Abstract
Developmental effects of a binary mixture of nanoparticles (NPs) composed of Titanium dioxide (TiO2) and iron oxide (Fe2O3) were identified in Daphnia magna using a chronic toxicity test. Survival, growth, reproduction, and age at first brood were measured throughout 21 days of exposure. Results from this study demonstrated that mixture exposure to NPs (TiO2 and Fe2O3) significantly affects the development of D. magna to maturation and disturbs the reproductive performance in a dose-dependent manner and beginning from the lower dose. Mortality recorded in control group (1 ± 0.365) was significantly lower than in experimental groups (6.94 ± 0.193, 7.19 ± 0.188, 7.62 ± 0.125, 7.62 ± 0.155 individual, in C1, C2, C3, and C4, respectively). Moreover, swimming performances (F4, 35 = 112.9, df = 4, p < 0.0001) and heart rate (F4, 25 = 19.37, df = 4, p < 0.0001) were also affected. In conclusion, the interaction of binary mixtures can exacerbate toxicological effects that have significant implications for appropriately assessing the ecotoxicological effects of emerging pollutants. Further investigation and the results reported in the present study will be useful in environmental policies.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In the last decade, the use of nanomaterials has continued to progress in different fields of emerging technologies such as electrical engineering, chemistry, materials science, and medicine (Nations et al 2011). Consequently, new research concentrating on improving their performance and suitability was developed. However, because of their extensive usage and inevitable leakage into the environment, there is a lot of concern about their adverse impacts (Liu et al 2022, Zergui 2023).
Titanium dioxide (TiO2) and iron oxide (Fe2O3) are two nanoparticles (NPs) widely spread and produced at 5000 and 55 tons per year in industrial processes, cosmetics, and the pharmaceutical industry (Piccinno et al 2012). They will reach a production rate of 2.5 million tons by the year 2025 (Wang et al 2019). In the aquatic environment, the accumulation of NPs of TiO2 from discharges (e.g., nano paint, cosmetics and sunscreens, food additives) can disturb the environment and affect aquatic organisms (e.g., phytoplankton, Daphnia magna, fish, and bacteria) (Zhu et al 2010b, Fan et al 2016, Novak et al 2018, Fan et al 2019, Liu et al 2019). Moreover, iron oxide NPs (Fe2O3) are also frequently used in several sectors, such as water treatment, magnetic storage, cosmetics, and the chemical industry) (Ates et al 2020). However, occupational exposure to iron oxide nanoparticles leads to health and environmental risks (Farsi et al 2021). The toxicity of TiO2 and Fe2O3 was established using animal experimentation according to OECD (Organization for Economic Cooperation and Development) guidelines (Buschmann 2013).
Literature shows that, D. magna is one of the most commonly used bio-models for aquatic toxicology tests due to its high sensitivity to environmental changes and chemicals, its large size compared to other species, and its ease of use in vivo (Gaiser et al 2009). Several previous studies reported the use of different nanoparticles types (ZnO, CuO, CeO2 and AgNPs) to assess their toxic effects on D. magna physiology and behaviour (Fan et al 2017, Dai et al 2020, Milenković et al 2021, Galhano et al 2022, Ribeiro Santos-Rasera et al 2022).
Prior research also indicates that the toxic effect of individual exposure to nanoparticles is widely known; however, the combined effects are unknown or poorly studied (Deng et al 2018, Forest 2021). Because it is important to consider that in real life, we are exposed simultaneously to mixtures of (NPs), generally in a continuous manner and at low levels, with possible additive, antagonistic, potentiation or synergistic activities of the resulting mixture. In this study, we hypothesize that the coexistence of the two NPs (TiO2 and Fe2O3) in different concentrations in the environment may affect the life history and behaviour of D. magna. After that, we evaluate the combined effects of TiO2 and Fe2O3 NPs on the physiological (mortality, life span, growth rate, and reproduction such as age of maturation, number of eggs, and heart rate) and behavioural (swimming speed) endpoints of D. magna.
