Abstract
The electrolysis of water is one of the most promising strategies to produce renewable fuels and it is important to develop an energy-conserving, low-cost and easily prepared electrocatalyst for oxygen evolution reaction (OER). In this work, Ni foam supported Co4S3 (Co4S3/NF) was fabricated by a facile one-step approach at room temperature and exhibited excellent OER performance in alkaline media. Specifically, the Co4S3/NF electrocatalysts showed a small overpotential of only 340 mV to reach a current density of 100 mA cm−2 and a Tafel slope of 71.6 mV dec−1 in alkaline media. More importantly, excellent stability was achieved under a constant current density of 100 mA cm−2 for 100 h and the OER performance of the catalyst was improved after 1400 cycles of linear sweep voltammetry tests in alkaline media. Furthermore, the underpinning mechanism of action was studied by measuring the change of valence states for different elements to elucidate the structural evolution and active species during the electrocatalytic process.
Export citation and abstract BibTeX RIS
1. Introduction
Water electrolysis is a promising strategy to generate high-purity hydrogen in large-scale industrial production, which is important for the green energy scenario and the safety of future energy [1–3]. Electrolysis of water consists of two half reactions, cathodic hydrogen evolution reaction (HER) and anodic oxygen evolution reaction (OER) [4]. In recent years, electrocatalysts for HER and OER have been intensively investigated and optimized in various electrolytes (acid, neutral and base) by different strategies, such as defect engineering, doping and confinement engineering [5–13]. Compared with HER with a two-electron transfer process, the pathway for the four-electron process, OER, is more sluggish with the breaking of O–H bonds and the formation of O–O bonds, which hinders the overall efficiency of the electrolysis of water [14–16]. Noble metal oxides, such as RuO2, IrO2, show excellent catalytic performance and are regarded as the benchmark for OER electrocatalysts [17, 18]. However, the high cost, scarcity and unstable features limit the application of noble metal-based catalysts in practical use. Thus, it is important to develop low-cost, high performance and stable electrocatalysts for OER.
Compared with noble metal-based OER electrocatalysts, transition metal-based materials have been explored largely due to their high activity and abundance in Earth's crust [19]. Much effort has been devoted to developing transition metals derivatives, such as Co [20], Ni [21], Mo [22], and Fe [23] oxides [24], hydroxides [25], nitrides [26], sulphides [27] and phosphides [28] for high-performance OER electrocatalysts. Currently, some transition metal-based electrocatalysts could even outperform the benchmark precious metal OER catalysts in regard to overpotential and stability [29, 30].
Transition metal sulphides (TMSs) were demonstrated as some of the most effective OER catalysts due to their unique physical and chemical properties. In addition, TMSs behave as small band gap semi-conductors and lead to good electrical conductivity [19, 31]. For example, Qu et al [32] developed a spinel MnCo2S4 nanowire array on Ti mesh by the sulphidation of MnCo2O4 precursor. The catalysts showed an excellent OER activity with a small overpotential of 325 mV at a current density of 50 mA cm−2 in 1 M KOH, similar to that of commercial RuO2. The Tafel slope of the material is 115 mV dec−1, which implies fast OER kinetics. The catalysts also showed great stability at fixed current densities of 20 mA cm−2 and 50 mA cm−2 for 100 h. Lee et al [33] developed a series of compositional tunable Cox Niy Sz by the template-assisted method. CoO@Co9S8 core–shell structure was achieved by the sulphidation of CoO and the addition of Ni precursor could promote the transition from Co9S8 to Ni9S8. Then the Cox Niy Sz could be achieved by controlling the amount of Ni precursors and the reaction time. The optimized Co9−x Nix S8 catalysts showed an overpotential of 362 mV at a current density of 10 mA cm−2 in 1 M NaOH and the corresponding Tafel slope is 74.7 mV dec−1. However, the instability and surface reconstruction behaviour of metal sulphides during OER requires further study to uncover the specific active species.
