Abstract
Bladder cancer (BC) is one of the commonest malignancies in the urinary system. Bladder cancer is divided into non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC) according to the depth of invasion. Besides, the prognosis of MIBC remains poor. Surgical resection combined with radiotherapy or chemotherapy is the standard treatment for MIBC. However, the major obstacle that hinders successful chemotherapy is its lack of tumor targeting. Here, we fabricated nanoparticles that respond to near-infrared laser irradiation in order to increase the drug accumulation at the tumor sites and combine chemotherapy with photothermal therapy to overcome challenges of bladder cancer treatment. IR780 and Doxorubicin (DOX) were loaded into albumin nanoparticles (IR780-DOX@Albumin NPs). In the process of IR780-DOX@Albumin NPs synthesis, the near-infrared molecule IR780 was used as the assembly molecular bridge. Under irradiation, the nanoparticles were decomposed due to the degradation of IR780 while the release of DOX increased. Nanoparticles can be ingested by tumor cells in a short time. The IR780-DOX@Albumin NPs were sensitive to near-infrared laser irradiation. Near-infrared laser irradiation can promote the release of the drugs from the nanoparticles and induce a photothermal effect, thus destroying the tumor cells. Given the excellent tumor-targeting ability and negligible toxicity to normal tissue, IR780-DOX@Albumin NPs can greatly increase the concentration of chemotherapeutic drugs in tumor cells. This study combines photothermal therapy and chemotherapy to treat MIBC, so as to avoid chemotherapy resistance, reduce the toxicity to normal cells, and achieve the purpose of improving the treatment of MIBC.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Abbreviations
| DOX | doxorubicin; |
| DTT | dithiothreitol; |
| HE | hematoxylin–eosin staining; |
| NPS | nanoparticles NMIBC: non-muscle invasive bladder cancer; |
| MIBC | muscle invasive bladder cancer; |
| PTT | photothermal therapy |
Introduction
According to the most recent registry of cancer in 2019, the incidence of bladder cancer ranks second among urinary system tumors, the fourth among male systemic malignancies, and the eighth highest mortality rate [1]. Bladder cancer is divided into non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC) according to the depth of invasion. Most bladder cancers belong to NMIBC (75%–80%), but MIBC is more malignant than NMIBC, which makes it one of the important factors threatening patients life [2, 3]. 20%–30% of patients will experience malignant transformation or even metastasis of tumors, such as lymph node metastasis, lung metastasis, etc [4–6]. Radical bladder resection combined with chemotherapy is the standard treatment for MIBC. The survival rate of patients with bladder cancer has not been improved significantly for a long time [7, 8].
Doxorubicin (DOX), an antitumor drug, is commonly used against bladder cancer. However, the clinical application of DOX is restricted by its cardiotoxicity, although it remains as one of the most effective chemotherapeutic antitumor agents since 1950s [9]. Its antitumor effect is achieved by intercalating into DNA helix and inhibiting the topoisomerase II enzyme activity [10–12]. IR780 is a lipophilic cationic near-infrared small molecule [13], generally in the form of iodide. Its maximum absorption peak is at around 780 nm. IR780 can penetrate deep into the tissue for imaging and obtain obvious signal contrast. Tumor cells of the spectrum are preferentially taken up and selectively enter the mitochondria of tumor cells. After IR780 is ingested by tumor cells, it can be excited by near-infrared laser to generate high temperature, thereby killing tumor cells and reducing damage to normal cells. Although IR780 possesses the above advantages, its hydrophobicity and phototoxicity still limit its use in tumor therapy [14, 15].
Photothermal Therapy (PTT) is a promising therapeutic approach by using laser light to heat contrast agents, leading to the thermal damage of tumor cells and making tumor cells degeneration and necrosis. [16]. Based on the good tissue penetration of near-infrared (NIR) laser, it is an ideal light source for photothermal treatment [17, 18]. The high temperature produced by photothermal therapy can change the permeability of biofilms, directly affecting the entry and exit of Na+, K+, Ca2+, adenosine triphosphate and some proteins. These factors can lead to the destruction of tumor cells; increase DNA damage and inhibit the repair of DNA damage. High temperature will not directly cause damage to the DNA chain, but can change the structure of chromatin and inhibit its synthesis, thereby inhibiting DNA repair. High temperature disorganizes the cytoskeleton, impairs cell function, and causes cell death [19].