2. Material and methods
2.1. Nanomaterials
All Nanomaterials (TiO2 and Fe2O3) were supplied as powders from the laboratory of chemistry (Guelma University Algeria) free of metals and organic impurities. The TiO2 NPs (product number: 637254, particle size: 25 nm, 99.7% trace metals basis). The Fe2O3 were elaborated at the laboratory of chemistry; it was 26 nm in size. The elaboration of α-Fe2O3 nanoparticles was carried out using mechanical grinding at from the elemental powder of hematite. The milling was performed in a Pulverizing 7 planetary mill (Fritsch, Idar-Oberstein, Germany) using two steel jars (stainless steel with a volume of 45 ml). The preparation of the charge (beads and powders) was carried out in a glove box under argon atmosphere. The mass ratio beads to powders were approximately 1/20 and the grinding speed was at 500 rpm. To minimize the effects related to the temperature increase inside the jars, the grinding was done with sequences of half an hour followed by 15 min of pause and this for 3 h. Before the exposure experiments, stock solutions were sonicated for 30 min (50 W L−1 at 40 kHz) and diluted with ultrapure water.
2.2. Culturing experiment
The freshwater crustacean D. magna used in this study were collected from a water pond in the Algerian park (Jardin d'essai du Hamma, Algiers; 36° 44' 53'' North, 3° 04' 34'' East). D. magna were maintained in unpolluted natural water at a constant temperature of 23.5 °C with a 16:8 h (light–dark) photoperiod at the University of Guelma, Algeria. D. magna cultures were maintained in 1 l glass beakers (20 individuals/beaker). The culture medium was renewed every 48 h, and daphnids were fed daily with Raphidocelis subcapitata at a concentration of (3 × 105 cell/mL). Third or fourth generation neonates (<24 h) from the same female were collected to eliminate variation between clones. Individual neonates of similar size were exposed to different concentrations of NPs: In the immobilization test, various concentrations of (TiO2 + Fe2O3) in a 1:1 fixed ratio (v/v), namely 0, 2.55, 7.75, 13, and 27.5 mg l−1, were used. These concentrations were represented as control, C1, C2, C3, and C4, respectively. For the chronic test, concentrations of 50% (TiO2 + Fe2O3) were employed in a 1/2:1/2 fixed ratio (v/v), specifically 0, 1.27, 3.87, 6.5, and 13.75 mg l−1, which corresponded to control, C1, C2, C3, and C4, respectively.
2.3. Immobilization tests
Immobilization tests were performed based on the OECD guideline 202 (OECD 2004).Tests were carried out using sixteen replicates randomly per concentration and negative control, with eight neonates with ≤ 24 h per replicate exposed in 50 ml glass beakers. Tests were maintained at room temperature of 20 ± 1 °C and 16:8 h light: dark photoperiod and daphnids were not fed during the entire experiment. After 12, 24, and 48 h, immobilization (i.e., inability to swim within 15 s after gentle agitation of the beaker) and mortality were assessed using a Leica microscope equipped with a digital camera (Leica, Wetzlar, Germany) and then recorded (Ozmen et al 2023). The concentration that caused the immobilization of 50% of the neonates (LC50) was calculated. Nominal concentrations of single exposures of TiO2 and Fe2O3 were (0.1 ; 0.5 ; 1 and 5 mgl−1) and (5 ; 15 ; 25 and 50 mg ml−1), respectively, were chosen according to their EC50 values and from previous modified published studies. Mixture concentrations of 100% (TiO2 + Fe2O3) used were (0; 2.55 ; 7.75 ; 13 and 27.5 mg L−1) of (control; C1; C2; C3; C4), respectively. Theses concentrations were chosen according to their EC50 values from the single exposures for each experimental setup.
