Abstract
We report a corrosion study on Al 7075—T651 surface. The purpose of the study is to use Graphene oxide—Polymethyl methacrylate (GO—PMMA) coating as corrosion resistant layer on Al 7075—T651, an alloy of aluminium mainly used in aerospace industries. The study is done with and without GO—PMMA coating on Al 7075—T651 respectively, by varying the exposure time of material (24, 48 and 72 h) with HCl in atmospheric condition. The formation of corrosion is resisted by the GO—PMMA coated samples. The effect is studied by material characterization and non-destructive evaluation (NDE) techniques. The formation of aluminium hydroxide in uncoated samples is confirmed by Raman spectroscopy and x-ray diffraction (XRD). Corrosion formation, GO—PMMA coating on plain and coated samples are clearly visible in Scanning Electron Microscope (SEM) study. Shift in current is observed in I–V Characteristics study, due to pitting in plain samples. Material loss is observed, to be low in GO—PMMA coated samples, using pulsed thermography. The work proves that the GO—PMMA coating is very effective in corrosion resistance.
Export citation and abstract BibTeX RIS
1. Introduction
In aircraft industries, corrosion is a very big challenge to deal with [1, 2]. The aircraft components are exposed to various environment which leads to many types of corrosion. The prolonged exposure to agents like industrial fluid, salt, moisture, internal problems such as condensation formation, leaking lavatories and galleys, can cause corrosion. To avoid such problems, corrosion resistive materials to be used. The commonly used materials in aerospace industries are Aluminium (Al) alloys such as Al 7075, 7068, 7050, 6061 and 6063. Despite all, aircraft or aerospace aluminium usually refers to Al 7075 because of its high strength to density ratio [3–5]. Al 7075 is extensively used in aerospace applications like fuselage stringers, frames, upper wing stringers, floor beams, seat rails, etc. Many studies have been taken to study corrosion in Al 7075 [6–8]. In Aluminium alloys, Al is the predominant metal. Al and its alloys are extensively used because of its low density and corrosion resistance property. Some alloying metals are copper, magnesium, manganese, silicon, zinc and tin. It is also used in satellites for space research purposes. In Al 7xxx series, Zinc is the main alloying metal. Al 7075 has good strength often compared to many steels, but less corrosion resistant. Based on the tempering of the material, there are different types like Al 7075—0, 7075—T6, 7075—T651, and 7075—T7, where '0' denotes it is annealed, 'T6' denotes it is solution heat treated and artificially aged, 'T651' denotes it is solution heat treated, stress relieved by stretching and artificially aged, 'T7' denotes it is solution heat treated and stabilized. In our study, we have chosen Al 7075—T651 because of its ultimate tensile strength and yield strength when compared to other tempered alloys [8, 9]. Its high machinability property gives the components a very high surface finish. Corrosion is a dangerous process which will result in failure of metal. It is the result of chemical reaction between the metal and environment. There are many types of corrosion that can occur in materials like uniform attack or surface corrosion, pitting corrosion, intergranular corrosion, galvanic corrosion, environmental cracking, flow assisted corrosion, high temperature corrosion, etc. Here we tried to use Graphene Oxide (GO) and Poly (methyl methacrylate) (PMMA) as coating medium to prevent corrosion in our research work, because of its compact filler-matrix interface and the presence of passivation layer [10]. GO is extensively used for its wide applications in resisting corrosion while other organic coatings have the demerits of high cost, difficult process and environment pollution [11–16]. GO is an oxide of graphene which has high oxygen groups. Ayesha kausar et al investigated that, GO has provided an inexpensive way to develop PMMA based functional materials. Many researches have proved that when GO is added, it improves mechanical properties, electrical properties, corrosion resistant property, etc [17–19]. It forms an anti-corrosive layer over the alloy to prevent it from corrosion. HCl is very commonly used agent to induce corrosion on the material [20]. We characterize the accelerated corrosion in GO—PMMA coated and without coated Al 7075—T651 alloy. The formation of corrosion in the material can be investigated by various characterization techniques like X-ray diffraction (XRD), Raman spectroscopy, Scanning Electron Microscopy (SEM), I–V characteristics and non-destructive evaluation (NDE) technique pulsed thermography. In our work, we extensively study the formation of corrosion in Al 7075—T651 with and without a corrosion resistive coating and prove that the GO—PMMA coating is very effective in corrosion resistance. Pulsed thermography technique shows the material loss is much less in GO—PMMA coated samples. XRD and Raman spectroscopy shows the absence of oxide formation in GO—PMMA coated samples which is confirmed in SEM images also.