Albumin is one of the most abundant plasma proteins and it keeps stable in the pH range of 4–9 and soluble in 40% ethanol. Even more, no deleterious effects were observed when it was heated at 60 °C for up to 10 h [20–22]. Albumin has various advantages, including biodegradability, non-immunogenicity, and biocompatibility. Therefore, the use of albumin as a nanoparticle carrier can overcome the biological safety deficiencies of other materials [23, 24]. We found that the nanoparticles carrying photosensitizer IR780 can produce photothermal and photodynamic effects under specific illumination, resulting in a significant anti-tumor effect [25].
Therefore, in this study, we taken advantage of the lipophilic cationic properties of IR780 to make it play the role of assembling molecular bridges, and constructed a drug delivery system with albumin as the backbone, which had controlled release of doxorubicin and IR780 and photothermal therapy. This delivery system can reduce the toxicity of doxorubicin and IR780, and has the function of fluorescence imaging.
Materials and methods
Chemicals and materials
Doxorubicin (DOX) and BSA were purchased from Aladdin co. (Shanghai, China). IR780 was purchased from Sigma-Aldrich co. (St. Louis, USA). MTT Cell Proliferation and Cytotoxicity Assay Kit was obtained from Beyotime (Shanghai, China). 1 × Phosphate buffer solution (PBS) and deionized water were used in the experiments. All other reagents were purchased from Nanjing Wanqing Chemical Glassware Instrument Company and used as received.
Synthesis of IR780-DOX@Albumin NPs
Firstly, we fully dissolve 100 mg BSA in 50 ml pure water, and add 100 μl of Dithiothreitol (DTT) solution (10 mg ml−1) to the BSA solution. After a period of reaction, DTT opens the hydrophobic space in the BSA. Then 1 ml of DOX in DMSO solution (1 mg ml−1) was slowly added to the BSA solution under ultrasonic condition. After well mixing, 1 ml of IR780 DMSO solution (1 mg ml−1), was slowly added to the aforementioned solution under ultrasonic condition, and a rotary evaporator was used to fully remove the organic solvent to obtain IR780-DOX@Albumin NPs.
Characterization of IR780-DOX@Albumin NPs
We measured the characterization of the nanoparticles before and after laser excitation. Particle size was measured by a Brookhaven BI-90Plus laser particle size analyzer. The UV spectra of IR780, DOX, and albumin nanoparticles were measured by an ultraviolet-visible spectrophotometer, to evaluate whether IR780 and DOX were encapsulated in the nanoparticles. Transmission electron microscopy was used to observe the morphology of the nanoparticles. Samples were prepared by dropping a suitable concentration of the nanoparticle solution on a 200 mesh copper mesh and dried overnight.
In vitro temperature curve
We have prepared free IR780 solution, albumin nanoparticle solution containing IR780 (IR780@Albumin NPs), albumin nanoparticle solution containing DOX and IR780 (IR780-DOX@Albumin NPs), and PBS solution (pH 7.4). The concentration of IR780 is 50 μg ml−1. At room temperature, 808 nm near-infrared laser irradiation was used to irradiate the samples for 0–150 s, and the photothermal effect of IR780-containing nanoparticles was evaluated by a temperature probe.
Cell culture
Murine bladder carcinoma MB49 cells were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. The cell line was maintained in RPMI 1640 cell culture media supplemented with 10% fetal calf serum (Hyclone, Logan, UT) and antimicrobial-antimycotic (Gibco/Invitrogen, Carlsbad, CA) in a humidified incubator at 37 °C in an atmosphere composed of 5% CO2.The cell line was transduced with the firefly luciferase gene by a lentivirus vector.
