Abstract
The use of nanoparticles as biomaterials with applications in the biomedical field is growing every day. These nanomaterials can be used as contrast imaging agents, combination therapy agents, and targeted delivery systems in medicine and dentistry. Usually, nanoparticles are found as synthetic or natural organic materials, such as hydroxyapatite, polymers, and lipids. Besides that, they are could also be inorganic, for instance, metallic or metal-oxide-based particles. These inorganic nanoparticles could additionally present magnetic properties, such as superparamagnetic iron oxide nanoparticles. The use of nanoparticles as drug delivery agents has many advantages, for they help diminish toxicity effects in the body since the drug dose reduces significantly, increases drugs biocompatibility, and helps target drugs to specific organs. As targeted-delivery agents, one of the applications uses nanoparticles as drug delivery particles for bone-tissue to treat cancer, osteoporosis, bone diseases, and dental treatments such as periodontitis. Their application as drug delivery agents requires a good comprehension of the nanoparticle properties and composition, alongside their synthesis and drug attachment characteristics. Properties such as size, shape, core-shell designs, and magnetic characteristics can influence their behavior inside the human body and modify magnetic properties in the case of magnetic nanoparticles. Based on that, many different studies have modified the synthesis methods for these nanoparticles and developed composite systems for therapeutics delivery, adapting, and improving magnetic properties, shell-core designs, and particle size and nanosystems characteristics. This review presents the most recent studies that have been presented with different nanoparticle types and structures for bone and dental drug delivery.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Nanoparticles
As medicine and biomedicine face numerous technological developments, nanoparticles (NPs) appear as promising resources for diagnoses and treatments. This technology leads to the development of systems in the size of cells and molecules present in the human body [1]. Due to their small size, shape, and surface properties, the use of nanoparticles in medicine includes features such as targeted delivery, contrast imaging, combination therapies, tissue engineering, bone and dental repair, and hyperthermia [2–4]. Furthermore, the use of nanoparticles as drug delivery agents presents multiple advantages. Firstly, nanoparticles can encapsulate medicinal agents and improve their biocompatibility. Once encapsulated, drugs can be transported to specific sites of action and released at organs or tissues at ease and controlled discharge. It reduces the required dose for the treatments and avoids ingestion, preventing undesirable side effects and aggression of organs such as the liver, kidney, and heart. Besides that, they can be produced on a large scale and reduce the toxic effects of medications [5–8]. Cancer therapies are one of the most studied applications of drug delivery nanoparticles [9], mainly because they turn the process of cancer cells treatment faster and less aggressive for patients, reducing treatment's toxicity leading to the potential use of currently limited medications for tumor therapies [10, 11]. Usually, nanoparticles employed in cancer treatments present magnetic properties and structures formed by polymers and lipids [12], facilitating the loading of drugs. In addition, radiation therapy or ultrasound techniques can also be modified by nanoparticles [13]. Moreover, studies have proposed them as great options for bone-target in bone cancer treatments, osteoporosis, bone regeneration, and dental applications and treatments Moreover, studies have proposed them as great options for bone-target in bone cancer treatments, osteoporosis, bone regeneration, and dental applications and treatments [5], presenting a potential for many other oral environment applications.
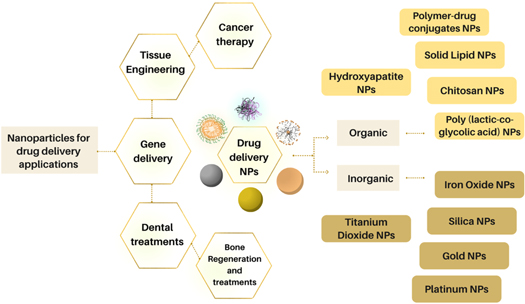
Nanoparticles are synthesized in different compositions, sizes, and structures and exhibit various properties depending on their purpose and necessity. Some of the most common drug delivery nanoparticles include polymeric nanoparticles, polymeric micelles, liposomes, silica, gold, silver, platinum, cerium, and titanium solid-lipid nanoparticles [14]; besides that, they can be classified as inorganic NPs, or organic NPs [15], which could show magnetic properties. Figure 1 presents some examples of the most used nanoparticles for drug delivery and bone treatments.
Figure 1. Drug delivery nanoparticles and some of their most common applications.
Download figure:
Standard image High-resolution imageMany investigations have recently modified NPs properties and features to enhance their benefits and applications. While on the one hand, some results indicate better biocompatibility of the nanoparticle due to coating changes, on the other hand, some types of modifications could lower properties related to magnetism in magnetic nanoparticles, for instance. This review aims to analyze some recent developments reported in drug delivery nanoparticles for bone and dental applications; and the different types of nanoparticles synthesized to be used as drug delivery agents for bone and dental targets, comparing types, obtained results, and characteristics.
2. Nanoparticles in bone treatments
One of the body's most important organs is bone; it sustains and protects organs and regulates hormones. Unfortunately, bone tissue may be affected by diverse diseases and skeletal disorders that affect mobility and lead to death. Usually, treatments require administering high drug doses and concentrations, which could provoke adverse effects. Nanotechnology and nanoparticles for highly localized treatments are an alternative to manage the delivery of these therapeutic agents and diminish toxicity in the blood [16].
NPs' innovative uses have various purposes in bone therapies. For instance, their small size, large surface area, facility to modify surface roughness, and wettability can effectively sustain bone regeneration when aligned with good cell adhesion and proliferation [17]. Furthermore, other investigations have shown that due to their potential for modification, different product types, and properties possibilities, nanoparticles can emerge as great therapeutic approaches and parts of scaffolds incorporating the composite properties and enhancing therapeutics' delivery [18–22]. Although commercial options are not available so far, the use of nanotechnology as therapeutic carriers is rising in different bone diseases applications such as bone regeneration, osteosarcoma, osteoporosis, and cancer treatments [23–25].
