Abstract
Recently, photodynamic therapy (PDT) has received a lot of attention for its potential use in cancer treatment. It enables the therapy of a multifocal disease with the least amount of tissue damage. The most widely used prodrug is 5-aminolevulinic acid, which undergoes heme pathway conversion to protoporphyrin IX, which acts as a photosensitizer (PS). Additionally, hematoporphyrin, bacteriochlorin, and phthalocyanine are also studied for their therapeutic potential in cancer. Unfortunately, not every patient who receives PDT experiences a full recovery. Resistance to different anticancer treatments is commonly observed. A few of the resistance mechanisms by which cancer cells escape therapeutics are genetic factors, drug–drug interactions, impaired DNA repair pathways, mutations related to inhibition of apoptosis, epigenetic pathways, etc. Recently, much research has been conducted to develop a new generation of PS based on nanomaterials that could be used to overcome cancer cells' multidrug resistance (MDR). Various metal-based, polymeric, lipidic nanoparticles (NPs), dendrimers, etc, have been utilized in the PDT application against cancer. This article discusses the detailed mechanism by which cancer cells evolve towards MDR as well as recent advances in PDT-based NPs for use against multidrug-resistant cancers.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The current need for therapeutic oncology is to develop a cytotoxic drug that effectively eliminates cancer cells while protecting vital host cells and their activities. If not for the issue of human cancer's resistance to chemotherapy or the drugs' non-specific cytotoxicity, cancer treatment would have been analogous to antibacterial treatment, in which complete eradication of infection is frequently observed. One of the key issues with cancer therapy is innate (inherited) or acquired resistance [1]. A tumor's initial inability to respond to a specific therapy is referred to as natural resistance, and the unresponsiveness of a drug that develops following an initial successful course of treatment is referred to as acquired resistance. Mutations that arise during treatment can also contribute to acquired drug resistance, as can a variety of other adaptive responses like elevated levels of the therapeutic target and stimulating substitute compensatory signaling pathways in previously sensitive tumors [2]. Drug resistance has been associated with a wide variety of molecular mechanisms, including elevated drug efflux rates, drug target mutations, and changes in drug metabolism [3]. Chemoresistance has also been linked to epigenetic changes and the impact of the surrounding tumor microenvironment (TME). Because cancer stem cells are inherently resistant to many therapeutic modalities, their presence in some situations has recently been linked to treatment failure [4].
One of the less invasive techniques that could be employed for cancer therapy is photodynamic therapy (PDT). PDT can be used as another option for surgical cancer treatment [5]. In this therapy, a photosensitizer (PS) that has been applied to the patient builds up in the tumor, where it causes significant damage when exposed to the right wavelength of oxygen and light, resulting in the death of cancer cells and tumor vascularity. PDT also triggers immune and inflammatory reactions against tumor cells. Currently, 5-aminolevulinic acid (5-ALA) is the most commonly used medication for PDT [6]. Unfortunately, not all patients experience full recovery post-treatment with PDT. The primary factor in anticancer PDT therapy failure is the development of resistance. The PDT resistance mechanisms attributed to the PS are likely similar to general drug resistance mechanisms and involve altered intracellular trafficking or efflux rates and altered drug uptake [7]. Alternately, upregulated antioxidant detoxifying enzymes and heat shock protein activation are linked to the deactivation of reactive oxygen species (ROS) and PDT resistance. An increased level of DNA damage repair and decreased PS levels in tumor cells may also lead to multidrug resistance (MDR).
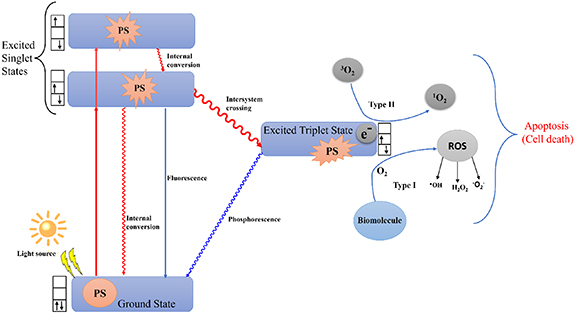
2. Photodynamic therapy (PDT)
The most widely employed treatment modalities for various types of cancer are surgery, chemotherapy, radiation therapy, and immunotherapy. Recently, small-molecule-based therapy has also been successfully applied [8]. However, these traditional treatments have major disadvantages in terms of their selectivity against cancer cells. Chemotherapy is frequently associated with systemic adverse effects and an elevated risk of recurrence following surgical excision of malignancies, whereas radiation therapy is constrained by the combined dose of radiation [9]. As a result, research has concentrated on creating alternative therapy approaches that are very specific and selective, safe, powerful, and economical. Additionally, it should only damage the cancerous cells while sparing the surrounding healthy cells and tissues. A new, non-invasive alternative to traditional tumor-ablative therapies for the treatment of a variety of malignancies is PDT [10]. PDT is made up of three main parts: (1) light, (2) PSs, and (3) oxygen in their molecular states (figure 1). PDT makes ROS more likely to form in two different ways. In the type I pathway, the PS moves electrons to make free radicals [11]. In the type II pathway scenario, it transfers energy to produce the highly reactive singlet oxygen (1O2) and the triplet ground-state molecular oxygen (3O2) [12]. A ground-state PS is propelled into a high-energy electronic state by the absorption of light (photon) [13, 14]. According to Lucky et al following intersystem crossing, the PS's excited singlet state can produce a persistently excited triplet state [15]. This excited triplet state can then come back to the ground singlet state by releasing energy in the form of fluorescence, heat, or other forms of energy. The excited triplet state transfers energy to surrounding oxygen molecules or other substrate molecules, which in turn induces the creation of singlet oxygen (1O2) or other ROS. When PDT is performed, the formation of ROS such as hydrogen peroxide (H2O2), hydroxyl radical (OH‧), and superoxide anion radical (O2‧) reacts with biological molecules such as lipids, DNA, and proteins to eradicate tumor cells by necrosis and/or apoptosis. However, the killing mechanisms of radiotherapy and chemotherapy are predominantly immunosuppressive, destroying tumor vasculatures as an anti-angiogenesis effect and stimulating the host immune system to find, locate, and kill any remaining tumor cells [16]. Additionally, PDT can be locally applied to a specific region of tumors by selectively illuminating the lesion without affecting the normal tissues. Therefore, PDT is much more advantageous than radiotherapy and chemotherapy, which exert toxicity. PDT is more effective than traditional treatments because it is less invasive, can be done more than once without causing toxicity, has better functional and cosmetic effects, has less long-term morbidity, and improves the patient's quality of life. PDT has been successful in treating skin cancer [17], Barrett's esophagus [18], head and neck malignancies [19], lung cancer, and bladder cancer [20] over the past 40 years. PDT is well tolerated by patients because of its selective action. PDT procedures are pleasant, and their straightforward application enables outpatient use.
Figure 1. Mechanism of photodynamic therapy which is induced by light radiation leading to reactive oxygen species generation mediated cell death.