2.4. Chronic toxicity test
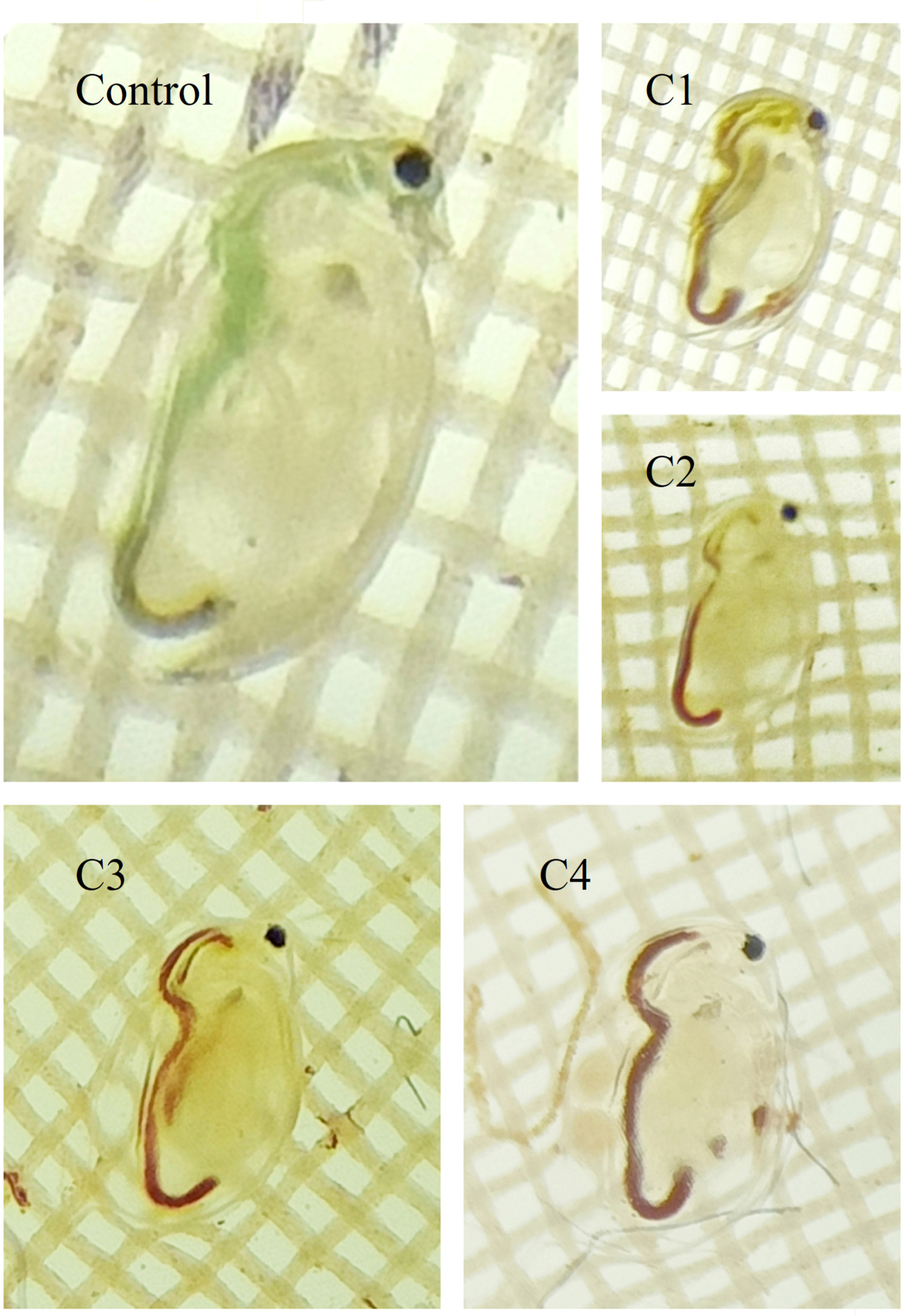
The chronic toxicity of the mix of NPs TiO2 + Fe2O3 was assessed according to an OECD standard protocol (OECD 211 1998) with modification. Based on the acute toxicity results, neonates (6–24 h old) where exposed for 21 days under the following concentrations that were chosen according to the acute test: 50% (TiO2+ Fe2O3) (control); 1.27 ; 3.87 ; 6.5 and 13.75 mg l−1 (figure 1).
Figure 1. Coloration changes in D. magna exposed to (TiO2 and Fe2O3) at four concentrations (C1, C2, C3, C4) and with Control treatment.
Download figure:
Standard image High-resolution imageIn this experiment, eight neonate (aged 6–24 h) were placed in each glass beakers containing 50 ml of test solution. A total of 16 randomly placed replicates of each concentration. Test containers were monitored every 24 h to record deaths or any anomaly. Daphnids were fed daily using Raphidocelis subcapitata at a concentration of (3 × 105 cell mL). Each adult (and offspring if present) was carefully separated from beaker and test solutions were replaced each three days. The incubation was carried out at a temperature of 23 °C, under the same photoperiod conditions as those used in the culture experiment. The chronic effect endpoints recorded were used to evaluate the reproduction of D. magna including the age of maturation, and the number of eggs per female. The number of mortality of adults in each treatment was recorded during the exposure period and life span.