Although rare, osteosarcoma bone cancer originates from mesenchymal cells and requires chemotherapy and surgery. It is fully understood that conventional chemotherapy has a significant issue: its toxicity and body system side effects. That being said, NPs as tumor-target drug delivery agents could be used as an option to achieve a controlled release in the body [26]. The use of nanoparticles as drug vehicles remains a challenge because of nanoparticles' lack of cancer cells target capability; however, studies have designed efficient and smart nanocarriers that could differentiate normal healthy cells from tumor cells. Mesoporous Silica nanoparticles (MSNs) loaded with doxorubicin (DOX) can have the addition of a poly(acrylic) acid (PAA) layer to increase pH sensitivity and biocompatibility, and also have the addition of a target ligand, such as concanavalin A (ConA). This procedure could bind NPs to cell-surface glycans, highly present in cancer cells, selecting tumor cells for a highly localized treatment. Studies have shown that this system could preserve the viability of healthy cells and increase the medicinal effect against tumor cells, compared to the free drug [27].
Another disease that treatments could be favored from MSNs is osteoporosis. A deterioration in the bone tissue characterizes this skeletal disorder due to a low bone mass, directing, consequently, to a high fragility in bones. It could be devastating for older patients, which are the most affected by osteoporosis, since a vertebral fracture, for instance, could lead to death or morbidity. Treatments typically include fracture prevention but are related to long drug exposure, leading to possible harms [28, 29]. So, to enhance and stimulate bone regeneration, a research study incorporated nanoceria, cerium in the form of oxide NPs, as a radical scavenger in MSNs, due to the silica release and reduced osteoclast activity due to nanoceria properties. These nanoparticles could decrease the usage of drugs and damaging side effects [30].
Bone infections, such as osteomyelitis, could be treated with nanoparticles too. Formulations using magnetic and gelatin nanoparticles loaded with gentamicin have a therapeutical potential to release the drug with a controlled profile and accelerate osteomyelitis recovery due to very localized treatment [31].
3. Nanoparticles in dental treatments
The use of nanoparticles in the dentistry field can enhance treatments at atomic and molecular levels. Thus, this technology usually applies NPs with a size range between 10 and 100 nm to improve the properties of conventional materials by adding functional groups pursuing advancements in diseases prevention, diagnosis, and treatments [32]. The study of nanoparticles applications in the dental field has reached areas such as dental implants, preventive and antibacterial nano dentistry, restorative dentistry, tissue regeneration, periodontics, oral cancers, dentin hypersensitivity, drug delivery, and others [33–36]. Some very common nanoparticles studied in these areas are titanium, silver, gold, iron oxide, silica, chitosan, zirconia, and zinc [37–39]. Furthermore, when employed as drug delivery agents in the oral environment, NPs can enhance dental restoration, antifungal and antibacterial action, and periodontal treatments [35].
Dental caries, a worldwide known problem that affects individuals of all ages, is a condition in which NPs could work as useful tools to reverse cavities caused by microbial colonization and demineralization. Usually, treatments include adding a fluoride agent in the affected cavities to help remineralize the tooth and prevent bacterial activity. Applying this agent in nanoscale could produce smaller treatment systems that are less sensed by patients and concentrate in a particular area [34, 40]. Calcium fluoride (CaF2) nanoparticles have been studied in bioadhesive films to overcome the limitations of conventional treatments and prevention methods such as toothpaste and mouth rinses that have a brief enduring period in the mouth. This application can increase the contact period of fluoride agents in affected areas and suppress bacterial and biofilm generation. In the study, CaF2 nanoparticles embedded in thiolated chitosan (TCS) biofilm were tested as potential treatments. After in vitro tests, the system showed a drug release time of up to 8 h and 28% of mucosal membrane permeation in 6 h after ex vivo tests, showing facilitation of fluoride agents delivery by nanoparticles application in bioadhesives [41]. The fluoride loading in chitosan nanoparticles was also investigated as a possible option for oral environment drug delivery by using sprays or mouthwash. Studies showed that even small amounts of fluoride could present a constant release in the mouth [42].
Another common dental disease triggered by bacterial pathogen and immune system response that leads to the release of toxins is periodontal disease. In its chronic stage, immune cells are activated, and the release of pro-inflammatory cytokines and reactive oxygen species leads to bone and periodontal fibers degradation. Therefore, the use of oral drugs such as anti-inflammatories and antibiotics is often necessary within invasive surgeries, leading to discomfort and adverse side effects. Nanoparticles delivering localized drugs could be an efficient and less invasive treatment method [43]. For instance, Backlund et al developed silica nanoparticles with the potential to release exogenous nitric oxide to overcome periodontal pathogenic bacteria [44].
Similarly, a fungal infection caused by Candida albicans, known as oral candidiasis, with existing treatments based on expensive and very unpleasant medicines with uncomfortable and harmful effects if swallowed, could have its challenges suppressed by the use of nanotechnology [45]. One of the main active agents of oral candidiasis treatments is fluconazole (FLZ). Although this agent interacts very well with different medications when used in conventional treatment methods, such as oral gels, mouth paints, and rinses, the mouth's remaining period is not very long. Furthermore, adhesive nanoparticles to deliver this agent could operate as a solution. To analyze this possibility, a study developed mucoadhesive eudragit (EUD) with FLZ nanoparticles coated with chitosan. The candidate nanoparticle did not exhibit cytotoxic effects, and with good stability, ex vivo and in vivo rabbits' tests results presented themselves as attractive options to reduce overall drug dosage and side effects, improving treatments options [46].