In this work, we report the fabrication of Ni foam supported Co4S3 nanostructures (Co4S3/NF) by a single-step wet chemistry synthesis method at room temperature, which does not require external heating or annealing processes and is a low energy-process. Meanwhile, the wet chemistry synthesis provides a promising route to synthesize TSM materials at room temperature for large-scale production, which has great potential for industrial application. Owing to the unique electronic structures that endow rich active sites, the Co4S3/NF catalysts showed a small overpotential of 340 mV to reach a current density at 100 mA cm−2 in 1 M KOH and with a small Tafel slope of 71.6 mV dec−1. Furthermore, the catalysts showed excellent stability and possessed a stable output at a large current density of 100 mA cm−2 for 100 h in 1 M KOH. The 1400 cycles of linear sweep voltammetry (LSV) tests showed an even better performance afterwards. The surface reconstruction behaviour behind the outstanding stability was investigated by ex-situ x-ray photoelectron spectroscopy (XPS), which showed Co3+/Ni3+-sulphate/sulphites species and the formed Co3+/Ni3+-(oxy)hydroxide contributed to the improved performance.
2. Experimental
2.1. Material
Cobalt chloride hexahydrate (CoCl2 · 6H2O) was purchased from Sigma-Aldrich (UK) Co., Ltd. Sodium thiosulfate (Na2S2O3) was purchased from Sigma-Aldrich (UK) Co., Ltd. Potassium hydroxide (KOH) was purchased from Sigma-Aldrich (UK) Co., Ltd. Ni foam were used as received. All chemicals were used as received without further purification.
2.2. Preparation of Co4S3/Ni(OH)2 supported on Ni foam (Co4S3/Ni(OH)2@NF)
The Co4S3/Ni(OH)2 catalysts supported on Ni foam were prepared by a one-step solution phase method at room temperature. The solution was prepared by dissolving 3 g CoCl2 · 6H2O and 0.2 g Na2S2O3 into 10 ml deionized (DI) water in a small bottle. Then the Ni foam (1 × 2 cm2) was put into the bottle and left at room temperature for one day. Finally, the as-prepared samples were washed with DI water three times followed by a vacuum drying process for 12 h. The obtained electrodes were denoted as Co4S3/NF. The amount of Co precursors was optimized by changing the amount of CoCl2 · 6H2O to 1 g, 2 g, 4 g, 5 g and 6 g while other steps were the same and the prepared electrodes were denoted as Co4S3/NF-1, Co4S3/NF-2, Co4S3/NF-4, Co4S3/NF-5 and Co4S3/NF-6, respectively. The loading mass of active materials are shown in table S1 (available online at stacks.iop.org/MFM/4/025001/mmedia).
2.3. Characterization
Scanning electron microscope (SEM) (Joel 6700) was applied to study the morphologies of materials and energy-dispersive x-ray spectroscopy (EDS) was applied to study the distribution of elements of the material. A STOE SEIFERT diffractometer with Mo x-ray source was applied to obtain the x-ray diffraction (XRD) patterns for understanding the phase information of catalysts. XPS (thermo scientific K-alpha photoelectron spectrometer) was applied to study the valence states of elements on the surface of the catalysts and the data were analysed by CasaXPS software.
2.4. Electrochemical tests
A three-electrode cell was used to measure the electrochemical performance of the prepared electrodes. A graphite rod and an Ag/AgCl (saturated KCl) electrode were used as the counter electrode and the reference electrode separately. The prepared self-standing electrodes were used as working electrodes directly. The Ar-saturated 1 M KOH solution was prepared and used as the electrolyte. A Gamry Interface 1000 potentiostat was used to conduct the electrochemical measurements. LSV data were collected at a scan rate of 10 mV s−1. The measured potentials versus Ag/AgCl electrode were converted into potentials versus reversible hydrogen electrode (RHE) by ERHE = EAg/AgCl + 0.197 + 0.059 pH. Tafel slopes were obtained by calculating the slope of corresponding replotted polarization curves. The long-term stability was evaluated by the chronovoltammetry measurement and 1400 cycles of LSV scans. Electrochemical impedance spectroscopy (EIS) was performed with frequencies from 100 kHz to 100 mHz. All the LSV measurements are presented with iR compensation.