Cellular uptake
The mouse bladder cancer cell line MB49 is used to evaluate the cell uptake efficiency. Cells incubated with IR780-DOX@Albumin NPs solution (both IR780 and DOX concentration are 1 μg ml−1) for a period of time, and the nanoparticles that had not entered the cells were washed away with PBS solution. The cells were fixed with 4% paraformaldehyde, and then placed under a confocal microscope for observation to analyze the cellular uptake of nanoparticles.
In vitro therapeutic efficacy of IR780-DOX@Albumin NPs
Incubating the mouse bladder cancer cell line MB49 with different concentrations of free IR780 solution, free DOX solution and IR780-DOX@Albumin NPs solution for a period of time, 808 nm laser was used to irradiate part of the cell area of the experimental group for 3 min, and then cultured for 24 h. The cell viability was evaluated by MTT assays by measuring the absorbance of the solution at 570 nm.
In vitro drug release
To study the release profile of NPs, an appropriate amount of IR780-DOX@Albumin NPs and DOX@Albumin NPs were put into a dialysis bag separately, and placed in an Erlenmeyer flask. The Erlenmeyer flask is filled with PBS solution (pH = 7.4) as a release medium and placed in a constant temperature vibrator. The release of IR780-DOX@Albumin NPs was also studied by irradiation with 808 nm laser. Samples (released solution) were collected at the set time point (0–72 h), and replenish the same amount of released medium. The release curve of the drug was got by measuring the characteristic absorption peak of DOX in the released solution according to the standard curve.
Establishment of a bladder cancer model in C57BL/6 mice
4–6 weeks old C57BL/6 female mice were anesthetized by intraperitoneal injection of sodium pentobarbital, and 2*106 MB49 cells were planted into the left abdomen subcutaneously of each mouse. The size of the tumor was measured every day. When observing the tumor growth to 100mm3, mice with a rounder tumor shape and little difference in volume were selected as an animal model for subsequent experiments.
Therapeutic efficacy of IR780-DOX@Albumin NPs using bladder cancer model
After selecting a suitable mouse bladder cancer model, we defined the tumor size at this time as V0. We injected saline, DOX@Albumin NPs (2.5 mg kg−1 DOX), IR780-DOX@Albumin NPs (2.5 mg kg−1 DOX, 4 mg kg−1 IR780), IR780@Albumin NPs plus laser (4 mg kg−1 IR780), and IR780-DOX@Albumin NPs plus laser (2.5 mg kg−1 DOX, 4 mg kg−1 IR780) groups into mice via tail vein injection. Each group contained five mice. After 48 h of administration, IR780@Albumin NPs plus laser and IR780-DOX@Albumin NPs plus laser groups were irradiated with 808 nm laser. The tumor volume was measured every 2 days after the administration and the body weight change of the mice was monitored.
In vivo toxicity and biosafety
In order to verify the biosafety of the nanoparticles, C57BL/6 mice were used here. Saline, DOX@Albumin NPs (2.5 mg kg−1 DOX), IR780-DOX@Albumin NPs (2.5 mg kg−1 DOX, 4 mg kg−1 IR780), IR780@Albumin NPs plus laser (4 mg kg−1 IR780), and IR780-DOX@Albumin NPs plus laser (2.5 mg kg−1 DOX, 4 mg kg−1 IR780) groups were injected into mice by tail vein. Each group contained three mice. After 48 h of administration, IR780@Albumin NPs plus laser and IR780-DOX@Albumin NPs plus laser groups were irradiated with 808 nm laser. After 2 weeks, the mice were killed, and the heart, liver, spleen, lung and kidney were taken out, and the organs were dehydrated and fixed, and then HE staining was performed for histological analysis.
Results and discussion
Synthesis of IR780-DOX@Albumin NPs
The synthesis process of IR780-DOX@Albumin NPs was illustrated in figure 1. Firstly, we dissolved albumin in pure water, while DTT was added to open the hydrophobic domain of albumin, and DOX can be loaded into albumin by hydrophobic interaction [26]. Secondly, we use the hydrophobic and electrostatic effect of IR780 to make it as a molecular bridge to tighten the spatial structure of albumin nanoparticles, and finally get IR780-DOX@Albumin NPs [27]. Under irradiation, IR780 in the nanoparticles will be degraded, and produce a photothermal effect, and also promote the release of DOX.