The application of nanoparticles could likewise improve endodontic treatments, also related to bacterial biofilms development. For instance, research studies using silver and zinc incorporating mesoporous calcium-silicate nanoparticles (Ag-Zn-MCSNs) applied these nanoparticles inside dentin to analyze adhesion and effects in the tooth. Results presented no adverse effects of nanoparticles on dentine mechanical properties, such as flexural strength and modulus of elasticity, and nanoparticles could penetrate dentinal tubules and aggregate to the surface, showing the potential for their application as drug delivery agents and improvement of therapies [35, 47].
Studies utilizing nanotechnology to enhance dental treatments are not limited to these disorders only. Dental implants, which are also susceptible to failure due to bacteria, could also take advantage of NPs properties. As the number of dental implants placed in patients worldwide increases, with a projection of reaching investments around 4 billion dollars by 2022, studies to avoid failure of this body implant attached to the bone expand too. Coating implants with nanoparticles would be an advantageous option to leave implant parts uncoated for osteoblasts formation, have controlled distribution for elements, and employ their therapeutic effects for peri-implantitis prevention [48–50].
Usually, plaque formation and infections due to bacteria and fungus are failure causes of dental implants because of the favorable environment for oral microorganisms. However, one study has developed silver nanoparticles conjugated to chitosan. This natural polysaccharide is known for its antibacterial characteristics, while silver is inert, biocompatible, and causes no harm to the body. In vitro analysis showed that the developed nanoparticles significantly reduced the formation of bacteria and the survival of microorganisms, so coating titanium dental implants with them could be a suitable alternative [51]. Another antibacterial agent option is the use of zinc NPs. zinc peroxide nanoparticles have been studied in vitro as alternatives for implants bacterias. Results indicated good inhibition of bacterias such as A. actinomycetemcomitans, P. gingivalis, P. intermedia, and F. nucleatum, which are favorable for the progression of implant issues and losses. Further studies aim to examine coating dental implants with these NPs, as options to contain implant failure [52]. Research studies have also coated Titanium implants with chlorhexidine-hexametaphosphate nanoparticles due to chlorhexidine's (CHX) good antimicrobial and antifungal characteristics. In vitro analysis demonstrated a soluble release of CHX and antimicrobial effect, indicating an alternative for improving dental implants [50]. Figure 2 exhibits some dental conditions and infections in which drug delivery nanoparticles could enhance therapies. Understanding the most promising nanoparticles and their recent applications and tests can benefit biomedical materials and pharmaceutical possibilities. The following sections present some of the most recent NPs studies related to oral and bone drug delivery.
Figure 2. Drug delivery nanoparticles options for some common dental diseases and infections.
Download figure:
Standard image High-resolution image4. Organic nanoparticles
Organic nanoparticles offer high biocompatibility and low toxicity, characteristics that qualify them as good options as drug delivery agents. The most studied organic NPs are polymeric and solid lipid nanoparticles; these nanocarriers can be found as polymeric micelles, vesicles, dendrimers, and nanoparticles with an average size from 10–1000 nm. Their easy synthesis process, well-defined structures, changeable size, good surface characteristics, hydrophobicity, and controlled drug release properties are the main reasons that entitle them as popular choices for the localized transport of therapeutics [53–56]. These organic nanocarriers can outstand when compared to inorganic materials. They have low toxicity, high biocompatibility, and valuable properties to encapsulate drugs and improve their bioavailability when referring to the delivery of therapeutics. On the other hand, these unique characteristics may also lead to a low-loading capacity on some carriers and a premature release [54, 56–58]. Table 1 presents some characteristics of the most popular drug delivery organic nanoparticles used for bone and dental treatment.
Table 1. Characteristics and toxicity of most used organic nanoparticles for drug delivery in Bone tissue and dental applications.
| Principal organic nanoparticles | |||
|---|---|---|---|
| Type | Characteristics | Toxicity | References |
| Hydroxyapatite | -Brittle bioceramic | -Non-irritating | [59–62] |
| -Good osteoconductive and osteoinductivity | -Non-toxic | ||
| -Potential as drug delivery carriers | |||
| Poly(caprolactone) | -Medical approved polyester | -Non-toxic | |
| -Hydrophobic | -Encapsulates and increases drugs'bioavailability | ||
| (PLC) | -Semi-crystalline | [63–66] | |
| -High blend-compatibility | |||
| Poly(lactic-co-glycolic) acid | -Hydrophobic | [67–69] | |
| -Diffusible and metabolized in the human body | -U.S. FDA approved | ||
| (PLGA) | -Negative surface charge | ||
| -Natural polysaccharide | -Biocompatible | [70–73] | |
| Chitosan | -Surface amine groups | -Low toxicity | |
| -Good physicochemical, and antimicrobial properties | -Cytotoxicity in some in vitro and in vivo | ||
| Polymer conjugated to Polyethylene glycol | -Improves hydrophilicity | ||
| -Electrically neutral | -Low toxicity | [74–76] | |
| (Polymer-PEG) | -High stability | ||
| Lipid/Liposomes | -Phospholipids membranes | -Many FDA approved structures | [77–79] |
| -Improving drugs' solubility may form different structures of phospholipids | |||
4.1. Hydroxyapatite nanoparticles
Apatite, one of the human body's main components, known as hydroxyapatite (HA) in vertebrae, is a biocompatible and bioactive mineral. It also maintains osteoconductive properties, a high affinity with some drugs, and absorbs osteoblasts. Many recent studies have developed HA nanoparticles for different bone-targeting applications related to therapeutics delivery [6]. To study these nanoparticles' in vitro behavior, one group synthesized HA NPs for in vivo applications and radiolabelled the particles with technetium-99m (99mTc) using hydrothermal syncretization to analyze the NPs uptake in the bone. The produced nanoparticles presented a surface area of 103.05 m2 g−1 with a mean porous diameter of 8.14 nm. The particles' mean size was 285.3  10.3 nm, and the 99mTc-HA NPs presented affinity with the bones and a long residence time in the bloodstream, with a half-life of 2 h. This high probability of reaching the bones with a higher affinity with bones after biodistribution analysis showed an increased bone uptake after 4 h. Results indicated that, even though some mice's organs absorbed the particles, they could have potential applications as nanocarrier agents [80]. Besides that, Shweta Pandey et al reported HA NPs synthesized by a wet-chemical precipitation method. The nanoparticles loaded with teriflunomide (TEF) and methotrexate (MTX) combined these drugs' good properties and efficacy while reducing their used amount and toxicity. The nanoparticles presented a smooth surface and spherical shape. So, after in vivo insertion in rat ankle, the carriers helped reduce inflammation and improved articular structure regeneration [81]. Another researcher reported sol-gel method produced HA nanoparticles that could be used as zoledronic acid (ZOL) delivery since this antiresorptive bisphosphonate has an excellent potential to improve bone regeneration. Because HA has a good absorbance of this drug with a strong affinity with bisphosphonates, both agents could function as drugs, and ZOL formulation could be reduced. The nanoparticles had a size range between 70 and 100 nm, with a not very regular shape before the drug loading, showing that this synthesis method might lead to a not very good size distribution for the nanoparticles. However, in vivo results in rats demonstrated that this drug formulation and localized delivery could improve bone regeneration, and this approach could benefit osteoporosis treatments in humans [82].