Download figure:
Standard image High-resolution image2.1. Light source
For a PDT reaction to take place, the PS is required to be turned on by a light source with a wavelength of <800 nm. Above 800 nm, PS cannot be turned on and cannot make enough singlet oxygen because its triplet state is beneath the energy threshold of singlet oxygen [21]. In the earlier days, photosensitization had been performed with high-power traditional metal halogen lights that put out radiation between 600 and 800 nm [22]. In the next generation of PDT, laser lights were used for photosensitization instead of gas lights. There are two types of lasers used in PDT: dye-lasers and diode-lasers. An organic dye molecule, such as rhodamine or kiton red, that produces light in the 600–650 nm spectral region serves as the lasing medium in dye lasers. This is done to coincide with a particular PS's absorption wavelength [23]. To prevent overheating, the dye substance is often a liquid that is constantly circulated. Due to circulation, only a part of the dye is ever lasing in the cavity at any given time. Light is produced by electron-hole recombination in semiconductor devices called diode lasers. Since diode lasers have a fixed wavelength, a different laser unit is required for each PS that absorbs at a different excitation wavelength. Irradiance from clinical diode lasers can reach 1 W cm−2. PDT commonly uses diode lasers with output wavelengths in the 415–690 nm range [23]. But light-emitting diodes (LEDs) are now widely used instead of lasers as light sources for photosensitization because they are easier to operate and last longer than lasers [24]. Due to the dynamic interactions between light, PS, and oxygen, PDT dosimetry is complicated. The clinical effectiveness of PDT is determined by the type of complex dosimetry used, the overall dose, the length of light exposure, the delivery mechanism, and the fractionation scheme. Direct dosimetry, explicit dosimetry, biophysical/biological tissue response monitoring, and implicit dosimetry are the four dosimetric methods that can be employed for PDT dosimetry. A single metric can combine two or more of the treatment parameters. PDT dosage is one such metric, which is calculated as the sum of the PS concentration and light fluence (J cm−2) [23].
2.2. Photosensitizers (PSs)
Scientists have studied three different types of PSs. At first, a hematoporphyrin derivative was used to detect tumors and emit fluorescence (figure 2). Later, Meyer-Betz found that porphyrins might also have phototoxic effects and could be used to kill cancer cells [25]. So, the first-generation PSs are porphyrins, hematoporphyrin, and their derivatives, such as Photosan and Photocan, as well as Photofrin. The monomeric, dimeric, and oligomeric parts of each derivative are different [26].
Figure 2. Chemical structures of commonly used photosensitizers for photodynamic therapy: porphyrin, hematoporphyrin, bacteriochlorin, phthalocyanine, methylene blue, and aminolevulinic acid.
Download figure:
Standard image High-resolution imageThe pure synthetic compounds made of an aromatic macrocycle are second-generation PSs such as chlorins, bacteriochlorins, benzo-porphyrins, and some common dyes such as rose Bengal (RB) [27], eosin Y [28], or methylene blue (MB) [29] that produce a high absorbance (85 000 M−1 cm1) at 664 nm. Third-generation PSs are distinguished by the fusion of second and first-generation PS with targeting ligands, such as antibodies, carbohydrates, amino acids, and peptides, or by their encapsulation within carriers like liposomes, micelles, and nanoparticles (NPs). This strategic approach aims to enhance the concentration of PSs specifically at the intended tumor sites, building upon the advancements seen in second-generation PS. Figure 3 represents the mechanism of utilizing third-generation PSs for cancer treatment using PDT. A list of clinically approved photosensitizers or under trial for PDT in cancers has been provided in table 1.
Figure 3. Schematic representation for the mechanism of tumor cell destruction by photodynamic therapy using third-generation photosensitizers.
Download figure:
Standard image High-resolution imageTable 1. List of photosensitizers clinically approved or under trial for PDT applications in cancer.
| Generic name/trade name | Chemical compound | Approved or in trail | Cancer types | References |
|---|---|---|---|---|
| Clinically approved drug | ||||
| (Photofrin®) | Porfimer sodium porphyrin | World wide | PDT of esophageal cancer, lung adenocarcinoma, endobronchial cancer, gastric and recurrent bladder cancers | [30] |
| Levulan | 5-Aminolevulinic acid | Worldwide | PDT of skin, bladder, brain, and esophagus cancers | [31] |
| Cysview® | Hexaminolevulinate (HAL) | EMEA & USA | PDT of skin and bladder cancer | [32] |
| Metvix® | Methyl ALA ester (MAL) | USA, Europe | PDT of actinic keratosis and basal cell carcinoma (BCC) | [33] |
| Laserphyrin® | N-aspartyl choline E6 | Japan | PDT of early centrally located lung cancer | [34] |
| Redaporfin® | Bacteriochlorin | USA | PDT of biliary tract cancer | [35] |
| Photogem® | Porphyrin | Russia | PDT of BCC | [36] |
| Photosens® | Aluminum phthalocyanine tetrasulfonate | Russia | PDT of breast, lung, stomach, and skin cancers | [37] |
| Photolon® | Chlorin E6 | Belarus, Russia, Kazakhstan, Ukraine | PDT of skin melanoma and mucosal malignancy | [38] |
| Photochlor® | 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH) | USA | PDT of BCC, head and neck cancer, and lung cancer | [39] |
| In clinical trail | ||||
| Verteporfin® | Chlorin | Approved in China and Norway, trial in the UK | PDT of BCC, lung and skin cancers | [40] |
| Temoporfin (Foscan®) | Chlorin | Approved in Europe, trial in USA | PDT of advanced head and neck cancer, lung, brain, skin, and bile duct cancers | [41] |
| Talaporfin® | Chlorin | Approved in Japan, trail in USA | PDT of lung cancer, colorectal neoplasms, liver metastasis, and glioma | [34] |
| Radachlorin | Chlorin | Belarus, Russia | PDT of skin cancer, nasopharyngeal sarcoma | [42] |
| Purlytin | Tin ethyl etiopurpurin | USA | PDT of metastatic breast cancer | [43] |
| Padoporfin (TOOKAD) | Palladium-bacteriopheophorbide | USA | PDT of prostate cancer Phase II and III | [44] |
| Motexafin lutetium (Lutex) | Texaphyrin | USA | PDT of breast cancer | [45] |
2.3. Limitations of PDT
Although PDT is one of the most effective techniques for the therapy and management of various types of cancer, its fundamental limitation is that it can only be used to treat superficial, flat lesions, initial lesions, or lesions accessible by endoscopic instruments. However, PDT cannot be used to treat solid, bulky, or deeply embedded tumors. This is because light cannot penetrate the deep tissues inside our body, and whole-body irradiation is not possible with the PDT technique. Moreover, the organic PSs in PDT do not exhibit high selectivity for cancer cells, and they have a complicated structure. It is easily degraded by enzymes and is not cost-effective. Although PDT is used for cancer treatment, it has toxic side effects. The PS does cause a significant amount of toxicity to the local tissue when exposed to light. Although reactive oxygen is thought to be the major cytotoxic agent in PDT, other ROS, like the superoxide anion and hydroxyl radicals, could cause biological damage to healthy tissue in the nearby locations around tumors. So, to solve the drawbacks of this classic PS, a better alternative, such as nanomaterial-mediated PDT, has been developed.
3. NPs and PDT
Nanomaterials can be synthesized from a wide variety of starting materials, which are both natural and synthetic. NPs are simple to create, allowing for the targeted delivery of several theranostic agents [46, 47]. NPs are extensively used as carriers for PSs in PDT [47]. This is because of the following factors:
- (1)Due to their high surface area-to-volume ratio, they can significantly enhance PS delivery to the cells being targeted.
- (2)If PS is enclosed in NPs, early PS discharge in healthy tissues and potential medication inactivation by plasma components may be prevented [48].
- (3)NPs loaded with PS can be transported efficiently through the circulatory system to the site of the tumor.
- (4)Compared to organic PS, NPs exhibit increased permeability and retention (EPR) within tumor tissue.
- (5)To enhance the biological distribution, pharmaceutical kinetics, cellular absorption, and targeting properties of the NPs, different functional groups or targeting moieties can be added to the surface of the particles.
Based on these above-mentioned properties, researchers have classified NPs as non-biodegradable and biodegradable NPs that can be used as PSs in PDT [49, 50]. Figure 4 shows different types of nonpolymeric and polymeric NPs applied in PDT.