3. Results and discussion
A series of Co4S3/NF in this work were synthesized via a simple and scalable one-pot wet chemistry synthesis method. Generally, specific amounts of CoCl2 · 6H2O and Na2S2O3 were dissolved in 10 ml of DI water in a 15 ml glass vial. Then a piece of Ni foam was immersed in the solution and kept at room temperature for 24 h. The colour of the electrode become darker after the reaction. Finally, the electrodes were collected and washed with DI water for several times. The as-prepared materials were used directly as self-standing OER electrodes. This method is energy-conserving, easy to operate and showed great potential for the large-scale fabrication of electrode materials.
Cobalt precursors (1 g, 2 g, 3 g) were applied to obtain different electrocatalysts and denoted as Co4S3/NF-1, Co4S3/NF-2 and Co4S3/NF, respectively. A digital photo of electrodes prepared with different amount of Co precursors is shown in figure S1. The morphologies of electrodes and the distribution of elements were studied by SEM and EDX. The material barely grows on nickel foam for Co4S3/NF-1, which could also be demonstrated by SEM images in figures 1(a) and S2. Figures 1(b) and S3 showed the SEM images of Co4S3/NF-2. The colour of the electrode is darker than Co4S3/NF-1 and clusters of nanoparticles could be seen grown on the Ni foam skeleton. However, the materials grow unevenly on the surface of the Ni foam, which could not provide sufficient active sites for OER and thus weaken the performance. When using 3 g of Co precursor, the materials grow evenly on the surface of Ni foam and the colour is the darkest which indicates uniform and dense growth of active materials on the electrode. The SEM images of Co4S3/NF (figures 1(c) and S4) provided excellent growth of the active materials on Ni foam without blocking the 3D porous structures of Ni foams, which could provide sufficient channels for the diffusion of electrolytes and promote the OER performance. The EDX mapping results showed a uniform distribution of the Co, S and Ni elements (figure 1(d)). Therefore, the optimum amount of precursor is 3 g, was used for further analysis unless otherwise indicated.
Figure 1. SEM images of (a) Co4S3/NF-1, (b) Co4S3/NF-2 and (c) Co4S3/NF. (d) Elemental mappings of Co4S3/NF.
Download figure:
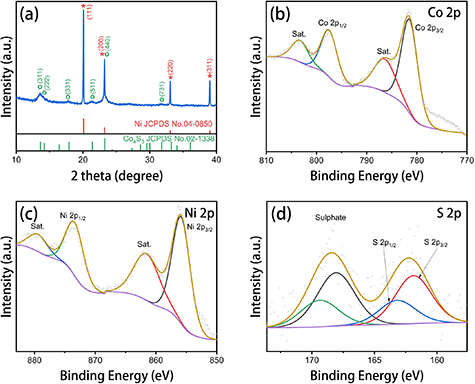
Standard image High-resolution imageXRD patterns of the Co4S3/NF electrodes are shown in figure 2(a). As can be seen, the XRD peaks of the electrodes at 20.1°, 23.2°, 33.1° and 39.0° could be indexed to (111), (200), (220) and (311) planes of Ni (PDF No. 04-0850). The XRD peaks at 13.6°, 17.9° and 21.4° were assigned to (311), (331) and (511) planes of Co4S3 (PDF No. 02-1338). The XRD results indicated the main structure of Co4S3/NF was Co4S3 with strong Ni peaks originating from the Ni foam.
Figure 2. (a) XRD patterns of Co4S3/NF. XPS spectra of (b) Co 2p, (c) Ni 2p and (d) S 2p of Co4S3/NF.
