Abstract
This short perspective summarizes recent findings on the role of residual lithium present on the surface of layered Ni-rich oxide cathode materials in liquid- and solid-electrolyte based batteries, with emphasis placed on the carbonate species. Challenges and future research opportunities in the development of carbonate-containing protective nanocoatings for inorganic solid-state battery applications are also discussed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
In recent years, Li-ion batteries (LIBs) have become the primary energy-storage technology, enabling portable electronics and electrifying transportation. State-of-the-art LIBs usually rely on the combination of a metal oxide cathode as lithium source, a graphite anode, a porous polymer separator and an organic carbonate based liquid electrolyte. To achieve high energy densities (>250 Wh kg−1) on a cell level, layered Ni-rich oxide cathode active materials (CAMs), such as LiNi1−x−y Cox Mny O2 (referred to as NCM or NMC in the battery community), are commonly employed and currently represent a hot topic in cathode R&D [1, 2]. However, it has been recognized both in academia and industry that surface residuals, primarily carbonate and hydroxide species, remaining from the synthesis (use of excess reagents) or formed during storage and handling, play a critical role on their processability and cyclability [3–7]. Especially the carbonate contaminants have been thoroughly studied in the past and shown to contribute to gas evolution via chemical (equation (1)) and/or electrochemical decomposition (equation (2)), which can lead to problems with battery performance and safety [4, 8–10]:
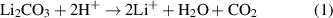
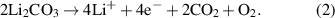
Through a combination of in situ gas analysis and isotope labeling experiments, it has been demonstrated that carbonate species are responsible for a large fraction of the released CO2 in the initial cycle [11–14]. Nevertheless, their effect on CO2 evolution during long-term cycling is minor (depending on the cycling conditions and other relevant parameters). Besides, it has been shown that the electrochemical oxidation of surface carbonates leads to the generation of reactive oxygen (singlet oxygen, 1O2), see equation (2) [15]. Similarly, reactive (lattice) oxygen is released from NCM cathodes at high degrees of delithiation or, in other words, at high states of charge (⩾80%), due to unfavorable phase transitions (layered to spinel and/or rocksalt) resulting from structural stability issues [8]. Apparently, this oxygen further contributes to CO2 evolution through follow-up reactions with the liquid electrolyte [15–17]. It should be noted though that a recent review article by Schürmann et al is questioning the electrochemical generation of 1O2 [18]. Regardless, for the application of Ni-rich NCM-type CAMs in high-performance LIBs, washing for the removal of residual lithium (followed by drying or post-annealing), without adversely affecting the lithium inventory and surface structure, is frequently required [12, 19–25].
In contrast to LIBs, surface carbonates on NCM have been shown to be beneficial to the cycling performance and stability of inorganic solid-state batteries (SSBs) with superionic lithium thiophosphate electrolytes [26, 27]. In particular, they act as a kind of protective buffer layer between solid electrolyte and CAM, thereby mitigating electrochemical degradation of the former and leading to improved reversibility and capacity retention. Via isotope labeling of the carbonate species, it has been found that in SSBs, as somewhat expected, carbonate contaminants are also responsible for CO2 evolution, while the accompanying oxygen release seems to cause SO2 generation through gas–solid reactions with the lithium thiophosphate (sulfide) electrolyte [27–31]. However, unlike in LIBs, the carbonate species are getting stepwise decomposed and the amount of gas evolution is much lower in SSBs [14, 28].
Lithium based transition metal oxide nanocoatings are typically applied to the CAM secondary particles' surface prior to their use in SSBs, with the most prominent example being LiNbO3 [32–35]. In general, protective coatings help to mitigate the formation of detrimental decomposition interfaces (similar to the anode and cathode solid electrolyte interfaces in LIBs) by preventing direct contact between solid electrolyte and CAM [30, 36, 37]. Sol–gel chemistry is a relatively simple and versatile tool for the preparation of nanocoatings. In this case, alkoxides commonly serve as precursors in low boiling solvents, such as alcohols, followed by heating at temperatures in the range 300 °C–500 °C to produce the oxide [32, 38–40]. Higher temperatures are avoided to prevent cation migration and interdiffusion [41–43].
Until recently it has been believed that a pure (clean) LiNbO3 coating is formed by wet chemical deposition. However, the surface layer rather has a hybrid structure consisting of LiNbO3 nanoparticles embedded in an amorphous matrix made from mostly Li2CO3, especially when the heating is done in air [26, 37]. Altering the preparation conditions and the Li:Nb ratio in the synthesis, the carbonate content in the protective coating has been successfully varied while keeping the Nb content constant. Ultimately this allowed for the identification of a sweet spot for maximum cell performance [44]. However, the authors of this study concluded that heating in oxygen is more beneficial. This is because the carbonate content is difficult to control precisely under (unstable) ambient atmosphere conditions. In addition, its effect on both coating microstructure and interfacial chemistry is not well understood and needs further study.
Previous literature reports revealed that this hybrid coating concept is also compatible with other materials than LiNbO3, for example, Li2ZrO3 and Li3BO3, demonstrating the great versatility in terms of chemical composition (coating formulation) and properties [45–47]. In all of these cases, the coating was capable of suppressing to different extents interfacial decomposition of the solid electrolyte at the contact points with the CAM secondary particles, which otherwise would lead to impedance buildup due to formation of insulating degradation products (oxygenated sulfur and phosphorus species etc) and capacity fade.
Taken together, these findings demonstrate the beneficial effect that carbonate-containing hybrid coatings may have on the cathode performance. However, their properties have largely been unexplored, but require thorough investigation to rationally improve cyclability, kinetics and lifetime. This also includes engineering of the micro- and nanostructures. From an analytical perspective, the particular role of the carbonate species remains unclear, possibly acting as a network former and adding mechanical flexibility to the coating and/or providing good bonding to the substrate (e.g. via the surface oxygen), without negatively affecting interfacial charge transport. Flexibility and bonding are important given that NCM CAMs, especially when rich in Ni, undergo distinct volume changes during cycling and the volumetric strain induced by unwanted side reactions [48–52]. Both can not only lead to particle fracture and mechanical separation between solid electrolyte and CAM, but can cause delamination of the coating, thereby generating reactive surfaces and facilitating lattice oxygen release. Apart from the increased (electro)chemical resistance and the potential impact on the chemo-mechanics, carbonate-containing hybrid coatings seem to enable high interfacial ionic conductivity, providing a multitude of parameters for tailoring the critical properties of protective coatings.
In summary, herein we have described recent findings on the introduction of carbonate species into coatings on CAMs, recently recognized to play a pivotal role in the performance of thiophosphate based SSBs. Surface contaminants cannot be neglected, but must be considered carefully in tailoring the coating chemistry and interfacial properties. Practically this can be achieved by taking advantage of the residual lithium during post-treatment of the surface layer or through direct reactions with reactive precursors, either in the liquid or gas phase [53–55]. However, detailed multiscale characterization (prior to and after battery operation) to gain insights into the efficiency and functionality of the nanocoating as well as into related cathode failure modes is challenging. Suitable analytical methods include differential electrochemical mass spectrometry, time-of-flight secondary ion mass spectrometry, x-ray photoelectron spectroscopy, x-ray absorption spectroscopy and advanced electron microscopy, to name a few.
Acknowledgments
F Strauss acknowledges financial support from the Fonds der Chemischen Industrie (FCI) through a Liebig fellowship. This work was partially supported by BASF SE.