Figure 4. Types of nanomaterials used in photodynamic therapy for cancer. Part of this figure has been drawn from published articles under the Creative Commons (CC-BY) license [51, 52]. Adapted from [51]. CC BY 3.0. Adapted from [52]. © IOP Publishing Ltd. CC BY 3.0.
Download figure:
Standard image High-resolution image3.1. Non-biodegradable NPs
NPs that are not biodegradable have gained much attention in the area of PDT as potential multimodal theranostic vehicles because of the unique traits they possess, including their optical characteristics, their form, their size, their porosity, and so on. Nonbiodegradable NPs encompass inorganic, metallic, or hybrid NPs.
When light interacts with metallic NPs, a phenomenon known as localized surface plasmon resonance (LSPR) occurs in which the free electrons in the metal collectively oscillate at a particular frequency [53]. The local electromagnetic field around the NP may be strengthened by this oscillation, increasing light scattering and absorption. Even though PSs themselves have relatively low absorption at the desired wavelength, they can effectively absorb the enhanced electromagnetic field when placed close to plasmonic NPs. This enhanced light absorption improves the chances that PSs will capture photons, which results in more effective photosensitizing molecule excitation. Table 2 represents different nonpolymeric NPs in PDT of cancers.
Table 2. List of different types of nonpolymeric nanoparticles and photosensitizers for photodynamic therapy in cancers.
| Nonpolymeric nanoparticles | Photosensitizers | Cancers | Light wavelength/intensity | References |
|---|---|---|---|---|
| AuNPs | Chlorophyll b (Chl b) | Human breast carcinoma cell line and liver carcinoma cell line (MCF7 & HepG2) | 650 nm/15 mW cm−2 | [54] |
| MSN | BODIPY | Human cervical cancer cell line (HeLa) | 455 nm, 518 nm and 655 nm/10–15 J cm−2 | [55] |
| Methylene blue | Breast cancer | 660 nm/15 mW cm−2 | [56] | |
| SPION | Protoporphyrin IX | Mouse mammary carcinoma cell line (4T1) | 632 ± 3 nm/5 mW cm−2 | [57] |
| TiO2 NPs | Porphyrin | Human breast carcinoma cell line MCF7 | 980 nm/0.72 W cm−2 | [58] |
| Phthalocyanine | HeLa xenograft tumor model | 420–800 nm/0.25 W cm−2 | [59] | |
| SWCNT | Chlorin e6 | Mouse squamous cancer cell line (SCC-7) | 630 nm/0.15 W cm−2 808 nm/1 W cm−2 | [60] |
| MWCNT | m-tetrahydroxylphenylcholrin | Overian cancer cell line (SKOV3) | 650 nm/125 mW cm−2 (for PDT) 808 nm/2.3 W cm−2 (for PTT) | [61] |
| GO NPs | Tetraphenylethelyne-Red | Bladder cancer cell line (UMUC3) | 450 nm/200 mW cm−2 | [62] |
| rGO NPs | Manganese dioxide (MnO2) | Human cervical cancer cell line (HeLa) | 980 nm/0.4 W cm−2 | [63] |
| C60 Fullerene | Black Phosphorous | Mouse mammary tumor cells (4T1) | 650 nm/0.5 W cm−2 | [64] |
| Tirapazamine (TPZ) | Cancer cell | 500 nm | [65] |
3.1.1. Gold nanoparticles (AuNPs)
AuNPs have played a significant role in the advancement of a synergistic hyperthermia-phototherapy technique because of their surface plasmon resonance (SPR) feature, their capacity to convert photoenergies into thermal energy for PDT applications, and their enzyme mimetic activity [66]. Based on these properties, a metal nano-organic framework was developed, where AuNPs act as catalysts like nano enzymes and the PS chlorin e6 is encapsulated to mitigate tumor hypoxia and reinforces PDT under 660 nm laser irradiation. This nano-delivery platform is stable in blood circulation and can easily penetrate and accumulate in tumors [66]. Similarly, in another study, AuNPs conjugated with 5-ALA showed enhanced PDT in cutaneous squamous cell carcinoma under 621 nm irradiation using LED for 1.5 h [67]. It was observed that 5-ALA-AuNPs not only suppressed cancer cell viability but also increased cell apoptosis and produced more singlet oxygen compared to PDT with 5-ALA alone [67]. In addition, to improve the PS's excitation effectiveness, LSPRs surrounding AuNPs may be utilized. Singlet oxygen generation in several AuNP morphologies has been investigated [68]. These include spherical AuNPs with hollow structures, gold nanocages (AuNCs), gold nanorods (AuNRs), and gold nanospheres [68].
As an optical contrast agent, AuNRs were used by Kuo et al to kill A549 cancer cells [69]. The cancer cells were exposed to PDT and hyperthermia simultaneously after their treatment with AuNR conjugated PS indocyanine green (ICG) under an 808 nm NIR laser for 30 s to 3 min. The results showed that the combined approach successfully eliminated cancer cells when compared to PDT or photothermal treatment (PTT) alone [70]. On the other hand, Wang and Lu hypothesized that coordinating an early-phase PDT impact with a late-phase PTT effect would boost the synergistic therapeutic efficacy of AuNPs [71]. To do this, they conjugated Ce6 molecules into polyethylene glycol (PEG) chains to create PEGylated AuNRs, which they evaluated in breast cancer cells in vitro and a xenograft model in mice using MDA-MB-435 [71]. After 4 h of intratumoral delivery of the nanoconjugates, researchers used a 671 nm laser (1.0 W cm−2) to treat the tumor for 6 min. Tumor volume was significantly reduced by laser therapy with Ce6-loaded PEGylated AuNRs compared to the respective control groups [71]. This research proved that the combinatorial method of PDT/PTT of AuNPs is more effective against cancer. A pH-stimulus-responsive drug delivery system based on chitosan-coated AuNPs was developed by Zhang et al [72]. This system too has applications for both PDT and PTT [72]. Due to the SPR absorption in the visible region and its nonlinear features, PTT using gold nanospheres can be accomplished using pulsed or continuous wave lasers in the near-infrared (NIR) and visible (Vis) wavelength spectrums [73–75].
3.1.2. Silica NPs (SNPs)
Different SNP varieties, including organically modified silica, mesoporous silica NPs (MSNPs), and Stöber SNPs, have recently been investigated as PDT vectors. This is because SNPs tend to be chemically inactive, have a porous structure that does not grow or change when the pH changes, and have a clear matrix that lets light in and out. They are very useful and can be made using a wide range of ingredients and easy-to-use synthesis methods. Changes can be made to the NPs' density, ability to spread out, size, and shape. Their surfaces can also be changed by several functional chemicals, polymers like PEG, or biomolecules that target cancer cells in particular. In an investigation, Cornell prime dots (C' dots), which are extremely tiny PEGylated fluorescent core-shell SNPs, were employed to provide a potential targeted platform for PDT usage in oncology [76]. MSNPs are better for delivering drugs because they have a large surface area and a high pore volume that can be reached [77]. Spherical and rod-shaped MSNPs were created to be used as third-generation PSs in a trial on the bladder cancer model using UM-UC-3 and HT-1376 cell lines. Cells were exposed to white light at 8.4 mW cm−2 for 40 min [78].