Download figure:
Standard image High-resolution imageThe elemental valence states of the as-prepared catalysts were further investigated by XPS. The high resolution XPS spectra of Co 2p, Ni 2p and S 2p for Co4S3/NF electrodes are shown in figures 2(b)–(d). In the Co 2p region, two peaks at binding energies of 781.7 eV (Co 2p3/2) and 797.7 eV (Co 2p1/2) with satellite peaks of 786.8 eV and 803.5 eV were assigned to Co4S3 species [34]. The two peaks at binding energies of 856.0 eV (Ni 2p3/2) and 873.7 eV (Ni 2p1/2) with satellites peaks of 861.9 eV and 879.8 eV were ascribed to surface oxidized Ni2+ species [35]. In the S 2p region, the peak at 161.9 eV (S 2p3/2) and 163.2 eV (S 2p1/2) can be assigned to S2− [36], which could be originated from Co4S3 species. The other two peaks at 168.1 eV (S 2p3/2) and 169.4 eV (S 2p1/2) could be assigned to a sulphate group [30], which may have come from the oxidation of residual Na2S2O3 during the reaction or from the storage in air.
The OER electrocatalytic performance of Co4S3/NF, Co4S3/NF-1, Co4S3/NF-2 and the pure Ni foam were examined in 1 M KOH electrolyte. Figure 3(a) presents the relevant polarization curves. Electrodes prepared with a larger amount of Co precursor showed better OER performance, which could be due to more active materials grown on the surface and more even distribution of the active materials. At a geometric current density of 100/300 mA cm−2, the overpotential of Co4S3/NF is 340/373 mV, which is better than that of Co4S3/NF-1 (385/430 mV) and Co4S3/NF-2 (370/420 mV). To obtain the kinetic information of the as-prepared electrodes, the corresponding Tafel plots are provided in figure 3(b). The Co4S3/NF electrode exhibits a Tafel slope of 71.6 mV dec−1, which is among the best reported electrodes and suggests more rapid OER catalytic kinetics (table S2). The OER electrocatalytic performance of Co4S3/NF, Co4S3/NF-4, Co4S3/NF-5 and Co4S3/NF-6 showed a very close OER performance (figure S5), which could be due to the saturated active materials supported on the Ni foam when preparing with more than 3 g cobalt precursors. To better understand the remarkable catalytic property of Co4S3/NF, the electrochemically active surface area (ECSA) was investigated using a typical cyclic voltammetry (CV) method (figures S6–S8). As shown in figure 3(c), the double-layer capacitance (Cdl) of Co4S3/NF is 3.96 mF cm−2, while the Cdl of Co4S3/NF-1 and Co4S3/NF-2 are 2.96 mF cm−2 and 3.25 mF cm−2, respectively. This result proved more active sites would be generated with increasing amounts of Co precursors, corresponding to the SEM results. Figure S9 showed the EIS results of different electrodes recorded in 1 M KOH under open circuit conditions. The inherent resistance values of Co4S3/NF, Co4S3/NF-1, Co4S3/NF-2 and pure Ni foam are 1.304 Ω, 1.351 Ω, 1.347 Ω and 1.275 Ω, respectively. The Co4S3/NF electrodes showed a small value of resistance which indicated a fast electron transfer ability. On the one hand, the surface reconstruction behaviour could increase the electron population on the metal centre and the conductivity could be enhanced [3]. On the other hand, the large surface area and large number of pores on Ni foam could promote the mass and electron transfer [5].
Figure 3. Electrocatalytic measurements of different electrodes for oxygen evolution in 1 M KOH. (a) The polarization curves of different samples; (b) Tafel plots derived from the curves in (a); (c) Cdl values of different electrodes; (d) long-term stability test at a constant current density of 100 mA cm−2; (e) polarization curves of Co4S3/NF at the 1st and 1400th cycles; (f) comparison of the overpotential to achieve 100 mA cm−2 for different electrodes.