2. Experimental
2.1. Material
The samples used in this study is Al 7075—T651 with standard composition [6]. It has Aluminium (Al) 87.1–91.4, Zinc (Zn) 5.6, Magnesium (Mg) 2.5, Copper (Cu) 1.5, Iron (Fe) 0.5, Silicon (Si) 0.4, Titanium (Ti) 0.2, Manganese (Mn) 0.3, Chromium (Cr) 0.25 and others 0.15 percentage of weight as composition.
2.2. Sample preparation
Al 7075—T651 is purchased from the commercial market. It is cut into pieces of equal size and shape for our study. Samples are prepared with and without GO—PMMA coating respectively. PMMA is purchased from sigma Aldrich chemicals. GO is synthesized by modified hummer's method. 0.5 g and 0.25 g of graphite powder and NaNO3 respectively are mixed with 12 ml of sulfuric acid. It is kept in an ice bath till temperature drops below 5 °C. Then temperature is increased to 35 °C and 1.5 g of KMnO4 is added to the mixture. It is stirred for 2 h. After the condition of solution is changed to paste, 23 ml of distilled water is added. Then it is heated to 90 °C for 15 min and 80 ml of water is added. Then 3 ml of H2O2 is added to the mixture and the final solution in appears in yellow color. The precipitate is washed with HCl and with doubly distilled water five times. It is then dried in a hot air oven at 60 °C for 48 h. 30 mg of obtained GO is mixed with 0.25 g of PMMA in 5 ml acetone to get a high viscous solution for coating. The prepared solution is coated on the Al 7075—T651 alloy and made to dry in an oven for 1 h at 80 °C. Since, the coating is done by pouring drops over the samples, we could not say the thickness accurately. So we can say approximate coating thickness around 0.1 mm.
2.3. Corrosion test
Corrosion is induced in the prepared samples using 3 M HCl. The prepared 3 M HCl is poured on all the samples for every half an hour, in atmospheric condition. Figure 1 shows the corrosion on uncoated Al 7075—T651 and GO—PMMA coated Al 7075—T651 sample. In this paper, codes are fixed to avoid repetition of words. 0 h, GP 0 h, 24 h, 48 h, 72 h, GP 24 h, GP 48 h, GP 72 h refers to corrosion free, corrosion free GO—PMMA coated, 24, 48, 72 h corroded uncoated sample, 24, 48, 72 h corroded sample with GO—PMMA coating respectively.
Figure 1. Schematic representation of behavior of samples. (a) Corrosion behavior on the uncoated sample, (b) Corrosion behavior on GO—PMMA coated sample.
Download figure:
Standard image High-resolution image2.4. Material characterization
XRD measurements are taken by Rigaku Ultima III X-ray diffractometer in the range of 10 to 90° with an interval of 0.05° using Cu Kα (λ = 1.54 Å). SEM study is done by Hitachi S-3000H scanning electron microscope. The Raman study is carried out in Horiba Jobin Yvon Labram HR Evolution Micro Raman spectrometer using laser of 532 nm wavelength and 5 mW power. The spectra are measured in the range of 50–500 cm−1. Pulsed thermography is carried at a maximum of 6000 Watt/sec. A fixed flash tube and a movable reflector is used. The cable length is 16.5'. The modelling light wattage is 650 with 120 V. The sensor type is InSb. Waveband is 1.5–5.1 μm. The pixel resolution is 320 × 256 and the pitch is 30 μm. Aperture is F/3 with a frame rate of 1 Hz to 380 Hz. Input voltage given is 12 V DC. Power consumption is 30 W/25 W. I–V characteristic study is carried out using keysight B2901A source measuring unit by applying −5 to +5 V on the sample and measure the corresponding current.