2.5. Effects of chronic exposure on D. magna growth
Growth rate was defined by the average increase in body length of individuals (8 ind of D. magna) in each measurement for each cup from the beginning of the experiment to the 21 days. The body length was measured from the anterior part of the head to the end of the body, excluding the caudal spine. It was calculated from photos taken every five days using a photo processing software image J.
2.6. Effects of chronic exposure on D. magna life span and mortality
The mortality rate (i.e., number of individuals dying at a specific time) was determined as the slope of the number of individuals dying versus time over the 21-day duration of the study. However, the life span was defined by the number of days of life of each individual in each cup throughout the period of the experiment.
2.7. Effects of chronic exposure on D. magna maturation and reproduction
Throughout the experiments, mature females were individually placed in separate cups, with one female per cup. These cups maintained the same conditions as the solution concentration, temperature, and daily feeding with Raphidocelis subcapitata. When the eggs hatched, we recorded the first brood's date and the number of eggs.
2.8. Effect of mix on behaviour (swimming performance and heart beat rate)
2.8.1. Swimming performance
After 48 h of exposure, we randomly selected eight individuals from each concentration group, including the control, within the NP mixtures containing various concentrations of (TiO2 + Fe2O3). This selection was made to assess their swimming speed. The individuals were transferred separately to transparent graduated cups of 100 ml of similar environmental conditions to that of the experiment. After stabilisation, the swimming distance was monitored every minute for 10 min, using a millimeter paper. The latter method was modified from the protocol used by Kim et al (2021).
2.8.2. Heart beat rate
After the immobilization test five neonates were randomly selected from each concentration groups and the control group to examine the heartbeat count in each exposure condition. The heart rate was recorded as a video file for one min using an integrated PC light microscope at 4× grossing (Optika microscopes, Italy). The heart rate was manually counted using a low-speed reading (×0.3) and repeated three times, with the mean values of each sample used for comparison (Kim et al 2021).
2.9. Statistical analysis
Statistical analyses were carried out with R 4.0.2 (R Core Team 2021). The normality, homogeneity assumptions, and residual patterns in the data were checked (Zuur et al 2007). A linear mixed-effects models was used to compare the growth rate of the species between concentration treatments including concentration treatments as fixed effect and cups as random intercept, Tukey's post hoc test with glht function was applied using multcomp package (Hothorn et al 2008) to reveal potential differences in the growth rate between the different treatments. A separate generalised linear mixed effect model with Poisson error distribution with Log link function was applied to assess the difference of all traits: life span (the number of days of life of each individual in each cup), age at maturation (time in days until first brood of each individual), eggs number at the first brood, and mortality of the species between the treatments concentrations. This was followed by Tukey post hoc analyses for pairwise comparison of the different nanoparticle concentration exposures. The models included trait as a response variable, treatments concentrations as fixed effect and females ID or cups (to account for over-dispersion) as random effect. All models were fitted using the R package lme4 (Bates et al 2015). ANOVA analyses were conducted to determine the effects of the nanoparticle concentrations treatments on the swimming performance and heart beats count. To evaluate which levels of the nanoparticle treatment differed we additionally carried out Tukey pair-wise tests. The data was presented as mean ± standard error (SE). The minimum threshold of significance retained is p < 0.05.
3. Results
3.1. Effects of chronic exposure on D. magna growth
Growth rate was defined by the average growth rate of body length of individuals in each measurement for each cup from the beginning of the experiment (21 days). ANOVA analysis of a general linear mixed effects model (LMER) showed a highly significant effect of treatment on growth rate of the species compared to the null model (χ2 = 637.78, Df = 4, p < 0.0001) (table 1 and figure 2). However, growth rate between the control group and the different nanoparticle concentration exposed groups showed statistically significant differences (table S1). Subsequent Tukey tests for pairwise comparisons of growth rate of length showed that the four concentrations of mixed nanoparticles exposures (C1, C2, C3, and C4) significantly affected the growth of length of D. magna compared to the control treatment, and the growth rate was higher at the control treatment. Individuals from the control group had an average growth rate of 0.0137 ± 0.0002 mm, as compared with the experimental group growth rates of 0.007 ± 0.0002 mm, 0.006 ± 0.0002 mm, 0.005 ± 0.0003 mm, 0.00453 ± 0.0004 mm, for C1, C2, C3, C4, respectively.