10.3 nm, and the 99mTc-HA NPs presented affinity with the bones and a long residence time in the bloodstream, with a half-life of 2 h. This high probability of reaching the bones with a higher affinity with bones after biodistribution analysis showed an increased bone uptake after 4 h. Results indicated that, even though some mice's organs absorbed the particles, they could have potential applications as nanocarrier agents [80]. Besides that, Shweta Pandey et al reported HA NPs synthesized by a wet-chemical precipitation method. The nanoparticles loaded with teriflunomide (TEF) and methotrexate (MTX) combined these drugs' good properties and efficacy while reducing their used amount and toxicity. The nanoparticles presented a smooth surface and spherical shape. So, after in vivo insertion in rat ankle, the carriers helped reduce inflammation and improved articular structure regeneration [81]. Another researcher reported sol-gel method produced HA nanoparticles that could be used as zoledronic acid (ZOL) delivery since this antiresorptive bisphosphonate has an excellent potential to improve bone regeneration. Because HA has a good absorbance of this drug with a strong affinity with bisphosphonates, both agents could function as drugs, and ZOL formulation could be reduced. The nanoparticles had a size range between 70 and 100 nm, with a not very regular shape before the drug loading, showing that this synthesis method might lead to a not very good size distribution for the nanoparticles. However, in vivo results in rats demonstrated that this drug formulation and localized delivery could improve bone regeneration, and this approach could benefit osteoporosis treatments in humans [82].
The HA nanoparticles' potential for drug delivery led to designing a system that simultaneously delivers two different drugs and acts as penetrating material in the bones by using HA nanoparticles grafted with MTX and poly(vinyl alcohol) (PVA) by emulsion polymerization technique. Them, the nanoparticles were loaded with gemcitabine (GEM), an anti-cancer drug. The in vitro studies for the drug release presented a conjugated release of about 25% after ten days, while for the physically loaded nanoparticles, it was around 60%. Besides that, in vitro studies showed a tendency to sequential delivery of GEM succeeded by MTS, increasing the potential for treatments and increasing therapeutics' efficiency [83]. Furthermore, the HA NPs may also present potential use in the oral environment. Planning to develop a system for cell proliferation and, consequently, bone regeneration in the oral environment Rajabnejadkeleshteri et al synthesized strontium fluor-hydroxyapatite Nanoparticles (F-Sr-HA) since fluorine helps in the proliferation of osteoblasts and strontium is known for improving gene expression in osteoblastic cells. The group used the precipitation technique and ph-Cycling method to achieve these nanoparticles. After cell incubation, the hydroxyapatite's added elements helped increase cell growth and differentiation, presenting potential dental applications [84].
4.2. Polymeric and solid lipid nanoparticles
Some examples of polymeric drug carriers for the delivery of therapeutics are polymeric nanoparticles, polymer carriers, lipid nanoparticles, and lipid-polymer-hybrid nanoparticles. Due to advancements in chemistry, the synthesis of diverse polymer-nanoparticles with very well-defined structures and new polymerization methods is feasible, with the outcome of ideal molecular weight distribution and better properties.
When referring to polymers, polymer-drug conjugates with covalent conjugation and polymer nanoparticles that can use non-covalent conjugations are achievable [85]. Simultaneously, Lipid nanoparticles have the advantages of easy large-scale production, biocompatibility, biodegradability, and low toxicity potential. They also improve the potential of both hydrophilic and lipophilic drug release [86]. Another prospect is to employ the qualities of both liposomes and polymers in the nanoparticles, creating lipid-polymer hybrid nanoparticles. This system increases encapsulation efficiency, has well-tolerated serum stability, and holds good targeting properties [87].
Liposomes formed by phospholipids bilayers were studied for Curcumin encapsulation and bisdemethoxycurcumin in nanoscale. The system was synthesized by a modified thin-film hydration method, and according to results, they exhibited great potential for use in some osteoblasts and drug delivery treatments [88]. Their dual nature allows encapsulating hydrophilic and lipophilic drugs in the core and bilayer. One study investigated how efficient the liposomes can be using a 3D bone marrow model. Testing different liposomal drug delivery systems for anticancer drugs presented great potential for future therapies [89]. Moreover, when attempting to update from a tissue level to a cellular level using bone strategies, Liposome NPs with sizes <90 nm were produced to be used as siRNA carriers. These aptamer-functionalized nanoparticles would facilitate some bone strategies, and as CH6 aptamer facilitated siRNA entry in osteoblasts and liposome scape of siRNA, this first study enhanced an RNA bone-based strategy, improving cellular level treatments [90].