3. Results and discussion
3.1. XRD measurements
The XRD measurements on the surface corrosion are taken from 10 to 90° range with an interval of 0.05°. Most intense peaks are analyzed instead of all the small shoulder peaks. There are several small peaks because of the oxide formation in the samples. Figure 2 shows the XRD measurements for 0, GP 0, 24, GP 24 and 48, GP 48, 72, GP 72. The peaks at 39, 45, 65, 78° corresponds to (111), (200), (220), (311) planes confirms the presence of aluminium in 0, GP 0, 24, GP 24 h samples respectively. Even though the samples 24 and GP 24 are corroded for 24 h by pouring HCl for every half an hour, there is no sign of oxide formation like Al2O3, α- Al2O3, γ- Al2O3 were identified in these samples. The peaks at 45, 65, 78° corresponds to (200), (220), (311) planes confirms the presence of aluminium in 48, GP 48, 72, GP 72 h samples respectively. The peaks at 21, 28, 30° corresponds to (110), (120), (004) planes in 48, GP 48, 72 h samples confirm the Al(OH)3 formation respectively [21, 22]. The XRD measurements when compared between GO—PMMA coated and uncoated samples, it is observed that the intensity of peaks are high in 24, 48, 72 h samples. So, we conclude that GO—PMMA coating on GP 24, GP 48, GP 72 samples are acting as corrosion resistance, which is observed by the low intensity of peaks in these samples. Several small peaks are identified which are due to the cracks that are formed in the material due to the corrosion. Since aluminium is the major element in this alloy, the probability of Al(OH)3 formation is high which is observed in the samples.
Figure 2. XRD pattern of all samples, (a) Peak indexing confirms the presence of aluminium in the samples respectively, (b) Peak indexing shows the Al(OH)3 formation in the samples.
Download figure:
Standard image High-resolution image3.2. Scanning electron microscope study
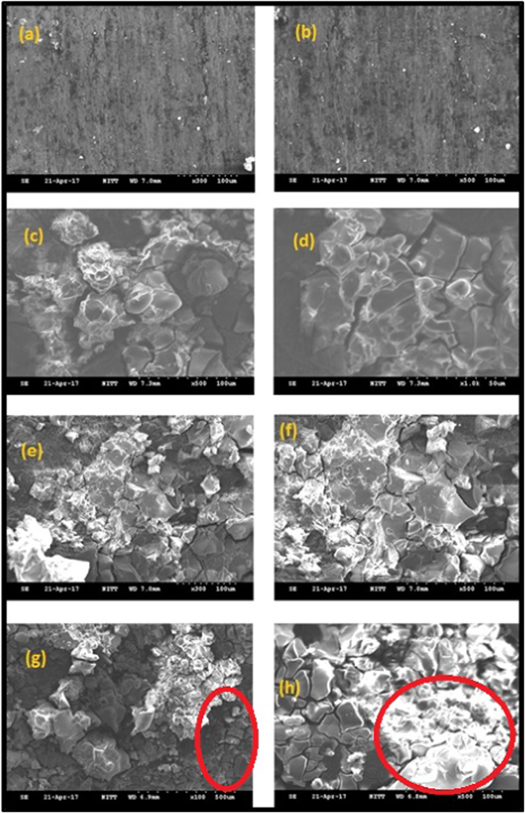
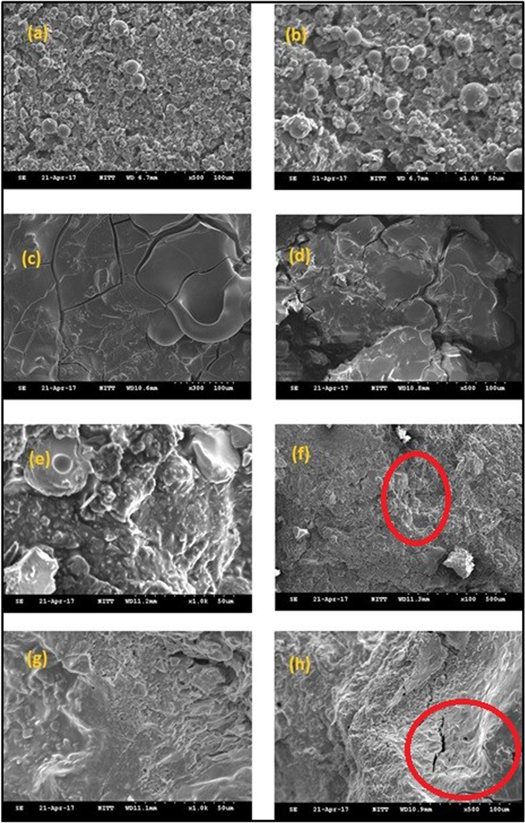
Figures 3 and 4 shows the SEM images of 0 h, 24 h, 48 h, 72 h, and GP 0 h, GP 24 h, GP 48 h, GP 72 h samples respectively. The structure of aluminium is very like other metals. It is malleable and ductile due to its polycrystalline structure. After the corrosion process, there are many differences in the phases formed in the base metal with and without GO—PMMA coating. Figures 3(a), (b) shows the 0 h sample which is corrosion free [23, 24]. Figures 3(c), (d) shows the 24 h sample in which the aluminium hydroxide formation is minimum. Various magnification shows the phase formation in detail. Figures 3(e), (f) shows the 48 h in which the formation is even higher than the 24 h sample. It clearly indicates the progress of corrosion on the surface of the sample with respect to time. Figures 3(e), (f) with 500× and 300× magnification shows how high the corrosion is induced in the sample. Figures 3(g), (h) shows the 72 h in which the material gone worse than the previous sample because of the high rate of corrosion induced in the sample due to more exposure time. Comparison of all images in figure 3 shows the phases formed and the progress of the phases formed in the sample with respect to time due to corrosion is clearly shown. Figures 4(a), (b) shows the GP 0 h sample. The coating looks like a bubble-like structure and is uniform in the sample. Figure 