Table 1. Summary results of the general linear mixed effects model assessing the effect of (mixed nanoparticles) concentrations on the growth rate of length of D. magna.
| Estimate | SE | Df | t value | p value | |
|---|---|---|---|---|---|
| (Intercept) | 0.0070476 | 0.0003229 | 40.2427914 | 21.829 | <2e-16 *** |
| Concentration C2 | −0.0006903 | 0.0004072 | 68.0000000 | −1.695 | 0.094623 |
| Concentration C3 | −0.0015111 | 0.0004072 | 68.0000000 | −3.711 | 0.000418 *** |
| Concentration C4 | −0.0025128 | 0.0004072 | 68.0000000 | −6.171 | 4.26e-08 *** |
| Control | 0.0066764 | 0.0004072 | 68.0000000 | 16.395 | <2e-16 *** |
The concentration C1 (1.27 mg l−1) is used as the baseline level for contrast calculation. C2 (3.87 mg l−1), C3 (6.5 mg l−1), C4 (13.75 mg l−1). *** indicates a probability significance: ***—p < 0.001; **—p < 0.01; *—p ≤ 0.05.
Figure 2. Growth rate of body length of individuals in each treatment group during the exposure period of 21 days to (TiO2 and Fe2O3) at four concentrations (C1, C2, C3, C4) and with Control treatment. Error bars represent the standard error. Means which are denoted by different letters (a, b, c, and d) indicate a significant difference between different treatments, and same letters denote non-significance (Tukey's post-hoc test, p < 0.05) (see: Supplementary Material, table S1).
Download figure:
Standard image High-resolution image3.2. Effects of chronic on D. magna life span and mortality
Life span was defined by the number of days of life of each individual in each cup throughout the period of experiment. ANOVA analysis of a general linear mixed effects model (GLMER) showed a highly significant effect of treatment on life span of the species compared to the null model (χ2 = 1229.8, Df = 4, p < 0.0001) (table 2 and figure 3(a)). The Tukey post-hoc analysis (table S2) indicated that the number of days in which the organism was in life decreased significantly from an average of 20.1 ± 0.188 days in the control treatment to 10.7 ± 0.503, 9.58 ± 0.514, 7.45 ± 0.418, 7.19 ± 0.440 days in C1, C2, C3, and C4, respectively.
Table 2. Summary results of the generalised linear mixed effects model assessing the effect of (mixed nanoparticles) concentrations on the life span, mortality, age of maturation, and reproduction of D. magna.
| Response variables | Estimate | SE | z value | p value |
|---|---|---|---|---|
| Life span | ||||
| (Intercept) | 2.31783 | 0.03997 | 57.987 | <2e-16 *** |
| C2 | −0.11324 | 0.03917 | −2.891 | 0.00384 ** |
| C3 | −0.36514 | 0.04202 | −8.689 | <2e-16 *** |
| C4 | −0.40038 | 0.04247 | −9.428 | <2e-16 *** |
| Control | 0.62769 | 0.03331 | 18.841 | <2e-16 *** |
| Age at maturation | ||||
| (Intercept) | 2.64415 | 0.07125 | 37.112 | <2e-16 *** |
| C2 | 0.11446 | 0.11008 | 1.040 | 0.29847 |
| C3 | 0.24623 | 0.12723 | 1.935 | 0.05295. |
| C4 | 0.36401 | 0.13199 | 2.758 | 0.00582 ** |
| Control | −0.74997 | 0.11801 | −6.355 | 2.08e-10 *** |
| Eggs number of_first brood | ||||
| (Intercept) | 1.0245 | 0.1601 | 6.398 | 1.57e-10 *** |
| C2 | −0.3314 | 0.2850 | −1.163 | 0.245 |
| C3 | −0.4367 | 0.3698 | −1.181 | 0.238 |
| C4 | −0.6190 | 0.4385 | −1.412 | 0.158 |
| Control | 1.4305 | 0.1752 | 8.166 | 3.20e-16 *** |
| Mortality | ||||
| (Intercept) | 1.93694 | 0.09492 | 20.407 | <2e-16 *** |
| C2 | 0.03540 | 0.13306 | 0.266 | 0.790 |
| C3 | 0.09449 | 0.13117 | 0.720 | 0.471 |
| C4 | 0.09449 | 0.13117 | 0.720 | 0.471 |
| Control | −1.93694 | 0.26741 | −7.243 | 4.38e-13 *** |
The concentration C1 (1.27 mg l−1) is used as the baseline level for contrast calculation. C2 (3.87 mg l−1), C3 (6.5 mg l−1), C4 (13.75 mg l−1). *** indicates a probability significance: ***—p < 0.001; **—p < 0.01; *—p ≤ 0.05.