Nevertheless, liposomes may not be very stable, and their drug loading capacity is not very high. In order to combine their good properties with polymeric advantages and overcome the instability of lipids and polymeric aggregation, Xiaoyan Wu et al studied NPs with a core consisting of poly(D,L-lactide-co-glycolide)-cholesterol with an alendronate-polyethylene glycol-lipid shell. These nanoparticles could be helpful for bone target and delivery of chemotherapy medicines reducing therapy toxicity, as proved in vitro and in vivo studies [10]. Some of the polymeric nanoparticles drug-systems recently developed for bone targeting and dental treatments are:
4.2.1. PLGA based nanoparticles
Poly(lactic-co-glycolic) acid (PLGA) nanoparticles are a promising nanosystem for bone treatments and regeneration. This FDA-approved polymer is biocompatible, biodegradable, easy to process, and one of the main components in copolymer nanosystems, which helps increase hydrophilic systems' properties. PEG-PLGA nanoparticles, for instance, have the advantage of avoiding hydrophobic interactions between proteins and polymers, and the use of poloxamers improves releasing control characteristics [91]. In one study, Nazemi et al reported PLGA nanoparticles that could sustain a controlled drug release for a more prolonged time. The nanoparticles loaded with Dexamethasone (DEX) and inserted in bioactive glass scaffolds presented smooth surfaces and a size range smaller than 100 nm. After studies, nanoparticles did not affect the characteristics of the scaffold, showing a high potential nanosystem for bone treatment applications within the delivery of therapeutics [92]. Another report using PLGA for bone-targeting drug delivery proposed an esterification reaction of hydroxyl of tetracycline (TC) and PLGA carboxyl, producing TC-PLGA NPs that could be attracted to the bone through a reaction between TC and hydroxyapatite. The results described a spherical shape and uniform nanoparticles, and in vivo studies suggested they could be very efficient in delivering hydrophobic drugs for osteoporosis treatment [6]. Duong Thanh et al reported another research involving PLGA, presenting targeted nanoparticles produced with alendronic acid-modified lipids using a PLGA core to encapsulate the chemotherapeutic drug Doxorubicin (DOX). The PLGA core acted as the particle skeleton and a reservoir for the drugs, while the lipophilic phospholipid layer was a passivation layer to stabilize and direct the nanoparticles to the target. The resulting nanoparticles had high stability in a colloidal state with adequate protein aggregation levels, also revealing that this nanomedicine method delivers higher doses of DOX and that alendronic acid provides a negative surface charge and sufficient hydration to nanoparticles representing a robust alternative for treatments [93].
PLGA nanoparticles loaded with 17β-estradiol (E2) coated with poly(vinyl alcohol) (PVA) were studied using different synthesis methods and comparing the best method for drug delivery nanoparticle performance. By using a combination of antisolvent diffusion with preferential solvation and an emulsification and solvent evaporation method, the nanoparticles presented sizes of 110 nm and 106 nm, respectively. Then, examining skin permeability, an antisolvent diffusion with solvation presented a good potential to develop nanoparticles helpful in treating osteoporosis [94]. Regarding dental applications, PLGA nanoparticles also could be applied to penetrate dental structures. Loaded with chlorhexidine (CHX), they have been investigated for restorative dentistry applications through dentinal tubules of demineralized dentin substrates. The study synthesized the particles using emulsion evaporation, with and without drug loading. Results showed that non-loaded NPs' average size was approximately 342.76 nm larger than drug-loaded PLGA nanoparticles. Besides that, the particles' morphology was spherical and smooth, presenting results of around 10% of the particles penetrating the tubules after 60 s, improving the possibilities of therapeutics delivery in dental applications [95].
4.2.2. Chitosan nanoparticles
Chitosan (CS) nanoparticles are also studied as drug delivery nanoparticle systems. This natural polysaccharide is biocompatible and biodegradable, facilitating cross-linking and drug encapsulation. Based on that, CS nanoparticles have been used to encapsulate silibinin and incorporated into alginate/gelatin scaffolds. The use of chitosan nanoparticles improved the scaffold's properties and prolonged the release of silibinin, making this strategy a prospect for bone treatments [96]. The use of these nanoparticles as drug vehicles and their potential for a controlled release, aligned with their ability to interact with cell membranes, has led to various CS-related studies [97]. Recently, the synthesis of CS NPs using ionic gelation technique and acid-base precipitation has been reported. The study produced risedronate functionalized chitosan nanoparticles (RISCNs) with a size range between 340 and 747 nm and presented them as excellent and beneficial options for osteoporosis treatment [98]. Similarly, developed chitosan nanoparticles loaded with minocycline have been applied in a collagen chitosan membrane for bone regeneration and inhibit bacteria colonization, improving future clinical treatments options [99].
Besides that, they have also been used in systems delivering genes for bone regeneration. For example, a study developed chitosan-gold nanoparticles with the gene c-Myb to be used as a coating layer in dental titanium implants to release c-Myb and improve osseointegration. in vivo and in vitro tests promoted bone formation and inhibited bone resorption. This strategy could enhance bone healing options and improve the integration of dental implants into the body [100]. Also, chitosan-polyethylenimine nanoparticles were developed to carry the hBMP-2 gene. chitosan is a good option for this type of delivery since it can protect the DNA from degradation. So, these particles could be applied in a zone with defects in the bone. The created particles presented a size around 162 nm, and although, according to in vivo data, the system did not fully restore the bone up to the last week of experiments, this is still a start for advances in bone regeneration [101].