Download figure:
Standard image High-resolution imageElectrochemical stability is an important factor when evaluating the performance of the electrocatalysts. A chronovoltammetry test was performed on Co4S3/NF at a fixed current density of 100 mA cm−2 for 100 h. The overpotential of Co4S3/NF increased by 3% from 406 mV to 419 mV after 100 h (figure 3(d)), which proved the excellent stability of the catalysts. It is to be noted that the electrolyte was not replaced or refilled during the stability test. Thus, the negligible change in overpotential could be due to the decreased area of the electrodes immersed in the electrolyte, which was caused by the consumption of the electrolyte under a large current density for long working times. Furthermore, figure 3(e) shows the LSV test of Co4S3/NF for 1400 cycles in 1 M KOH. Surprisingly, an enhanced OER performance was achieved after the 1400th cycle. The overpotential was even lowered by 20 mV at 100 mA cm−2 and a lower onset potential could also be noted after stability test.
To further analyse the remarkable stability of Co4S3/NF, an ex-situ XPS test was performed to study the change of valence states of the elements before and after OER tests at a constant current density of 10 mA cm−2 for one day. To avoid further possible oxidation of the materials, the electrode after the OER stability test was stored in a vacuum box before XPS analysis and transferred to XPS test chamber within 5 min. According to figure 4(a), by comparing the change of XPS binding energy before and after the reaction, the peaks of Co shifted to a lower binding energy, which could be due to the transformation from Co4S3 to Co3+ [37]. It is reasonable that the Co2+ species in Co4S3 are oxidized to Co3+ during the OER reaction. It was also observed that there was a change of binding energy of Ni before and after the reaction. The peaks of Ni shifted to a higher binding energy, which could be due to the transformation from Ni2+ to Ni3+ [38, 39]. The transformation of Co and Ni could also be proved by the polarization curves of the 1st cycle of Co4S3/NF as shown in figure S10. Two oxidation peaks could be seen around 1.25 V vs RHE and 1.38 V vs RHE, which correspond to the oxidation peaks of Co and Ni [33]. These oxidation peaks disappeared after 1400 cycles of the LSV, which proved the total transformation of Co2+ and Ni2+ species. In the S 2p spectra, the original S2− peak of the Co4S3 disappeared after the stability test. The changes in S XPS spectra indicates the catalyst surface undergoes a reconstruction process as the OER proceeds. The surface of the catalysts seems to change from Co4S3 to Co3+/Ni3+-sulphate/sulphites and Co3+/Ni3+-(oxy)hydroxide species during the OER process in the alkaline medium [30]. The XRD result of sample proved the formation of Co-sulphites species after OER stability test while the Co4S3 species remained, which could indicate the surface of Co4S3 undergoes reconstruction process (figure S11). The morphologies of materials did not show significant changes after OER stability test (figure S12).
Figure 4. Ex-situ XPS spectra of (a) Co 2p, (b) Ni 2p and (c) S 2p of Co4S3/NF electrodes before and after the OER for one day.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, a facile and energy-conserving method to obtain Ni foam supported Co4S3 at room temperature was developed. Superior electrocatalyst performance was achieved with an overpotential of 340 mV at 100 mA cm−2 and a Tafel slope of only 71.6 mV dec−1 in alkaline media. Excellent stability was realized with a negligible change in overpotential at a constant current density of 100 mA cm−2 for 100 h. The LSV curves showed that the catalytic performance become even better after 1400 cycles, demonstrating excellent stability. Ex-situ XPS tests proved the surface reconstruction process of the catalyst and showed the Co3+/Ni3+-sulphate/sulphites and Co3+/Ni3+-(oxy)hydroxide species could be the more active species as the OER electrocatalyst. This work provides cost-effective OER electrocatalysts with high performance and stability and provides a useful strategy to produce transition metal sulphides at room temperature.
Acknowledgments
The work was supported by Engineering and Physical Sciences Research Council (EPSRC, EP/V027433/1, EP/L015862/1, EP/R023581/1) and the Royal Society (RGS\R1\211080; IEC\NSFC\201261). S Z thanks the funding support from China Scholarship Council/University College London for the joint PhD scholarship.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.