4(b), higher magnification of 1000× shows even clearer. Figures 4(c), (d) shows the GP 24 h sample, when compared with 24 h sample shows an improvement in the attempt of making GO—PMMA coating a corrosion resistant one. The phase formation is not much dense as formed in 24 h sample. Figures 4(e), (f) shows the GP 48 h sample in which the oxide formation is much suppressed by the coating on the sample. It is clearly identified with various magnification sizes. Figures 4(g), (h) shows the GP 72 h sample in which phase formation is high due to high exposure time, because of coating the fusion of coating and phase formed is observed highly with various magnification. In overall comparison between figures 3 and 4, it is clearly identified that the attempt to make GO—PMMA coating as a corrosion resistant is achieved as the oxide formation is suppressed in coated samples.
Figure 3. (a), (b)—0 h, (c), (d)—24 h, (e), (f)—48 h, (g), (h)—72 h. The phase formation is increasing with respect to time. (g) Cracks propagated in the surface because of higher exposure time, (h) High phase formation because of higher exposure time. Marked spaces show that the formation of cracks which leads to pitting like corrosion.
Download figure:
Standard image High-resolution imageFigure 4. (a), (b)—GP 0 h, (c), (d)—GP 24 h, (e), (f)—GP 48 h, (g), (h)—GP 72 h. The phase formation is suppressed due to GO—PMMA coating which acts as corrosion resistant in the samples. (e) It is the higher magnification of area marked in (f). The phase formation is visible at 1000× magnification only. (h) Cracks are identified only at the phase formed in the samples and not on the material.
Download figure:
Standard image High-resolution image3.3. Raman spectroscopy study
Figures 5(a), (b) shows the Raman data for 0 h, GP 0 h, 24 h, GP 24 h, 48 h, GP 48 h, 72 h, GP 72 h respectively. The Raman shift for 0 and GP 0 h are identified at 1342, 1593 and 1342, 1582 cm−1 respectively [25, 26]. It confirms the presence of aluminium and GO in 0 h and GP 0 h sample respectively. In 24 h sample, a small peak and a strong peak are identified at 1645 cm−1 and 3365 cm−1 respectively. But in GP 24 h sample, a strong band at 3365 cm−1 is only identified which denotes that it avoids noises at 1645 cm−1 region which act as corrosion resistant medium. In 48 and GP 48 h sample a strong band is identified at 3377 cm−1 regions. GP 48 h avoids the peaks formed at 1366 and 1666 cm−1 in 48 h, indicates it is acting as a corrosion resistant medium. In 72 and GP 72 h sample a strong band is identified at 3437 cm−1 regions. GP 72 h avoids the peaks formed at 1354 and 1642 cm−1 in 72 h, indicates it is acting as a corrosion resistant medium. In this alloy, Aluminium is the major constituent, Zinc is the second major element. Since aluminium is around 90% of material, oxide formations like Al2O3, Al(OH)3 is highly probable. In all the samples, a strong band is identified at 3300–3400 cm−1 region which denotes the formation of Al(OH)3 in all the samples due to the corrosion induced in the material [27]. It is also observed that when comparing between GO—PMMA coated and uncoated sample, the intensity of peak is more in uncoated sample than GO—PMMA coated sample. It denotes that the GO—PMMA coated layer is acting as a corrosion resistant layer which is the main aim of this work.