4.2.3. Other organic drug delivery nanoparticle
One alternative employed to improve hydrophilic systems is polyethylene glycol (PEG) as a shell or core material. As an option for carrying hydrophobic drugs, Jinsong Liu et al produced polyethylene glycol-poly(ε-caprolactone) (PEG-PCL) nanoparticles linked to (Asp)8, a biodegradable and biocompatible peptide sequence with high bone affinity. The curcumin-loaded (Asp)8-PEG-PCL nanoparticles were easily permeable, accumulated in the bone niche, showed robust antitumorigenic characteristics, and could be good options for treatments [102]. Another study conjugated alendronate (ALN) on PEG, creating a hydrophilic structure. This polymer was attached to calcium phosphate nanoparticles, and this system could be useful in drug delivery since it has a high affinity with bone-tissue. After in vivo tests, the nanoparticles did not provoke hyperemia, hyperplasia, or other concerns in the rats' hearts, liver, kidneys, and lungs, confirming these particles' high biocompatibility [103]. Another polymer that appears as an option for nanoparticles studies is poly(ethylene sodium phosphate) (PEP.Na), which has an affinity with bone substrates. Furthermore, compared to conventional polyesters, functionalization is more straightforwardly achieved in this material. A study synthesized cholesteryl-terminated poly(ethylene sodium phosphate) nanoparticles by a solvent evaporation technique; a helpful technique for developing particles composed of a hydrophobic end on the interior and a hydrophilic polymer on the exterior. The Cholesteryl-PEP.Na NPs presented a hydrophilic chain and a hydrophobic cholesteryl end. The particles showed an affinity with hydroxyapatite, offering great potential for carrying an anti-cancer agent in localized treatments [104].
Poly(ε-caprolactone) (PLC) nanoparticles coated with chlorhexidine (CHX), also called Nano-PCL/CHX particles, were investigated as drug delivery nanocapsules through dentinal tubules. The Nanocapsules were synthesized by interfacial polymer deposition. As a result, the particles presented a smooth spherical morphology with a CHX core and a PCL shell. Results showed that while loaded particle size was significantly smaller than unloaded, an increase in the loaded Nanocapsules' drug content led to a decrease in size. The nanocapsules penetrated the tubules by using a gently air-blown and, with a gradual degradation, released significant amounts of CHX. Afterward, this system could have the potential for prospective clinical dentistry [105]. Rudnick-Glick's group reported bisphosphonate nanoparticles (BP) as another example. BP has a high affinity with HA. The research delivered a blood half-life time of approximately 5 h, and after trials on female mice, some therapeutics were found in the kidneys and lungs, showing that reducing the number of drugs in the human organs still needed some improvements [106].
5. Inorganic nanoparticles
Inorganic Inorganic nanoparticles as drug delivery agents have been produced in different materials. They can include in their composition noble metals such as gold, silver, or other metals; some examples are zinc oxide (ZnO), copper (CuNPs), and iron oxide (Fe3O4). Usually, they present some important characteristics such as hydrophilicity, stability, low toxicity, response to the immune system, availability, biocompatibility, and inertness. In addition, these nanoparticles can also present magnetic properties, improving particular applications [54, 107, 108].
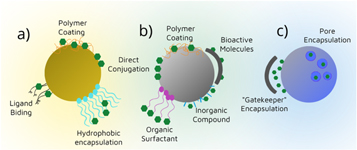
Even though inorganic nanoparticles present good chemical and mechanical resistance, it is essential to conjugate or coat them to enhance their characteristics. These new systems facilitate drug loading, diminish toxicity, and hamper nanoparticles penetration [57, 109], and at the same time modify the particles' surface, as seen in figure 3. Table 2 presents some characteristics of the most recently studied inorganic nanoparticles for drug delivery in bone-tissue and dental applications.
Figure 3. Inorganic nanoparticles surface modification possibilities, (a) gold, (b) iron oxide, and (c) silica nanoparticles.
Download figure:
Standard image High-resolution imageTable 2. Characteristics and toxicity of most used inorganic nanoparticles for drug delivery in Bone tissue and dental applications.
| Principal inorganic nanoparticles | |||
|---|---|---|---|
| Type | Characteristics | Toxicity | References |
| Silica | -Use growth in biomedical devices | -Non-toxic | [110–114] |
| -Anti-agglomeration properties | -Studies show that more investigation could be necessary | ||
| -Easily tunable | |||
| -Porous possibilities | |||
| Gold | -Easily tunable | -Toxicity highly | [115–118] |
| -High surface-area-to-volume ratio | dependent on particles size and cell type | ||
| -Controllable size, composition, and functionality | |||
| Superparamagnetic Iron Oxide Nanoparticles (SPIONs) | -Superparamagnetic | -in vivo studies necessary | |
| -High saturation field | -Accumulation potential | 119–122] | |
| -Aggregation tendency | -Toxicity may be dependent on size and coating, fate studies still necessary | ||
| -Controllable size | |||
5.1. Silica nanoparticles
Silica nanoparticles can be extremely helpful in boosting flavonoids' solubility and application in human body treatments. A study encapsulated isoliquiritigenin to mesoporous silica NPs to observe the drug release in vivo. These NPs could be applied in bone therapies for diseases such as cancer or inflammations, enhancing therapeutic characteristics of the flavonoids. The nanoparticles were added to mouse bone marrow-derived macrophages and mature osteoclasts, the study presented a significant cellular uptake, and the nanoparticles significantly diminished mature osteoclasts. Results showed that this association led to a much better response in inhibition of RANKL osteoclast formation, proving the nanoparticles to have a great potential in bone loss prevention and inflammation treatments [123]. Another study developed silica nanoparticles coated with polyethylenimine. Silica NPs have the advantage of a high loading capacity, so the pores were loaded using osteogenic peptide ostentation. After injection of the system in the femoral bone marrow of mice, the nanoparticles transported and delivered the siRNA and ostentation, exhibiting significant efficacy and achieving an effective silencing effect. Confirming this system's high potential for osteoporosis treatments using gene therapy [124].