To target cancer cells precisely, MSN was functionalized by a biomolecule that can bind to cancer cells. The absorption of the NPs by receptors would be enhanced if the particles were functionalized to target certain receptors that are overexpressed on the surface of cancer cells in many malignancies. Based on this idea a unique MSN was made where the water-soluble PS and cancer cell-targeting mannose covalently attached to the mesoporous silica matrix for PDT application in MDA-MB 231 breast cancer cells. Results showed that without irradiation MSN conjugated with mannose and PS had only 19% cytotoxicity but when cells were irradiated under 630–680 nm NIR exposure at 6 mW cm−2 for 40 min 99% cell death occurred [79]. On the other hand, tests on nude tumor-bearing mice were used to assess in vivo biodistribution of protoporphyrin IX (PpIX)-silica NPs loaded with the DID (dioctadecyl tetramethyl indodicarbocyanine chloro benzene) tracer [80]. Up to 24 h after the NPs' tail vein injection, photographs of the biodistribution were taken. The three cancer models under research (HCT 116, A 549, and glioblastoma) showed significant tumor uptake of PpIX-silica NPs with good phototoxicity under 630 nm NIR irradiation at 4 mWcm−2, but the peak accumulations occurred at different times: 2 h for glioblastoma models, 16 h for A549, and 20 h for HCT 116 models. Other types of SNPs have been used to encase PS in PDT [80]. In another report, silica nanospheres with a 130 nm diameter that are hollow and porous were synthesized [81]. The NPs were created by carefully hydrolyzing N-(b-aminoethyl)-a-aminopropyl triethoxysilane under the influence of ammonia. Hypocrellin A was not surface adsorbing but rather embedded, according to fluorescence quenching experiments. It was found that the particles have high thermal and light stability. These SNPs were efficiently absorbed by HeLa cancer cells and had higher PDT efficacy than hypocretin A.
3.1.3. Magnetic NPs
Out of all nanomaterials and NPs, superparamagnetic iron oxide NPs (SPIONS) are the ones that have been studied most profoundly in combination with PDT [82]. In a study, iron oxide (Fe3O4) NPs, or MNPs, were conjugated with Zn phthalocyanine derivatives. At first folic acid (FA) and amine-functionalized magnetic NPs were covalently linked to Complexes 1 (Zn mono cinnamic acid phthalocyanine) and 2 (Zn mono carboxyphenoxy phthalocyanine), respectively [83]. Next, they were evaluated in the human breast carcinoma cell line MCF7 for enhanced PDT at a fixed irradiation dosimetry of 170 J cm−2 at 680 nm. These complexes exert significant singlet oxygen generation and better bioavailability and toxicity on MCF7 cells [83]. Huo et al synthesized iron oxide (Fe3O4) NPs in the presence of triphenylphosphine (TPP)-grafted dextran. Here Fe2+/Fe3+, ions have a magnetic resonance imaging (MRI) effect [84]. Next, the PSs protoporphyrin IX (PpIX) and glutathione (GSH)-responsive mPEG-ss-COOH are grafted on Fe3O4@Dex-TPP NPs to form Fe3O4@Dex/TPP/PpIX/ss-mPEG NPs [84]. After internalization, these nano platforms increase the oxygen concentration in tumor cells by the Fenton reaction and produce ROS around the mitochondrial surface under illumination with a 637 nm laser which leads to disruption of mitochondrial membrane permeability. Therefore, this nano platform can serve as a Fenton reaction-assisted PDT for tumor therapeutic efficacy [83, 84]. In another study, a magnetic core and silica layer were combined to create hybrid nanomaterials. A single or double silica coating was applied for the synthesis of SPIONs. Following its entrapment in the silica layer, the PS molecule methyl blue decomposed on the surface of SPIONs. Upon exposure to light of a visible wavelength (λ = 532 nm or λ = 633 nm), this hybrid nanomaterial successfully generates singlet oxygen and highlights the potential use of MNPs in PDT [85]. The use of ferroptosis inducers in ferroptosis therapy to cause lethal lipid peroxidation and kill tumor cells is an intriguing option for the administration of chemotherapy for cancer. Chen et al created a NP-driven system where ferric oxide (Fe3O4) and chlorin E6 (Ce6) are combined in polylactic-co-glycolic acid to create a potent ferroptosis-photodynamic anticancer treatment [86]. The Fe3O4-PLGA-Ce6 NP is capable of breaking down in the acidic TME, releasing Ce6, ferrous (Fe+2), and ferric ions (Fe+3). The Fenton reaction may be carried out by extracellular hydrogen peroxide (H2O2) and release ferrous and ferric ions to create hydroxyl radicals (OH‧) and induce ferroptosis in cancer cells [87]. When exposed to laser light under 660 nm, 0.5 W cm−2 for 3 min, the discharge of Ce6 may promote the production and accumulation of ROS to provide PDT that can improve ferroptosis in 4T1 breast cancer cells [87]. Additionally, this Fe3O4-PLGA-Ce6 nanosystem has good MRI characteristics, extremely effective anticancer activity, and high in vivo biocompatibility [87].
3.2. Semiconductor NPs
In the present, semiconductor NPs are also used as PDT PSs. The gap between the valence and conduction bands should be filled by stimulating light energy. The energy of excitation can be transferred to an organic PS or molecules of oxygen in their ground state using a fluorescence resonance energy transfer mechanism.
3.2.1. Titanium dioxide NPs
Titanium dioxide (TiO2) is an oxide of titanium that is found in nature. It is also called titania. TiO2 is used in many different ways, such as photodegradation, photocatalysis, cleaning up the environment, dividing water for hydrogen fuel, reducing CO2, making surfaces that clean themselves, electrochromic gadgets, sensors, and cheap solar cells. TiO2 has lately been thought of as a possible photosensitizing compound for PDT due to its low toxicity, biochemical inertness, notable biocompatibility, and unusual photocatalytic properties [88]. Photoinduced hole-electron pairs are made when UV radiation with higher energy than the band gap of TiO2 excites electrons in TiO2's valence band. The strong reduction and oxidation characteristics of these photoinduced electrons and holes allow them to interrelate with nearby oxygen and water molecules to produce ROS [89]. Based on these properties, TiO2 NPs were utilized for PDT of cancers. The survival of human cervical cancer cells (HeLa) was reduced by TiO2 NPs upon solar and ultraviolet (UV) irradiation [89]. When these TiO2 nanocrystals were doped with metal (cobalt) and non-metal (nitrogen) the photoactivation of doped-TiO2 NPs was significantly enhanced in the Vis/NIR region [89]. However, these nanocrystals were not significantly cytotoxic compared to PEGylated undoped-TiO2. Therefore, this study showed that water-soluble PEGylated TiO2 NPs may be a good candidate for the PDT of cervical cancer cells [89]. Afterward, multiple cell lines of human carcinoma, including monocytic leukemia cells (U937), colon carcinoma cells (Ls-174-t), bladder cancer cells (T24), adenocarcinoma cells (SPC-A1), glioma cells (U87), and breast carcinoma cells (MDA-MB-468, MCF-7), were investigated to discover the accurate mechanism of TiO2 NPs' UV-induced phototoxic effect [90]. TiO2 NPs have only rarely been used in vivo for PDT applications. This is because TiO2 is insoluble and forms aggregates in the physiological environment; as a result, it is easily recognized by cells and removed from circulation, which in turn reduces its accumulation in the tumor.