Figure 1. Schematic illustration of the synthesis and working principle of IR780-DOX@Albumin NPs.
Download figure:
Standard image High-resolution imageCharacterization of IR780-DOX@Albumin NPs
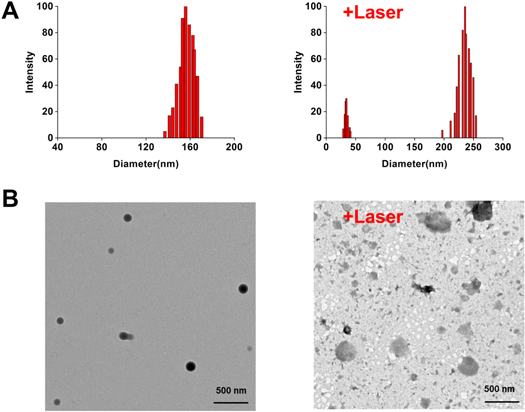
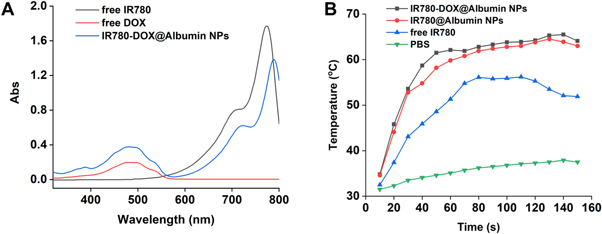
The IR780-DOX@Albumin solution was spotted on the carbon-film copper net and dried overnight, and then the shape and size were observed by transmission electron microscope; Dynamic Light Scattering (DLS) was used to characterize the size and distribution of nanoparticles; the average particle size of IR780-DOX@Albumin is 155.9 nm (figure 2(A)). After 808 nm laser is used to irradiate the nanoparticles, the average particle size of IR780-DOX@Albumin is 235.7 nm, because laser irradiation accelerates the disintegration of nanoparticles. The morphological changes of nanoparticles are shown in figure 2(B). By measuring the UV absorption spectra of IR780, DOX, IR780-DOX@Albumin nanoparticles, it can be found that IR780-DOX@Albumin nanoparticles have characteristic absorption peaks at 497 nm and 780 nm, which proved that IR780 and DOX were successfully encapsulated into albumin nanoparticles (figure 3(A)).
Figure 2. Characterization of IR780-DOX@Albumin NPs. (A) Particle size distribution data of IR780-DOX@Albumin NPs before and after laser irradiation. (B) Morphological changes of IR780-DOX@Albumin NPs under transmission electron microscope before and after laser irradiation.
Download figure:
Standard image High-resolution imageFigure 3. (A) Optical properties of free DOX, free IR780 and IR780-DOX@Albumin NPs. (B) Temperature rise curve of PBS, free IR780, IR780-DOX@Albumin NPs and IR780-DOX@Albumin NPs after laser irradiation.
Download figure:
Standard image High-resolution imageIn vitro temperature curve
Firstly, different concentrations of nanoparticle group and control solution were irradiated by using 808 nm laser, while a temperature detector was used to detect the temperature change, the temperature curve of the nanoparticles was draw after laser irradiation, to evaluate the photothermal effect of nanoparticles. We found that IR780-DOX@Albumin NPs and IR780@Albumin NPs increased significantly after the 808 nm laser irradiation. About 60 s later, the photothermal reaction of the nanoparticles reached the peak temperature and could be maintained for a period of time (figure 3(B)).