5.2. Gold nanoparticles
Due to their high biocompatibility and low toxicity, gold nanoparticles (AuNPs) are promising candidates for the delivery of therapeutics in bone treatments. In one investigation, a group created Gold NPs conjugated with pamidronate alongside alendronate (ALD) and analyzed cells' uptake and viability after the insertion of nanoparticles. The particles exhibited great potential for diminishing osteoclasts' viability and good characteristics for treating osteoporotic conditions. Although further analysis of toxicity cell behavior is still necessary to direct how the treatment's potential can change after in vivo analysis, this approach could be promising for a novel treatment strategy [125]. Besides that, the modification of gold nanoparticles' surfaces can expand their applications as target particles in bone-tissue. A research study developed AuNPs with ALD to facilitate the drug's delivery. These gold nanoparticles presented about 30 nm diameter and a synergistic effect of suppressing and differentiation of osteoclasts better than by using isolated ALD after mice insertion analysis [126]. When being modified, gold nanoparticles can interact with different functional groups, for instance, hydrophobic, anionic, and thiol groups. This principle was confirmed by Donghyun Lee et al when gold nanoparticles had their surface easily grafted with N-acetyl cysteine (NAC), a thiol group. Since these nanoparticles are good candidates for promoting bone regeneration, they were encapsulated in a hydrogel of gelatine and tyramine with human adipose-derived stem cells to facilitate their application in treatment spots. This composite did not affect the advantageous differentiation properties of the AuNPs, and the system's biocompatibility was confirmed [127]. Another advantage of gold nanoparticles is their interaction with other functional groups such as phosphines and amines. In an analysis, a group synthesized gold nanoparticles conjugated with vitamin D. The particles developed by the conjugation of Vitamin D and thiol-functionalized polyethylene glycol presented an average size of 60 nm, with a not very high cellular uptake, probably due to their size. On the other hand, conjugated nanoparticles presented good osteogenic differentiation and no toxicity via the cell-viability test. This approach could be a suitable carrier for osteoporosis treatment [128].
5.3. Non-magnetic nanoparticles
Another type of nanoparticle design was an oxygen vacancy-rich tungsten bronze nanoparticle (Nax WO3), produced using a pyrogenic decomposition process for photothermal therapy. The synthesized materials were oleic acid, 1-octadecene, ammonia, and sodium tungstate dehydrate (Na2 OW4.2H2 O), resulting in nanocrystals of a cube-like shape with 150–200 nm in length. These particles presented outstanding photothermal ability and stability, helping decrease tumor cells in vivo tests. These approaches demonstrate that powerful techniques are under development and study, and nanomaterials might have a significant future in localized therapeutics [129].
5.4. Magnetic nanoparticles
Magnetic nanoparticles are created using metallic materials and their oxides. They maintain unique properties due to a difference in bulk materials associated with their high surface-to-volume ratio. Besides that, some of their properties are saturation magnetization, coercivity, blocking temperature, and relaxation time. These magnetic properties are influenced by their syntheses and features such as particles size, shape, and composition; and also the presence of a core-shell design [130, 131].
Superparamagnetic nanoparticles stand among the most investigated magnetic nanoparticles for different drug delivery bone-targeting applications. They are attractive options because of their properties, characteristics, and facility to be positioned and directed to a specific area with the presence of a magnetic field [132–134]. When this magnetic field is removed, they present no magnetic properties allowing better control for specific applications. In addition, these nanoparticles present good properties such as non-toxicity for humans and cost-efficiency [8, 131, 135]. Besides that, to increase NPs stability and biocompatibility and decrease agglomeration due to their high specific area, these particles are usually coated with biocompatible materials, forming many different nanoparticles and systems [136].
Different researches have studied using these and other magnetic particles as drug delivery agents. In order to enhance penetration, cell interaction, and magnetic properties, different shell-core designs have been studied. Common architectures in the magnetic nanoparticles are core-shell structures with a shell of polymer molecules, metallic nanomaterials, carbon, silica, ligands, or proteins [137, 138].
5.4.1. Shell-core designs in magnetic nanoparticles
One coating material used for different application results is poly(lactic-co-glycolic) acid. Mrudhula Baskaran et al developed magnetic nanoparticles of Capsaicin coated with PLGA. First, the group prepared nanoparticles using a co-precipitation technique with ferric chloride and ferrous chloride dissolved in degassed deionized water and ammonium hydroxide. Next, a dispersion of ethanolic solution of capsaicin was created over the magnetic nanoparticles, generating magnetic capsaicin nanoparticles. Then, PLGA dissolved in methylene chloride was added to the magnetic fluid, forming a PLGA coating in the particles. In the end, their size range was between 50 and 100 nm, demonstrating that this synthesis method does not provide a perfect size distribution for the particles; however, encapsulation of the drug by PLGA increased its solubility, therefore can be an excellent option to increase the bioavailability of Capsaicin in vivo [5].
Silica coating is also a possibility for these particles coating. In order to compare magnetic properties differences after various coating methods, Victoria et al developed superparamagnetic iron oxide nanoparticles (SPIONs) that presented a silica-coated shell (SPION/SiO2) and also a carbon-coating shell over these silica-coated particles (SPION/SiO2/C) using hydrothermal synthesis. Particles aged for one year and presented 13 nm of size average. The group demonstrated that the saturation magnetization values for the coated nanoparticles were higher than for the SPIONs before the treatments, meaning better magnetic properties can be achieved with the correct shell-core design. Furthermore, these results demonstrated that the coating shell acted as a protective agent, minimizing inter-particles dipolar coupling and maintaining the single-domain structure of the particles. This indicates that even with the addition of a paramagnetic weight that could decrease the magnetic properties, the hydrothermal process presents itself as an excellent method to compensate for the negative contribution of SiO2/C and increases the superparamagnetic properties of the SPIONs [139].