Figure 3. Daphnia magna individual responses traits in each treatment group during the exposure period of 21 days to (TiO2 and Fe2O3) according to the four concentrations (C1, C2, C3, and C4) and with Control treatment. (a) Longevity (days), (b) Mortality, (c) Age at maturation, (d) Number of eggs at the first brood. Error bars represent the standard error. Means which are denoted by different letters (a, b, c, and d) indicate a significant difference between different treatments, and same letters denote non-significance (Tukey's post-hoc test, p < 0.05) (see: Supplementary Material, table S2, S3, S4, S4).
Download figure:
Standard image High-resolution imageMortality rate (number of individual deaths in specific time) was obtained as the slope of number of died individuals versus time over the 21-day experimental period. Mortality of D. magna occurred in all exposure treatments including the control treatment with (20.48%). The mortality rate of D. magna increased significantly with the increase of mixed nonoparticles concentrations (χ2 = 62.32, Df = 4, p < 0.0001) (table 2 and figure 3(b)). The assessment of all pairwise combinations using the Tukey contrast test (table S3) revealed that the control group was the only group with a statistically significant difference in mortality rate compared to the four concentrations groups. Individuals from the control group had an average mortality of 1 ± 0.365 individual, as compared with the experimental group mortality of 6.94 ± 0.193, 7.19 ± 0.188, 7.62 ± 0.125, 7.62 ± 0.155 individual, in C1, C2, C3, and C4, respectively.
3.3. Effects of chronic exposure on D. magna maturation and reproduction
The concentration of nanoparticle affected the age at which the species became mature (χ2 = 81.15, Df = 4, p < 0.0001). The age of maturation differed between treatments (table 2 and figure 3(c)). Subsequent Tukey tests for pairwise comparisons (table S4) of age at maturation showed that the four concentration of mixed nanoparticles exposures (C1, C2, C3, C4) significantly impacted the date of the first brood compared to the control, increasing it to (14.1 ± 0.438, 15.8 ± 0.521, 18 ± 1.05, and 20.2 ± 0.479 days), respectively. The Tukey test revealed that the age at maturation was significantly greater in concentration C4 when compared to concentrations C1 and C2.
The concentration of nanoparticle affected egg number at the first brood of D. magna (χ2 = 141.91, Df = 4, p < 0.0001) (table 2 and figure 3(d)). The average number of eggs at the first brood in the control treatment was 11.6 ± 0.80 eggs. The Tukey post-hoc analysis (table S5) indicated that the four concentration of mixed nanoparticles exposures (C1, C2, C3, C4) significantly impacted the number of eggs at the first brood of D. magna compared to the control, decreasing it by (77%, 83%, 85%, and 88%), respectively. Notably, no difference of number of eggs was found between the four concentrations (table S5).
3.4. Swimming performance and heart beat rate
The mixed nanoparticle affected the swimming performance (F4, 35 = 112.9, df = 4, p < 0.0001) (figure 4(a)). Swimming speed differed significantly between the control treatment and the four concentrations treatments, the swimming speed decreasing from 0.942 ± 0.0178 mm min−1 of the control treatment to (0.569 ± 0.0346, 0.504 ± 0.0125, 0.417 ± 0.0258, and 0.285 ± 0.0190 mm/min) for (C1, C2, C3, C4 treatment), respectively. There was a significant difference in swimming speed between the concentration C4 and the other three treatments concentration (C1, C2, C4), and it was lower in C4 (table S6).
Figure 4. Individual's responses traits in each treatment group after 48h of exposure to (TiO2 and Fe2O3) according to the four concentrations (C1, C2, C3, and C4) as well as the Control treatment. a) Swimming speed (mm/min), b) Heart beats/min. Error bars represent the standard error. Means which are denoted by different letters (a, b, c, and d) indicate a significant difference between different treatments, and same letters denote non-significance (Tukey's post-hoc test, p < 0.05) (see: Supplementary Material, table S6, S7).
