulombic efficiency (>99.95%) up to 200 cycles. Usually, the Li∣LiFePO4 cell presented the good cycle performance, but some cycle numbers exhibited a larger amount over 150 mAh g−1 in the charging process. An electrochemical corrosion of the stainless steel current collector may be contributed to the charging capacity. Although further research is required to identify the increased capacity in charging, the fabricated freestanding electrolyte films normally provided stable battery operations with the high capacity.
Figure 3. Cycle performance of a single-layered Li∣LiFePO4 cell using a freestanding electrolyte film with 85 vol% LiFSI/EMI-FSI. Charging (Red circles) and discharging (Blue triangles) were performed at 1 C (0.6 mA cm−2) under 35 °C. Green squares represent coulombic efficiency.
Download figure:
Standard image High-resolution imageLiFSI/EMI-FSI volume fraction dependence on impedance spectra
Here, we discuss a rate-determining step in the fabricated lithium batteries, which was identified from impedance spectroscopy. Figure 4 shows the impedance spectra of the Li∣LiFePO4 cells using the freestanding electrolyte films with a variety of volume fractions. The obtained impedance spectra exhibited three notable features. First, the real axis values at the high-frequency intercept were monotonically decreased from 42.3 to 3.8 Ω as increasing the volume fraction of the LiFSI/EMI-FSI from 75 to 85 vol% in the freestanding electrolyte films. This shift of the intercept should reflect the ionic conductivity of the freestanding electrolyte films. Second, each spectrum consists of a semicircle in the high-frequency region, which is assigned to two interfacial responses from the LiFePO4/electrolyte and Li/electrolyte interfaces. The interpretation of a semicircle is described based on the time-domain modeling21 (See Supplemental material). The interface resistances exhibited values in the ranges between 31.4 and 56.7 Ω. Interestingly, the interface resistance is independent of the volume fraction of the LiFSI/EMI-FSI in the quasi-solid-state electrolytes. This result suggests that a solvation structure is unchanged when the LiFSI/EMI-FSI is solidified on the fumed silica nanoparticles in spite of an electrostatic interaction. This consideration should be supported by focusing on a transference number of the lithium ion in the quasi-solid-state electrolytes. The free-standing electrolyte films exhibited the lithium transference numbers of approximately 0.3 in whole ranges in the volume fractions, where the transference numbers were evaluated by PGSE-NMR measurement. In addition, through Raman spectroscopy measurement, noticeable changes in the peak derived from the solvation were not confirmed (See Supplemental material). This result indicates that the solvation structure should remain even after the solidification of LiFSI/EMI-FSI. Third, a dramatic increase in the resistance was observed at lower frequency regions when the volume fraction of the LiFSI/EMI-FSI decreased to below 80 vol% as shown in Fig. 4b. A straight line at the lower frequencies in the spectrum corresponds to a Warburg impedance. The increased Warburg impedance should originate from the concentration polarization resistance. This origin is reasonable by considering the low self-diffusion coefficients of mobile ionic species in the freestanding electrolyte films (<80 vol%). In contrast, for >80 vol% LiFSI/EMI-FSI, the Li∣LiFePO4 batteries showed similar values in the Warburg impedance, suggesting that the rate determining step should be not the QSE freestanding films but others such as a passivation film over Li, LiFePO4, or LiFePO4 bulk inside. At least, the impedance of the Li anode should be negligible as evidenced by the impedance spectrum for a Li symmetric cell (See Supplemental material). Although further research is required to identify the origin of the Warburg impedance in the Li∣LiFePO4 cells using QSEs with > 80 vol% LiFSI/EMI-FSI, these results indicate that tuning of the volume fraction of the LiFSI/EMI-FSI in quasi-solid-state electrolyte plays a key role on the control of ionic conductivity and solidification of LiFSI/EMI-FSI and, thus, a high content of the ionic liquid in the quasi-solid-state electrolyte is indispensable when fabricating solid-state lithium batteries with a high power density.
Figure 4. (a) Impedance spectra at the 50% of the density of state of discharge for the single-layered Li∣LiFePO4 cells using freestanding electrolyte films with a variety of volume fractions ranging from 75 to 85 vol%. Batteries were discharged at 35 °C and 0.1 C to the 50% of the density of state after charging at 0.1 C, then the impedance spectroscopy was performed. From top to bottom in (a), volume fractions of the LiFSI/EMI-FSI in the quasi-solid-state electrolytes are 85 (red circles), 82.5 (orange circles), 80 (green circles), 77.5 (blue circles), and 75 (black circles), respectively. (b) Impedance spectra of the cells using the freestanding electrolyte films with 77.5 vol% and 75 vol% within the frequencies ranging from 1 × 10−2 to 5 × 105 Hz. The top figure in (b) is the same impedance spectrum as the second one from the bottom in (a). The bottom figure in (b) is the same impedance spectrum as the bottommost one in (a). The numbers of 10n (−2 ≤ n ≤ 4) in the figures indicate the frequencies.