3.2.2. Zinc oxide NPs
ZnO has a similar photocatalytic activity and band gap energy (3.2 eV) as TiO2. It has been established that ZnO quantum dots, which have an average diameter of 11.6 nm, produce singlet oxygen as well as other forms of ROS when exposed to blue (400–500 nm) light [91]. There was no triplet signal in the ZnO samples that had not been exposed to radiation. Considering application in combination photothermal theory (PTT) as well as PDT for cancer, Vasuki and Manimekalai investigated the anticancer effect of ternary modified ZnO nanocomposites with NIR absorbance on the MCF-7 breast carcinoma cell line [92]. The nanocomposites showed significant cytotoxicity against cancer cells, and therefore, they can be used for combined cancer therapy [92]. In another study, it was shown that ZnO nanorods (NRs) can be used as a PS and biomarker for PDT on cervical cancer cell lines. This is because ZnO NRs are a source of NIR light for deep tissue penetration and have more surface plasmon resonance light absorption than ZnO NPs [93]. Through the use of laser scanning confocal microscopy and fluorescence spectroscopy with excitation at 488 and 514 nm wavelengths, it was possible to analyze the fluorescence and light activation of ZnO NRs in cancer cells. The fluorescence of the ZnO NRs was conjugated with the well-known PDT PS 5-ALA, and the results demonstrated that ZnO NRs are potent tumor necrosis candidates under the right light activation and are a good candidate for PDT of malignancies [93]. According to studies, Ultraviolet A radiation (20 mW cm2 for 15 min) combined with ZnO NP doses of 0.2 and 2 g ml−1 can effectively destroy cancer cells by causing late apoptosis and necrosis. In this aspect, Hariharan et al synthesized PEGylated ZnO NPs conjugated with doxorubicin (DOX), which generates ROS under UV irradiation for potential anti-tumor activity [94]. It has also been reported that ROS are produced when light energy is absorbed by PEG-functionalized Ag-doped semiconductor NPs of ZnO and transferred to water and molecular oxygen molecules in the biological environment [95, 96].
3.3. Carbon nanomaterials
There are three different types of carbon nanomaterials, such as zero-dimensional (0D)–structure fullerene, one-dimensional (1D) carbon nanotubes (CNTs), and two-dimensional (2D) graphene. Carbon nanomaterials are widely used in electronic and electrical fields, biosensors, medical treatments, environmental applications, etc. Due to their unique structure and large surface area, carbon nanomaterials, especially CNTs and graphene, are widely used in drug delivery for cancer therapy [97].
3.3.1. Carbon nanotubes (CNTs)
Graphene sheets roll up CNTs into hollow cylindrical structures with both ends open. The carbon atoms are exclusively organized in rings similar to those found in benzene. The armchair, zigzag, and chiral structural representations of CNT include allotropic forms of both sp2 planar and sp3 cubic [98]. CNTs are classified as single-walled CNTs (SWCNTs), which have an inner diameter of 1–3 nm and an outer diameter of 2–100 nm, and multi-walled CNTs (MWCNTs), which have an inner diameter of 1–3 nm and an outer diameter ranging from 0.2 to several micrometers, respectively, based on the layer formation [97]. CNTs play a special function as nanocarriers for medicines, polymers, PSs, and particular ligands that target siRNA and DNA [97]. SWCNTs penetrate the cells directly, whereas MWCNTs enter through the endocytosis pathway. Based on this property, CNTs are widely used in cancer therapy as novel drug carriers. To treat cancer effectively and specifically, researchers are now concentrating on combination therapies using CNTs and PDT. To improve solubility and bioavailability and to target only cancer cells, PSs are combined with CNTs. For efficient PDT of colon cancer cells, Sundaram and Abrahamse created SWCNTs combined with hyaluronic acid (HA) and chlorin e6 (Ce6) [98]. This nanocomposite induces morphological changes in colon cancer cells using PDT at a fluence of 5 J cm−2 and 10 J cm−2 after 24 h followed by LDH cytotoxicity and cell death [98].
3.3.2. Graphene, graphene oxide (GO), and reduced graphene oxide (rGO) NPs
A 2D network of carbon atoms with sp2 hybridization makes up graphene. Graphene undergoes an oxidation-reduction process to produce rGO and GO. The finding that graphene can be used as PSs in PDT and effectively kill cancer cells has been demonstrated in several studies [99, 100]. Considering its capacity to absorb light in the NIR range, graphene has been the subject of extensive in vivo and in vitro studies into its potential use in cancer imaging and phototherapy [101, 102]. Hosseinzadeh et al developed a nanocomposite for PDT using GO and MB and evaluated its cytotoxicity on the triple-negative breast cancer cell line MDA-MB-231 by MTT assay [103]. In dark conditions cell viability was reduced by up to 60% for the PS using a concentration of 20 µg ml−1; however, under irradiation with a red LED light of 630 nm for 30 min, a reduction of up to 80% was obtained using the same dose of PS [103]. This is because, in the presence of red LED light, MB-GO nanocomposites could penetrate more inside the tumor cells and exert toxicity. rGO NPs have also been used in PDT for cancer in the last few years. To test the photodynamic activity in the human breast cancer cell line MCF7, Vinothini and coworkers (2020) created an rGO-based nanocomposite with magnetic NPs (Fe3O4), camptothecin, 4-hydroxycoumarin, and allylamine [104]. The rGO hybrid exerts cytotoxicity by producing ROS against MCF7 cells under 365 nm of laser irradiation of 20 mW cm−2 for 3 min [104].
3.3.3. Fullerenes
Fullerenes (also known as bucky balls) are closed convex polyhedra composed of an even number -of carbon nanostructures with a high triplet yield, and a high molar absorption coefficient and are made of sp2 hybridized carbon atoms. Fullerenes have multiple types but the most renowned forms are C60, C70, etc because of their important uses. Fullerenes are particularly effective at creating photoexcited singlet oxygen (1O2) in organic solvents or hydrophobic environments, but in an aqueous environment, they convert to type 1 photochemistry and produce HO‧ and superoxide anions [105]. Although pristine C60 fullerenes are insoluble in water, the cage can be easily functionalized with polar groups such as carboxylic acids and quaternary amino groups to increase solubility and biological compatibility [105]. Various pristine C60 fullerenes as well as their functionalized derivatives, have been extensively used as PSs for in vitro cancer cell killing [106]. Fullerene C70 is also used in PDT of cancers due to its extended π system [107]. Guan and his team created fullerene C70 nanovesicles (abbreviated FCNVs) using C70-oligo ethylene glycol-Ce6 and comprised of both hydrophilic and hydrophobic components [46]. These constructs efficiently absorb light in the NIR region within 660 nm (20 mW cm−2) and exert strong anticancer activity in the lung carcinoma cell line A549 [46]. Fullerene C60 dyads are also reported for PDT in cancers. In one study four novel glucose-BODIPY-fullerene dyads with styryl units were created. The organic detergent Tween 80 helps to produce nanomicelles, which measure 14–17 nm [108]. When exposed to UV light, glucose-BODIPY-fullerene nanomicelles (14–17 nm) effectively produce singlet oxygen and ROS [108]. K562 human chronic myelogenous leukemia suspension cells were used to examine the molecules' in vitro anti-cancer efficacy under the influence of light and the absence of light for PDT [108].
Porphyrin-fullerene dyads have gained increasing interest due to their interesting optoelectronic properties and their potential applications in PDT. These dyads are created by joining organic molecules that donate electrons, like porphyrins, with molecules that accept them, like fullerene derivatives [109]. The efficient intramolecular photoinduced charge separation that the donor-acceptor dyads can undergo has sparked extensive research on the practical use of these materials. Additionally, it has been demonstrated that fullerene functions as a quencher, minimizing the photobleaching of the porphyrin component during PDT. A PS's therapeutic efficacy may be diminished due to photobleaching, which is the irreversible destruction of PSs upon exposure to light. In overextended PDT treatment sessions, the fullerene aids in preserving the stability and activity of the PS by quenching the excited states of the porphyrin. In 2021, Vallecorsa et al assessed the in vitro photoactivity of a porphyrin coupled to a fullerene and four meso-substituted porphyrins. The results suggest that the porphyrin-fullerene dyad TCP-C 604 has potential in PDT application as compared to fullerene dyad alone under 600 nm NIR radiation with a fluence rate between 10 and 220 mJ cm−2 and the power density 0.5 mW cm−2 [110] Although porphyrin-fullerene dyads show great promise in anticancer applications, it is important to note that further study is required to improve their characteristics and guarantee their safety and efficacy in clinical settings.