Download figure:
Standard image High-resolution imageSix individuals were randomly selected from each group for heart rate testing. Although the mixed nanoparticle affected the heart beat rate (F4, 25 = 19.37, df = 4, p < 0.0001) (figure 4(b)).
Interestingly, the heart rate did not show significant changes at low concentrations in C1 and C2 compared to the control (319 ± 0.40, 321 ± 0.63, 305 ± 0.65 beats/min), respectively (table S7). There was a significant difference between C4 and all treatments, it was significantly higher in C4 with 79 beats/min more than control, and higher with (60, 58, and 30 beats/min) more than C1, C2, and C3, respectively.
4. Discussion
In contemporary toxicology, it is well-recognized that the impact of a molecule can exhibit significant variation when it interacts with other molecules, whether they belong to the same family (such as nanoparticles, pesticides, endocrine disruptors, etc) or not. This interaction can result in additive, antagonistic, potentiation, or synergistic effects within the resulting mixtures. For nanoparticles, the physicochemical properties, concentrations, window and duration of exposure are factors that can play a crucial role in determining their uptake, distribution, metabolism, excretion, and overall toxicity.
Unfortunately, the existing data on nanoparticle toxicity in Daphnia primarily relies on 'research grade' materials, often involving simple combinations to be able to interpret the results. To gain a more realistic understanding of nanoparticle behavior in aquatic environments and accurate assessments of their potential effects, it is imperative to study the impact of environmental conditions on their degradation.
Here we report that D. magna growth was inversely proportional to the concentration of nanoparticles, which is in accordance with data reported by Zhu et al (2010a), when exposure to nTiO2 alone at 0.5 to 5 mg l−1 inhibited D. magna growth. Furthermore, in their study, Mendoza-Villa et al (2023) found a significant decrease in the length of D. magna when exposed to TiO2 NPs alone at four out of the five tested concentrations (37.5 mg l−1, 75 mg l−1, 150 mg l−1, 300 mg l−1, and 600 mg l−1). This contrasts with the findings reported by Lee et al (2009), who used lower doses of 7 and 20 nm TiO2 alone. This difference in exposure conditions likely explains the absence of developmental effects. However, the toxicity of the mixtures including Ag+ concentrations and ZnO NPs and TiO2 NPs combinations was greater than the inherent toxicity of each component, indicating a synergistic impact (Park et al 2019).
The effectiveness of nanoparticles (NPs) in conjunction with cationic surfactants can generate mixtures of metal-oxide nanoparticles and surfactants, which exhibit remarkable sorption capabilities (Guerranti and Renzi 2015). Mixtures of [(ZnO or TiO2) and Triton] increases effects of nanoparticles on D. magna (Renzi and Blašković 2019). The combination of graphite-diamond nanoparticles with the fungicide thiabendazole demonstrated synergistic toxic interactions at low concentrations, likely due to the enhanced bioavailability of the fungicide facilitated by the nanoparticles. However, at higher doses of nanoparticles, an antagonistic effect was observed. This phenomenon was attributed to the aggregation of nanoparticles, which ultimately reduced the bioavailability of the fungicide (Martín-de-Lucía et al 2019).
From previous studies, and despite the use of NPs in remediation strategies against pesticides pollution (Akash et al 2022), nanoparticles, specifically sized titanium dioxide (nTiO2), have been shown to enhance the toxicity of pesticides (malathion, permethrin, and pirimicarb) in D. magna. This effect is likely attributed to the efficient degradation of pesticides and the potential formation of toxic by-products induced by photocatalysis (Lüderwald et al 2020). The impacts of NPs on the maturation and reproduction parameters of D. magna were also affected by other parameters such as the food availability. Sun et al (2022) reported that exposure to ZnO nanoparticles at high concentrations with low zooplankton (Chlorella) quantities can exacerbate the negative effect on the life history traits (survival time, body length at maturation, and offspring per female) of D. magna. Conversely, some other life history traits such as time to maturation and time to first brood exhibited an opposite trend in response to the nanoparticles. In addition, it was observed that higher zooplankton abundance mitigated the adverse effects of ZnO nanoparticles on D. magna. In the same way, mixtures of toxic Microcystis and ZnO NPs at several concentrations can provoke developmental alteration and body length at maturation, decreased the number of neonates in the first brood, total offspring, and number of broods per female in D. magna, without any effect on the times to maturation (Wang et al 2019).