Download figure:
Standard image High-resolution imageRate capability of bipolar-type lithium batteries
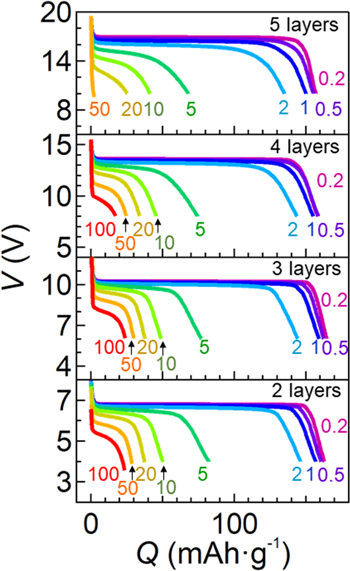
Finally, we demonstrate high rate capabilities of high energy density bipolar-type lithium batteries. Figure 5 shows the discharging curves of the bipolar-type lithium batteries with a variety of the number of stacked layers using the freestanding films with the 85 vol% LiFSI/EMI-FSI. In all bipolar-type lithium batteries, the capacity retention at various C rates was very close except at 50 and 100 C for the quintuple-layered bipolar-type cell. The capacities with over 96% based on that at 0.2 C were retained at 1 C. Further, the double-, triple-, fourfold-, and quintuple-layered batteries exhibited voltage plateaus of 6.7–6.8, 10.1–10.2, 13.4–13.6, and 16.4–16.9 V, respectively. These values were in good agreement with that of the single-layered cell (3.37–3.4 V) multiplied by the number of stacking layers. This result indicates that the fabricated bipolar-type cells exhibited very small overpotentials. Note that over 95% and 86% of the capacities were retained even at 1 C and 2 C among all the fabricated bipolar cells. In addition, the bipolar-type batteries could discharged at 100 C (60 mA cm−2) except for the quintuple-layered cell. Packing energy densities were evaluated based on the energy of a loaded LiFePO4 active material divided by weight of the complete coin cell. The evaluated packing energy densities of the single, double, triple, quadruple, and quintuple cells were 0.26, 0.53, 0.79, 1.02, and 1.24 Wh kg−1, respectively. This result indicates that the increase in a number of the stack of cell components led to a monotonic increase in the energy density. Thus, using the freestanding electrolyte films, the bipolar-type lithium batteries exhibited good battery performances without any short-circuiting.
Figure 5. Rate capabilities of Li∣LiFePO4 bipolar-type batteries fabricated by stacking cell components up to quintuple layers in a single package. The freestanding electrolyte films with the 85 vol% LiFSI/EMI-FSI were used for those bipolar-type lithium batteries. From top to bottom of the figures, the numbers of the stack of Li∣LiFePO4 cell components were quintuple, quadruple, triple, and double, respectively. Bipolar-type lithium batteries were discharged at a variety of C rates ranging from 0.2 to 100 C after charging at 0.1 C under 35 °C. The numbers with the same colors as the discharging curves indicate the C rates.
Download figure:
Standard image High-resolution imageConclusions
We fabricated the quasi-solid-state electrolytes using the LiFSI/EMI-FSI liquid electrolyte and fumed silica nanoparticles for a high power density of all-solid-state lithium batteries. The increase in the volume fraction of the ionic liquid in the quasi-solid-state electrolyte led to the increase in the ionic conductivity and the self-diffusion coefficient. The quasi-solid-state electrolytes with the 85 vol% LiFSI/EMI-FSI exhibited the ionic conductivity at 10.2 mS cm−1 and the self-diffusion coefficient of 3.3 × 10−11 m2 s−1 for lithium-containing species at 35 °C, respectively. Low volume fractions of the ionic liquid in the quasi-solid-state electrolytes led to the decrease in the ionic conductivity and the self-diffusion coefficient, which degraded the rate capability due to high concentration polarization resistances. Fabricated Li∣LiFePO4 batteries exhibited a high retention capacities even at 1 C compared to that at 0.1 C. The bipolar-type lithium batteries exhibited the high rate capability and the monotonic increase in the packing energy density as increasing stacking layers of the cells. These results pave the way to the fabrication of high energy and power density all-solid-state lithium batteries using quasi-solid-state electrolytes containing ionic liquids.
Acknowledgments
This research was supported by Hitachi, Ltd.