In vitro therapeutic efficacy of IR780-DOX@Albumin NPs
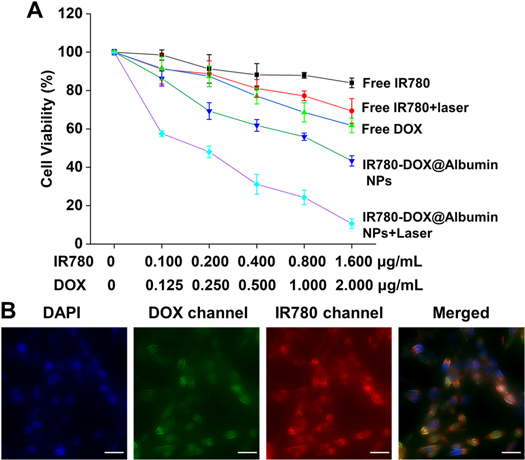
We use the MTT assay to evaluate the anti-tumor therapeutic effect of IR780-DOX@Albumin NPs in vitro. It can be seen from figure 4(A) that under the same concentration of IR780-DOX@Albumin NPs and free drugs, the killing effect of IR780-DOX@Albumin NPs on tumor cells is significantly better than that of free drugs on tumor cells. The free IR780 and nanoparticle groups were irradiated with 808 nm laser, and it was found that the free IR780 and nanoparticle groups had significantly increased therapeutic effects on tumor cells. The results confirmed that the nanoparticles had a good photothermal treatment effect.
Figure 4. Cell viability and uptake of IR780-DOX@Albumin NPs. (A) In vitro.
Download figure:
Standard image High-resolution imageCellular uptake
In order to verify the uptake of IR780-DOX@Albumin NPs by cells, MB49 cells and IR780-DOX@Albumin NPs were co-cultured for 12 h, and then observed by a confocal microscope. It could be seen from figure 4(B) that IR780-DOX@Albumin NPs can be effectively taken up by MB49 cells and were mainly distributed in the cytoplasm cytotoxicity and therapeutic efficacy of IR780-DOX@Albumin NPs. (B) Observing cellular uptake in DOX channel, IR780 channel, DAPI and merge vision. Scale bars = 20 μm. (eyepiece: 1×, objective lens: 20×).
In vitro drug release
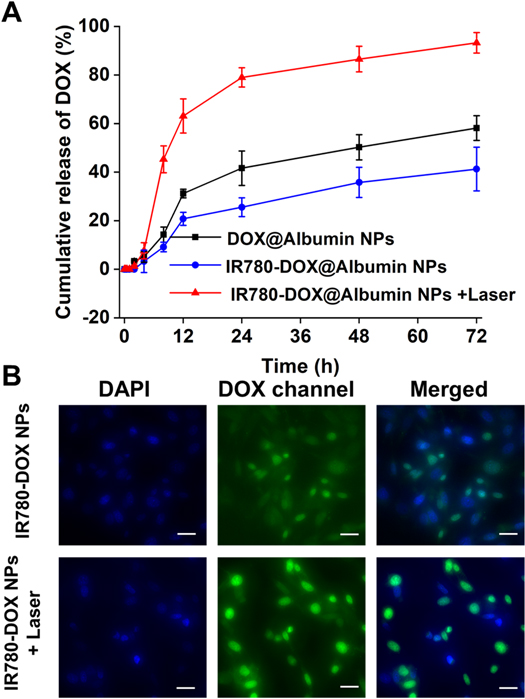
An in vitro drug release study was conducted to confirm the controlled-release pattern of DOX under 808 nm irradiation (figure 5(A)). It can be clearly found that as time increased, the cumulative release of DOX in vitro increased. More importantly, the laser triggered the photothermal effect of IR780 in the nanoparticles, and the cumulative release of DOX drugs showed a significant growth (figure 5(B)). It was worth noting that the cumulative DOX release of DOX@Albumin NPs was more than that of IR780-DOX@Albumin NPs in the same period. We speculate that this phenomenon was related to the role of IR780 in nanoparticles. IR780 acted as a molecular bridge in the nanoparticles, making the space structure of the nanoparticles more compact, and DOX was not easy to release. This experiment verified the nature of nanoparticles in a controlled release manner, and DOX can be triggered by near-infrared laser irradiation. From the results of drug release experiments in vitro, we can combine photothermal therapy with chemotherapy drugs to synergistically kill bladder cancer cells.