3.4. Biodegradable NPs
Biodegradable NPs are mainly polymeric NPs. Polymeric NPs are naturally occurring, exhibit greater photodynamic efficacy for tumor killing, and have reduced photosensitivity [111]. Polymeric NP encapsulation of PSs can significantly improve their solubility and dispersibility in a hydrated environment, enhancing their pharmacokinetic properties. Additional paybacks of utilizing biodegradable NPs made of polymers include their capacity to carry a respectable payload of different agents to the tumor while protecting it from early blood leakage and maintaining photoactive agents' and fluorophores' photostability. These agents include therapeutic PS and diagnostic PS. Several types of polymeric NPs have been used for PDT in cancers. Table 3 represents different polymeric NPs and PS in PDT of cancers.
Table 3. List of different types of polymeric nanoparticles and photosensitizers for photodynamic therapy in cancers.
| Polymeric nanoparticles | Photosensitizers | Cancers | Light wavelength/intensity | Reference |
|---|---|---|---|---|
| CNPs | Zinc Phthalocyanine | Cutaneous squamous cell carcinoma | 660 nm/5 J cm−2 | [112] |
| Protoporphyrin Ix | Adenocarcinoma | 635 nm/0.2 W cm−2 | [113] | |
| HSA NPs | Indocyanine green | Oral squamous cell carcinoma (OSCC) | 808 nm/2 W cm−2 | [114] |
| Chlorin e6 | Bladder cancer | 660 nm/0.15 W cm−2 | [115] | |
| Gelatin NPs | Zinc phthalocyanine (ZnPc) | Mouse macrophage carcinoma cell line (J774 A-1) | 660 nm/0.2 W cm−2 | [116] |
| Chlorin E6 | Mouse mammary tumor model | 650 nm/580 mW cm−2 | [117] | |
| PAA NPs | Methylene blue | Prostate cancer | 630 nm | [118] |
| Liposomal NPs | HPPH | Triple negative breast cancer | 660 nm/50 mW cm−2 | [119] |
| 2,2-bis(hydroxymethyl)propionic acid (Bis-MPA) hyperbranched PEG-OH dendrimers | Pyropheophorbide a | Mouse mammary tumor cells (4T1) | 660 nm/1 J cm−2 | [120] |
| PAMAM & PEG dendrimers | Chlorin E6 & indo cyanine green | Mouse mammary tumor cells (4T1) | 660 nm/0.1 W cm−2 (for PDT) | [121] |
3.4.1. Chitosan
Chitosan is a polymer. It is obtained from chitin, which is usually found in crab and insect shells. It is made up of N-acetyl-glucosamine and glucosamine repeating units. Chitosan has a positive charge and is unable to dissolve at pH 7, but it dissolves at an acidic pH [122]. The amphiphilic nature of chitosan has promoted them to serve as carriers for hydrophobic anticancer therapeutics [123]. For PDT, the PS was either confined inside the inner core of self-constructed NPs of chitosan or it formed a covalent or ionic connection with chitosan, which was then assembled into a NP. Chitosan NPs (CNPs) can encapsulate protoporphyrin IX (PpIX), a PS, and vitamin B9 (PpIX-B9) and transport it to tumor cell masses for effective PDT [124]. In a different investigation, ICG-loaded hydrophilic sulfhydryl NPs of chitosan (SA-CS-NAC@ICG NPs) were developed by Yang et al using a self-assembly and self-crosslinking method for PDT [125]. Traditional PS carriers have drawbacks due to their poor chemical stability, inadequate loading, and single-responsive PS release. These drawbacks are resolved by these unique pH/GSH multi-responsive chitosan NPs. ROS are reduced by the SA-CS-NAC@ICG NPs when exposed to 808 nm laser light. In vitro cell experiments verified that these novel NPs had a great capacity for cellular absorption, low toxicity, and effective cancer inhibition [125]. Chitosan NPs with ALA derivatives, such as prodrugs 5-ALA and 8-ALA, have the synergistic effect of combining conventional PDT with electrochemotherapy for the treatment of melanoma cancer [126]. In a different study, a nano-code delivery of chitosan/tripolyphosphate (CS-TPP) was made by combining a PS 5-ALA and methylenetetrahydrofolate dehydrogenase 1-like (MTHFD1L) shRNA for PDT. By triggering apoptosis and producing ROS in vitro under 635 nm, 10 J cm−2, this CS-TPP-(shMTHFD1L-ALA)-PDT demonstrated potent anticancer efficacy in oral squamous cell carcinoma (OSCC) [127].
3.4.2. Albumin
Human serum albumin (HSA) is an acidic, positively charged plasma protein with a wide variety of roles. HSA is a globular, amphoteric protein that maintains its structure between pH 4 and 9 [128]. NPs created using HSA have recently attracted attention as prospective theranostic drug carriers, especially for the administration of lipophilic drugs. Hydrophobic medicines can be transported by HSA-based NPs without the use of potentially hazardous solvents [128]. Additionally, HSA-based NPs can target the tumor by endogenous albumin pathways with massive doses of chemotherapeutic medications and can avoid the problem of the accrual of drug NPs elsewhere in the body. Abraxane® (albumin-bound paclitaxel NPs) is another example of a medication that utilizes HSANP-binding technology. In a medical facility, this technique is employed to provide paclitaxel for the treatment of pancreatic, breast, and pulmonary malignancies [128]. For the combined phototherapy and chemotherapy of breast cancer, flexible hollow human serum albumin NPs (HHSA) were produced and coupled with two distinct DOX therapeutics and the PS chlorin e6 (ce6) [129]. In in vitro conditions, it reduces the survival of 4T1 breast cancer cells without irradiation but it has remarkably higher toxicity under 660 nm irradiation. Additionally, these HHSADOX-ce6 NPs lower tumor metastasis in vivo [129]. ICG, a NIR fluorescent dye, has demonstrated significant possibilities in cancer PDT and PTT [130]. However this dye has some limitations that make it difficult to use effectively in PDT/PTT, such as the fact that it is insoluble in water, has a brief half-life, and has non-targeting accumulations. This is why Liu et al developed PEGylated-HSA-ICG-TAT to penetrate the nucleus of cancerous cells and get around ICG's drawbacks in the treatment of tumors [130]. They demonstrated that compared to free ICG, these ICG-loaded NPs had a greater cellular absorption rate and a stronger PDT/PTT impact. Moreover, these ICG-loaded NPs were easily metabolized in normal mice with no toxicity but in tumor-bearing mice, these NPs showed a significant reduction in tumor growth [130]. A similar NIR dye, IR780, and HSA NPs (HSA@IR780@DTX), which are loaded with docetaxel (DTX), were created for targeted imaging and PTT/PDT together with chemotherapeutic treatment of castration-resistant prostate cancer [131]. In this study, prostate cancer cells were irradiated with a 1 W cm−2 808 nm laser for 2.5 min and produced a large amount of ROS. In another study, a tumor-targeted multifunctional theranostic medication for PTT and PDT applications was made using four therapeutically approved substances: artesunate (Arte), folic acid (FA), HSA, and ICG under a single NIR irradiation. The produced nanocomposites (FA-IHA NPs) displayed good physiological and photo-stability, excellent cellular absorption, and tumor accumulation in vitro and in vivo [132]. Additionally, when this nanocomposite (FA-IHA NPs) were irradiated under 808 nm NIR (1 W cm−2) Arte was released in the tumor cell and showed a chemotherapeutic effect [132]. C086 is a derivative of Curcumin and it is an inhibitor of heat shock protein 90 (HSP90). It has stronger antitumor activity than Curcumin. Based on this property He et al developed a C086-loaded HSA NP for PDT against cancer cells [133]. The anti-tumor PDT activity of C086@HSA NPs is applied to the human cervical cancer cell line Hela. Upon 10 min blue light exposure at 450 nm (5 mW cm−2), it was found that C086@HSA NPs caused apoptosis and cell cycle arrest at the S and G2/M phase in HeLa cells. C086@HSA NPs were also tested for in vivo anticancer activity employing bright blue light (80 mW cm−2) using PDT in a 4T1 tumor-bearing mouse model [133].