Figure 5. Raman spectra for all samples, (a) bands at 1645 cm−1 is not there in GO—PMMA coated sample which shows it is acting as a corrosion resistant layer, (b) bands at around 1350 and 1650 cm−1 is not there in GO—PMMA coated sample which shows it is acting as a corrosion resistant layer.
Download figure:
Standard image High-resolution image3.4. I–V characteristics study
I–V characteristics study gives the behavior of the material within the electric circuit with respect to the voltage applied. The circuit diagram is shown in figure 6(a).
Figure 6. I–V characteristics curves, (a) Circuit diagram, (b) I–V curves of 24, 48, 72 h samples, (c) I–V curves of GP 24, 48, 72 h samples.
Download figure:
Standard image High-resolution imageFigure 6(b) clearly indicates the shift in the curve between 24 h, 48 h, 72 h samples. Figure 6(c) shows the variation in the curve for GP 24 h, GP 48 h, GP 72 h samples. The variation is because of the oxide formation which is formed on the surface. When time prolongs it starts to be worn out which results in a shift in current because of the pitting formed that is confirmed in SEM. When time prolongs it starts to be worn out which results in a shift in current [28]. It is observed from 24 h to 72 h samples. In figure 6(c), because of GO—PMMA coating on the samples, the oxide formation is not much observed on the samples as like previous samples. There is no much difference in profile but with variation in current scale. It shows passivation behavior of GO—PMMA coating, which resists oxide formation in the samples. Thus, we come to conclude that more compact filler matrix GO—PMMA coating used in our work reduced much oxide formation to act as corrosion resistant layer.
3.5. Pulsed thermography study
In pulsed thermography, the heat pulse from the flash lamb reaches the material and penetrates through it. There are two modes in thermography, namely transmittance and reflectance mode. We have used transmittance mode of thermography for our study. The diffusion of heat through the material is recorded. If there is no defect, the temperature decreases uniformly. Instead if there is any defect, there will be changes in the temperature variation pattern accordingly. We have calculated temperature difference between defect area (corroded area) and non-defect area (uncorroded area), material loss and running contrast to show the variation in the temperature decay pattern between the accelerated corrosion in both GO—PMMA coated and uncoated sample. The thermal conductivity of the Al 7075—T651, k is 130
W
m−1
k−1
, specific heat capacity, C is 960 J kg−1
k−1
, and density
ρ
is 2810
kg
m−3
. The heat pulse given is 6 KJ for 2 ms for every sample. The dimensionless Fourier number for the heat transfer is  [29]
[29]
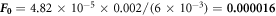
The diffusivity ( α ) is the ability of a given material to transmit a temperature variation.
 where C is the specific heat and it is calculated as [30–32]
where C is the specific heat and it is calculated as [30–32]
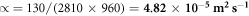
The temperature variation pattern is shown in figure 7
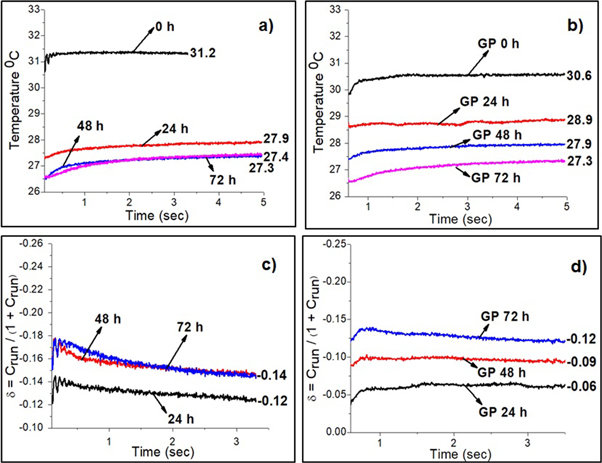
Figure 7. (a) Temperature variation pattern in 0 h, 24 h, 48 h, 72 h; (b) Temperature variation pattern in GP 0 h, GP 24 h, GP 48 h, GP 72 h; (c) Material loss in 0 h, 24 h, 48 h, 72 h; (d) Material loss in GP 0 h, GP 24 h, GP 48 h, GP 72 h.