Figure 5. (A) The cumulative release of DOX from IR780-DOX@Albumin NPs under laser irradiation. (B) Observing cellular uptake in DOX channel, DAPI and merge vision. Scale bars = 20 μm. (eyepiece: 1×, objective lens: 20×).
Download figure:
Standard image High-resolution imageTherapeutic efficacy of IR780-DOX@Albumin NPs using bladder cancer model
A mouse bladder cancer model have been established to evaluate the in vivo therapeutic effects of nanoparticles on bladder cancer. After selecting a suitable mouse bladder cancer model, we defined the tumor volume at this time as V0. We injected saline, DOX@Albumin NPs, IR780@Albumin NPs + laser, and IR780-DOX@Albumin NPs plus laser groups into mice via tail vein. The volume of the tumor and body weight were measured and recorded (figure 6(A)) (figure 6(B)) every two days. When the tumor of the mouse was too large on day 16th (V/V0 > 20), the mouse was immediately killed and stopped recording (because of animal experiment ethics). Experimental results show that the group IR780-DOX@Albumin NPs plus laser have an excellent therapeutic effect on bladder tumors in vivo. The daily increase rate of V/V0 in the IR780-DOX@Albumin NPs + laser group was about 5.05% in the saline group. It was worth noting that the laser-irradiated IR780@Albumin NPs group inhibited tumors well in the early stage, but due to the lack of the effect of DOX, the V/V0 increased significantly in the later stage. IR780-DOX@Albumin NPs had a good photothermal and tumor suppression efficiency, which achieved by near-infrared lasers to further suppress bladder cancer.
Figure 6. Therapeutic efficacy of IR780-DOX@Albumin NPs using bladder cancer model. (A) Construct an animal model of bladder cancer, inject different therapeutic drugs into the tail vein, and measure the diameter of the tumor and (B) body weight. (C) In vivo organ safety evaluation. Scale bar = 100 μm. (eyepiece: 1×, objective lens: 5×).
Download figure:
Standard image High-resolution imageIn vivo toxicity and biosafety
To evaluate the toxicity of the nanoparticles at the whole-body level, major organs were harvested for histological analysis. There were no obvious accumulation of nanoparticles and functional damage. The major organs (heart, liver, spleen, lung, and kidney) of all the groups showed no noticeable histological changes (figure 6(C)), suggesting the nanoparticles have no toxicity to the organs. Thereby, IR780-DOX@Albumin NPs have the possibility of clinical application
Conclusions
In this study, IR780-DOX@Albumin NPs were synthesized to control the release of DOX under the action of PTT to treat bladder cancer. We verified the biocompatibility, in vitro targeting, and photothermal properties of the nanoparticles at the cellular level. At the same time, we studied the combination therapy and the toxicity of the nanoparticles to normal tissues and tumor tissues at the animal level. IR780-DOX@Albumin NPs can be taken up by bladder cancer cells more than normal cells, so they have the less toxicity to normal cells and tissues. This study combines the advantages of nanoparticle carriers, and IR780 acting as a molecular bridge which made the structure of the nanoparticles more compact, to solve the problems of photosensitizers insoluble in water, poor photostability and high toxicity, and reduce the toxicity of photosensitizer nanoparticles to normal cells. Based on the optical treatment technology of nano-photosensitive materials, PTT treatment effect of IR780 and the tumor-killing effect of DOX were exerted to improve the treatment efficiency of bladder cancer.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Declarations
Ethics approval
All mice received care in terms of the guidelines for the Care and Use of Laboratory Animals and were used in terms of the Institutional Animal Care regulations and Use Committee (IACUC) of Nanjing University. All animal experiments were approved by the Administration Committee of Experimental Animals in Jiangsu Province and the Ethic Committee of Nanjing Drum Tower Hospital.
Consent for publication
Not applicable
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors have declared that no competing interest exists.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81972388, 81772710), The Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Key Medical Discipline (Laboratory) (ZDXKB2016014).