It is also possible to coat magnetic nanoparticles with the drug intended to be delivered by the system. Dextran - iron oxide nanoparticles were synthesized using a co-precipitation with hydrazine hydrate to reduce NPs toxicity and facilitate drug delivery using the particles' magnetic properties [140]. Also, they can be synthesized and present a mesoporous form. For instance, Yachao Jia et al presented Fe3O4 nanoparticles synthesized using stabilized oleic acid by a co-precipitation method. The nanoparticles presented a diameter of 55 nm and excellent biocompatibility. These mesoporous nanoparticles are ideal options for bone regeneration, and according to the rat model tibial distraction osteogenesis, further studies based on therapeutics and DNA/siRNA delivery could be promising [141]. Studies have also observed how these magnetic nanoparticles could be modified and used as composite agents for drug delivery applications. Combining their properties with other nanoparticles' properties can lead to diverse outcomes to facilitate various bone applications.
5.4.2. Magnetic nanoparticles as part of composites for drug delivery
One composite option is the combination of magnetic nanoparticles with HA NPs. For instance, a magnetic and HA nanoparticles composite using NaOH solution degassed with N2 into a FeCl2 solution has been studied. The particles presented an average diameter of 30 nm and could be used in a Rod-like HA particle. These nanoparticles' heating characteristic is their advantage, which could be used for cancer treatment under a magnetic field [130]. In addition to this combination, nanocrystalline HA particles blended with magnetite (Fe3O4) forming Fe3O4−HA nanoparticles have also been studied. At first, the magnetic nanoparticles were prepared by alkaline coprecipitation of ferric and ferrous chloride in an aqueous solution. Then, the study compared the magnetic properties of the Fe3O4 uncoated nanoparticles and the combination with HA, and while the pure magnetite saturation magnetization value presented was 20.639 emu g−1, the Fe3O4−HA nanoparticles presented a value of 7.34 emu g−1, leading to a decrease in the saturation magnetization curve. Furthermore, according to in vitro test results, combined nanoparticles enabled good cell proliferation and have potential hyperthermia, a technique for generating heat in an alternating magnetic field [142]. Another composite was developed using PLGA with SPIONs forming a SPION-PLGA nanoparticle. First, the SPIONs were prepared using thermal decomposition, then the combined SPIONs and PLGA particles were loaded with bovine serum albumin (BSA). The final particle obtained was a core-shell type formed by a BSA core and a PLGA shell that contained the SPIONs well distributed. Analysing the hysteresis curve of the particles, the produced SPIONs presented a saturation magnetization value of 47.8 emu g−1, while the combination of these nanoparticles to form the composite remained with the superparamagnetic properties, however with a decreased value where different sizes of BSA/SPION-PLGA presented values of 1.5, 2.3 and 1.5 emu g−1. The particles' saturation magnetization value was higher because the portion of SPIONs was higher in their compositions. Furthermore, the study also revealed that the BSA and proteins separated from the SPIONs did not affect the nanoparticles' inter-particle distance, thus the magnetization values [143].
Not only SPIONs can be used as magnetic nanoparticles in nanocomposites, but cobalt ferrite (CoFe2O4) nanoparticles too. They are considered one of the essential components of the spinel ferrite magnetic family since they present good properties such as chemical stability, good saturation magnetization values, high permeability, and last but not least, the presence of Co2+ ions, which leads to excellent therapeutic characteristics and antibacterial activity. A recent study used these nanoparticles synthesized by sol-gel combustion to form a core-shell composite with Mg2SiO4, a phosphorite bioceramic with outstanding mechanical properties and potential for bone restoration. The scaffolds were prepared with the core-shell nanoparticles, and scaffolds presented cell viability and proliferation potential along with a prolonged drug release time [144].
6. Conclusion
This brief review summarized some of the most recent studies on improving and developing drug delivery nanoparticles directed to bone-targeting and dental applications. At first, observing the provided data, it is noticeable that there is still a need for improvements in nanoparticles as dental penetration materials, and they have limitations. Besides, while many studies have developed drug targeting applications, there is still little information and results in vivo.
Moreover, analyzing the research studies, it is seen that there is a high dependence on the application, health problem, and the synthesis that is preferred for each type of particle and still a big challenge for medicine to find an ideal material and nanoparticle option for delivering medical components to bone tissues. Most studies change the synthesis method, and usually, the results do not present a perfect size distribution. Also, in the investigations related to magnetic nanoparticles and their applications, it is seen that some kinds of coating and surface modification decrease the magnetic properties of the particles, demonstrating the importance of more developments in this area resulting in larger applications.
Besides that, clearly, for most nanoparticles applications on delivery of therapeutics, even with mice models and in vivo studies, further developments, analysis, and results still need to come before this becomes a validity for medical treatments. Nevertheless, nanoparticles have been studied as great options, and nanotechnology is still a promising path for medicine.
Altogether, the study provided the different types of nanoparticles that have been developed in the last five years and how the properties and characteristics of the nanoparticles changes based on their synthesis. This study aimed to provide a view on how nanoparticles for bone-targeting and dental targeting have the potential for therapies applications and how essential it is to develop new technologies to provide a better quality of life for patients and decrease drug toxicity.
Acknowledgments
This work was supported by the New Frontier Seed Grant Program (2020-2021) from The Network for Canadian Oral Health Research (NCOHR).
Data availability statement
No new data were created or analysed in this study.