3.4.3. Gelatin
Gelatin is a proteinaceous polymer that can be partially hydrolyzed from a pig or bovine collagen. It is a water-soluble macromolecule that is frequently used as a food additive. Gelatin NPs are now applied for PS delivery [134]. For in vivo PDT, the hydrophobic PS's water solubility must be increased, and its accumulation in tumor tissue must be increased. So Son et al have conjugated choline e6 (Ce6) with gelatin NPs for PDT applications [135]. The resulting conjugates were more water-soluble for an elongated period than hydrophobic Ce6, and upon laser irradiation under 658 nm (0.3 W, 200 J), they could produce singlet oxygen and destroy tumor cells. Additionally, the conjugates displayed an extended presence in the circulatory system and an exceedingly amplified assemblage in tumor tissue in vivo. Lee et al have developed self-assembled gelatin NPs containing both pheophorbide a (Pba) and thiabendazole (TPZ) to study the synergistic effect of drugs and their efficient delivery in tumor cells in vivo [136]. Gelatin polymer chains were coupled with PEG and Pba to create amphiphilic structures for self-assembly in aquatic conditions. These NPs were then loaded with TPZ to create TPZ-Pba-NPs. After laser irradiation at 95.1 J cm−2, the efficacy of combination therapy employing TPZ-Pba-NPs was assessed in SCC7 tumor cells, and it was found that TPZ exerts toxicity in hypoxia. Additionally, in tumor-bearing mice, the in vivo therapeutic efficacy of combination therapy using TPZ-Pba-NPs was assessed [136].
3.4.4. Polyester and polyacrylamide NPs
Biopolyesters are biocompatible naturally occurring or synthetic polymers. PLA made from D- and/or L-lactic acid monomers (PLLA, PDLLA), poly(-caprolactone), poly(hydroxyalkanoates), and poly(lactic acid with glycolic acid) copolymers (PLGA) are examples of naturally occurring polymers. Poly(orthoesters), poly(-amino esters) (PbAE), and poly(-hydroxy esters) are examples of synthetic polymers. Hydrophobic PLGA or PCL NPs that trap hydrophobic PSs are examples of simple polyester-based NPs produced for PDT applications. When second-generation PSs were coupled with PLGA NPs, they demonstrated good photodynamic activity, tumor-inhibiting activity, improved 1O2 production, and increased plasma circulation duration. In one work, PLGA NPs were synthesized and conjugated with IR780 PSs for increased PDT against osteosarcoma HOS cells. These constructed NPs induce apoptosis and ferroptosis in HOS cells via excessive accumulation of ROS [137]. Similarly, in another study, Toluidine Blue was coupled as PSs in PLGA NPs (TB@PLGA), and it was proven that TB@PLGA NPs may trigger apoptosis via a photodynamic mechanism and cause widespread necrosis of tumor cells when exposed to 660 nm laser irradiation. Furthermore, it was discovered that these TB@PLGA NPs have an excellent tumor suppression effect in vivo [138]. For increased PDT of malignancies, PLGA NPs are employed for the regulated release of PS MB with a PARP inhibitor, veliparib in a very low concentration [139]. These VMB NPs showed no toxicity in the dark but when they were irradiated with visible light they showed significant toxicity against B16F10-Nex2 cells [139].
PEG is a polymer that is used to conjugate polyester NPs and micelles for drug entrapment in both hydrophobic and hydrophilic environments. PEGylated polyesters, such as PEG-PLGA, PEG-PCL, and PEG-PLA, are used to make a variety of NPs. PEGylated polyesters in nano PDT have been tried as a delivery strategy for hydrophobic PSs by several researchers [111]. Trimethylene carbonate-based monomers were used to create light-responsive polycarbonates (LrPC) and PEGylated LrPC (LrPC-PEG) before being loaded with the PSs 5, 10, 15, and 20-tetrakis(m-hydroxyphenyl)chlorin (mTHPC) [140].
Polymerization of a nanoemulsion matrix yields hydrogel-like nanostructures known as polyacrylamide (PAA) NPs. Biodegradable polyacrylamide NPs have become prevalent in cancer therapeutics such as PDT, fluorescence imaging, and cancer targeting [141]. Both biodegradable and non-biodegradable cross-linkers can be used to produce hydrophilic PAA NPs and can have their surfaces functionalized with targeted ligands. The two simplest approaches for conjugating a PS into PAA NPs are encapsulation and post-loading, and depending on the PS's water solubility, both procedures may have varying effects on PDT activity. When PAA NPs are coupled with hydrophilic PSs like MB and its derivatives, they produce more 1O2 under 808 nm laser at a power density of 1.5 W cm−2 for 2 min and increase cell death [142]. In another work amine-functionalized PAA NPs were loaded with hydrophobic PSs such as HPPH and NIR fluorescent organic dyes (NIRFDs). These NIRFDs can be excited in the first or second NIR windows of tissue optical transparency (NIR-I, ∼700–950 nm and NIR-II, ∼1000–1350 nm) but the PS HPPH cannot absorb light. The result shows that these nano-shell conjugates produced the most 1O2 and exhibited the highest phototoxicity in vitro and in vivo [143]. In an earlier study, tetrasulfonato-aluminum phthalocyanine-entrapped NPs coated with a second porphyrin-based PS and polylysine-bound tetrasulfonato-aluminum phthalocyanine (PCNP-P) were synthesized and were loaded with photodynamic PS. These two polyacrylamide-based NPs produce huge ROS in HT29 cells when irradiated with 7 J cm−2 laser light and have good therapeutic potential [144].
3.4.5. Liposomal NPs
Concentrated phospholipid vesicles known as liposomes have several bilayered membranes composed of lipids derived from either natural or artificial sources. They make ideal therapeutic carriers since they have the unique capacity to store hydrophilic medicines in their aqueous interior and substances that are hydrophobic in their inner layers [145, 146]. Different PS has been used in specific commercial liposomal formulations, including benzoporphyrin derivative (BPD)-MA, mTHPC, and zinc phthalocyanine (Zn-Pc) [147]. A PS-lipid combination called porphyrin-phospholipid leads to persistent, non-exchangeable inclusion into the liposome bilayer. Liang et al used nanoscale (20 nm) iron oxide-loaded porphyrin-grafted NPs of lipid to create a theranostic nano platform (Fe3O4@PGL NPs) [148]. These nano platforms generate ROS to kill cancer cells and transfer Fe ions into tumor cells via the Fenton reaction [148]. In a different study, DOX, an effective chemotherapeutic agent, was added to porphyrin-imbedded lipid NPs for cooperative chemo-PDT. PGL-DOX NPs established exceptional cellular absorption, chemo-photodynamic reactions under 650 nm laser (0.2 W cm−2, 10 min), and fluorescent imaging abilities in HeLa and PC3 cell lines. Porphyrin's usual ability to generate ROS with lower irradiation meaningfully blocked tumor growth in vivo once it united with the lethal potentials of the anticancer drug DOX [149]. Therefore from these studies, it can be stated that liposomal/lipid NPs can be used in PDT of cancers with suitable PSs.