Download figure:
Standard image High-resolution imageFrom figure 7(a), it is observed that the after the initial high heat pulse, the temperature continuous to follow a steady state. The temperature reaches a steady state at 31.2, 27.9, 27.4, 27.3 °C in 0 h, 24 h, 48 h, 72 h respectively. Since 0 h is uncorroded sample, it transmits high heat but in case of corroded samples, the oxide formation reduces the temperature transmitted through the material. The more the oxide formation, the less the temperature is. From figure 7(b), it is observed that after initial high heat pulse, the temperature reaches a steady state at 30.6, 28.9, 27.9, 27.3 °C in GP 0 h, GP 24 h, GP 48 h, GP 72 h respectively. On comparing figures 7(a) and (b), we observed that because of more oxide formation very less temperature difference is identified after steady state in without coating samples but in GO—PMMA coated sample, the temperature difference is identified higher than that the uncoated samples which supports our work on using GO—PMMA coating as corrosion resistant layer. Here, because of less oxide formation, the temperature variation is identified higher. The temperature difference, running contrast, material loss are calculated by the following equations [29].
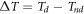
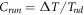
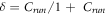
 indicates the temperature difference,
indicates the temperature difference,  denotes temperature in non-defect area (corrosion free area),
denotes temperature in non-defect area (corrosion free area),  denotes temperature in defect area (corroded area),
denotes temperature in defect area (corroded area),  indicates running contrast,
indicates running contrast,  indicates material loss.
indicates material loss.
In figure 7(c), the material loss for 24 h, 48 h, 72 h varies from −0.12 to −0.14 but the material loss is less in GO—PMMA coated samples, GP 24 h, GP 48 h, GP 72 h, which varies from −0.06 to −0.12 in figure 7(d). Material loss is a serious factor in corrosion. The more the material loss is, the quicker the material will go worse. So, by using the GO—PMMA coating on the samples the material loss is reduced due to more compact filler-matrix interface. Thus the presence of passivation layer clearly indicates it is acting as a corrosion resistant layer.
4. Challenges and future scope
Many researches are going on across the globe in using GO for anti-corrosion applications. However challenges for researchers are developing a uniform layer over the surface of application. The future scopes of present work are
- Developing a mechanism for uniform coating over the surface
- Applications over marine based alloys, Al 5052, etc.
- Corrosion study using phased array technique.
- Study of corrosion protection with localized electro chemical technique.
- Study of corrosion for longer time inhibition.
- Future research should also focus on more advanced characterization techniques and more basic techniques to clarify the mechanism of protection further.
5. Conclusion
Graphene Oxide based materials are opening an interesting field of research in current situation. It has a wide range on application in steel industries, aerospace industries, etc. This work demonstrated the GO—PMMA coating on Al 7075—T651 as an anti-corrosive coating. The results presented will be useful for development of next level of research in near future. Major outcomes are summarized below.
- XRD studies confirmed the presence of aluminium in the sample as it is the major composition of the alloy used. It also confirmed the presence and absence of Al(OH)3 formation in the plain and GO—PMMA coated samples respectively.
- Raman data supports XRD and confirmed the presence of Al(OH)3 in the corroded samples.
- SEM study indicated the difference between the phases formed in 0, 24, 48, 72 h and GP 0, GP 24, GP 48, GP 72 h samples.
- I—V characteristics study helped to find shift in current due to corrosion.
- Pulsed thermography study helped to find the material loss between coated and uncoated samples. Finally, it is confirmed that GO—PMMA coating used in our research work acts as a mechanism of protection to resist corrosion.
Data availability
The raw and processed data required to reproduce these findings cannot be shared at this time due to legal or ethical reasons because for the first time, we report GO—PMMA coating as an anti-corrosive layer in Al 7075—T651. So, we fear somebody may copy our work.