This study assessed the harmless harmful effects of nanoparticles, through examining locomotion-based behavior which is a highly sensitive measure (Bownik 2017). Cerium Dioxide Nanoparticles (CeO2 NPs), which are used in industry as oxidation catalysts, gas sensors, polishing materials, and UV absorbers, affect the swimming performance of Daphnia similis and Daphnia pulex after 1, 10, and 100 mg L−1 exposures for 48 h. Furthermore, exposure to 1 mg L−1 induced a decrease of 30% and 40% of the swimming velocities in D. pulex and D. similis, respectively. However, at elevated concentrations, the swimming velocities of D. similis were more severely affected (with a reduction of 60% at 10 mg L−1 and 100 mg L−1) compared to those of D. pulex (Artells et al 2013). Similarly, exposure to Ceria Chitosan NPs can affect behavioural endpoints related to swimming (average speed, distance moved, and activity time) and induce hyperactive behaviour by increasing the average speed and acceleration without increasing the distance moved (Villa et al 2020).
Exposure to TiO2 increases swimming speed proportionally with the exposure duration (from 8.2 ± 0.9 mm/min for 3 h to 21.6 ± 2.8 mm/min for 48 h) and inversely proportionally with concentrations (0.1, 1.0 and 10 μg mL−1) (Park et al 2022). The heart rate and swimming distance were both linearly affected by the TiO2 nanoparticle concentration (Chung et al 2016). Previous research has documented that the induction of oxidative stress can lead to negative impacts on the heart rate, swimming speed, and reproductive capabilities of D. magna (Bownik and Stępniewska 2015, De Felice et al 2019, Park et al 2022).
5. Conclusion
Our findings demonstrated that exposition to binary mixtures of NPs (TiO2 and Fe2O3) significantly affects the development of D. magna to maturation and disturbs their reproductive performance and behavior. The intensity of the effects is proportional to the concentrations of the mixtures. Mortality recorded in the control group was significantly lower than in the experimental groups. Moreover, swimming performances and heart rate were also affected. The results from the investigation and the analysis of data indicated that the exposure of D. magna to a combination of NPs, even at minimal concentrations, significantly affected various aspects of their life history and overall organismal fitness.
In natural ecosystems, chemicals are commonly found in mixtures, and the absence of a comprehensive method for studying the combined effects of chemical pollutants and other stressors reflects the existing divisions among scientific disciplines. Moving forward, it is evident that a paradigm shift is required to address the challenges posed by chemical mixtures in the context of environmental research and management. Interdisciplinary collaboration should be promoted among ecologists, toxicologists, chemists, and other relevant fields in order to build an integrative and unified approach to investigating the cumulative impacts of chemicals in ecosystems. We may overcome the limits of particular disciplines and get a more comprehensive grasp of the ecological implications of chemical mixtures by uniting our skills and resources. The reported results have significant implications for appropriately assessing the ecotoxicological effects of emerging pollutants.
Acknowledgments
The authors would like to express their sincere gratitude to Professor Moussa Houhamdi for his valuable comments and insights on the manuscript.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Author contributions
Amira Chorfi: Investigation (Data collection, and experiments performing), Formal analysis, Writing—original draft.
Hichem Amari, Soufyane Bensouilah, Zinette Bensakhri: Investigation (Data collection, and performing the experiments).
Rabah Zebsa, Sofiane Boudalia, Samira Bensoltane: Conceptualization, Supervision, Data curation, Formal analysis, Funding acquisition, Project Administration, Methodology, Writing—original draft– Review & Editing.
Mohamed Djekoun: Conceptualization, former supervisor, Methodology.
All authors have read and agreed to the published version of the manuscript.
Financial support
This work is part of Project PRFU-2022 (Code number: D00L02UN240120220002), and this work is funded by the Algerian Ministry of Higher Education and Scientific Research and the Directorate General for Scientific Research and Technological Development (DGRSDT). The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Conflicts of interest
The authors have stated that they have no conflicts of interest to declare.
Supplementary data (0.1 MB DOCX)