3.4.6. Dendrimer NPs
Dendrimers have received a considerable amount of interest as PS vectors because of their unique architecture. It contains several functional groups on the surface that can connect various molecules or functional moieties. The size of these molecules and their lipophilicity can be modified to improve cellular absorption and tissue biodistribution. PAMAM dendrimers are the most promising and well-characterized dendrimers. PAMAM dendrimers are used in PDT for the transportation of PS in tumor tissues. They are frequently used as templates for preparing tiny NPs. To create self-assembled NPs, PAMAM dendrimers are treated with poly(ethylene glycol) cholesterol. The hydrophobic core of the NPs is enclosed with PS chlorin e6 (Ce6) and MnO2 to increase the PDT. When exposed to a 670 nm laser, the developed DPCCM NPs act like enzymes that can catalyze the oxidation of H2O2 to produce O2 and exert an enhanced PDT effect [150]. Kojima et al used PEG-attached dendrimers to create nanocapsules with PSs for use in PDT [151]. They created two PEG-attached dendrimers from poly(amidoamine) (PEG-PAMAM) and poly(propylene imine) (PEG-PPI) and employed two PSs, RB and protoporphyrin IX (PpIX) at the same time. When compared to free PpIX, the combination of PpIX with PEG-PPI demonstrated efficient cytotoxicity by producing high levels of singlet oxygen under 530 nm light using a Xe lamp (400 W m−2) and efficiently delivering this singlet oxygen to mitochondria [151]. Along with PAMAM dendrimers, other dendrimers like PPI, ALA, and anionic phosphorous dendrimers are also studied for PDT applications [152].
3.5. Nanoformulations of tetrapyrrole
To carry the tetrapyrrole-based PS for drug delivery systems, many nanocarriers have been created [153]. Bacteriochlorins are made up of tetrapyrrole, which is made up of two pyrrole and two reduced pyrrole units joined together by methine connections, with the two reduced pyrroles positioned diagonally across from one another [153]. Gomes et al investigated bacteriochlorophylls-loaded NPs synthesized by solvent evaporation. It was observed that this approach achieved 69% encapsulation [154]. It was also found that ROS generation was also enhanced and thus therapeutic efficacy [154]. Pantiushenko et al developed a novel nanomaterial composed of non-sulfur bacteriochlorophylls and their derivatives using AuNPs [155]. Gold NPs loaded with PS had a longer circulation time and better tumor absorption than free PSs due to nonspecific passive targeting. Ostroverkhov et al investigated the immobilization of a PS based on bacteriochlorin on a magnetic NP [156]. These bacteriochlorin-loaded magnetic NPs demonstrated cancer cell localization during in vitro testing, followed by photoinduced toxicity.
4. Mechanism associated with the development of drug resistance in cancer cells
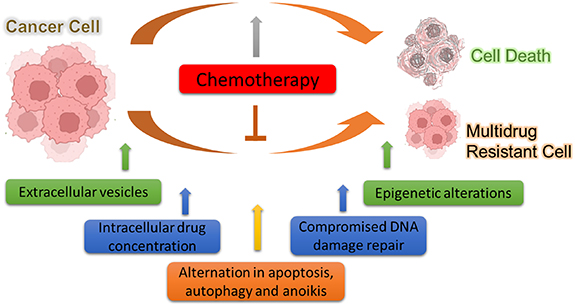
Until now, the most prevalent cancer therapies are chemotherapy, which includes one or combinational drugs as part of the conventional treatment regime. These drugs either slow down or stop cancer cells from proliferating, infiltrating, and spreading [157]. However, a significant problem in clinical oncology is the effectiveness of chemotherapy, as drugs may fail to eliminate cancer cells for several reasons. Many times, cancer patients exhibit either inherent or acquired chemoresistance i.e., failing to respond to anticancer medications [158]. As per statistical data, the development of chemo/drug resistance is responsible for more than 90% of cancer patients' deaths [159]. Because of the nearly universal prevalence of resistance to single medicines, several medications are typically employed [160]. Cancer cells can become resistant to multiple anticancer drugs depending on the mode of action of the drug and its structures. This effect is known as MDR and this makes the treatment strategy very difficult for clinicians. This challenge is particularly significant because most of the chemotherapeutic drugs only have a small therapeutic window and a narrow range of doses that will have a beneficial effect on the undesirable side effects [160]. Many individual factors could contribute to the development of MDR such as tumor-related, host-related, and host–tumor interactions as discussed below (figure 5).
Figure 5. Intrinsic mechanisms by which cancer cells develop multidrug resistance towards chemotherapy.
Download figure:
Standard image High-resolution image4.1. Factors related to host
4.1.1. Drug resistance due to genetic variation
From several clinical studies on identifying molecular markers and studying genetic patterns in these years, it has been well established that genetic variability in patients accounts for a major difference in drug response among individuals. At the same time, gene variations in cancer cells also play an important role and are responsible for drug resistance to chemotherapy. Phase I drug metabolizing enzymes inactivate various chemotherapeutic drugs by hydrolysis, reduction, and oxidation [161]. Humanoid cytochrome P450 (CYP) consists of more than 50 enzymes that play a role in inactivation via the metabolism of Phase I drugs among which CYP2D6 is one of the key enzymes. Tamoxifen which is a commonly used chemotherapeutic drug for breast cancer is metabolized by CYP2D6 to produce endoxifen and 4-hydroxytamoxifen which are therapeutically ineffective and thus lose their therapeutic activity against breast cancer [162]. From research findings, it is evident that patients with a combination of some non-functional allele could decrease the metabolism of the breast cancer chemotherapeutic drug tamoxifen which could reduce the chances of disease-free survival (DFS) or recurrence-free survival and thus lowers the therapeutic efficacy [162]. Plasma membrane transporters are essential for both the absorption and efflux of several chemotherapeutic medicines, and they are closely related to drug resistance. Most drug's intracellular concentration is controlled by the big solute carrier (SLC) family of membrane transporters, which affects drug absorption. Contrarily, drug efflux is caused by the ATP-binding cassette (ABC) superfamily, and some of its members also take part in intracellular drug accumulation. These ABC transporter proteins which are controlled by ABCC1, ABCB1, or P-glycoprotein (p-gp) play a significant role in the development of drug resistance as they determine the drug distribution and absorption by limiting the passage of drugs across the cell membranes [162]. Among different drug efflux proteins, ABCB1 has shown the highest clinical significance for the development of drug resistance from patient samples.
Many chemotherapy drugs, including platinum compounds, have cytotoxic effects that are caused by the production of various DNA adducts [163]. These adducts cause intra and inter-strand breaks, which stop DNA replication and cause cell death. However, various DNA damage repair mechanisms, such as nucleotide-excision repair and base-excision repair (BER), are available to cells to fix these defects. The clinical effectiveness of various medications, including cisplatin (CP), depends on the cancer cell's capacity to repair DNA damage caused by chemotherapy drugs. One of the members of the BER protein family 'XRCC1' can detect and repair DNA damage induced by CP [164]. As a consequence, cancer patients having upregulation of XRCC1 have shown decreased therapeutic responses towards CP and drug unresponsiveness. However, there are few cases of clinical studies where there is no connection observed between CP resistance and XRCC1 or genotypic relevance [164, 165]. In conclusion, several genetic markers have been studied for biomarkers of drug resistance in cancers, but the findings suggest that although some of them are linked with genetic variants but not all could be linked to it. Additionally, there must be other factors that have a key role in the development of drug resistance.
4.1.2. Drug interactions with another drug
Drug effectiveness or side effects are observed in case of parallel uptake of another medication which is linked to the pharmacodynamic and pharmacokinetic effects. Since cancer patients frequently take multiple medications, including anticancer medications, supportive care medications (such as antibiotics, anxiety relievers, gastrointestinal acid-reducing agents, and cholesterol-lowering statins), and other medications, they are particularly vulnerable to show these drugs non-responsiveness due to their inactivation by other [166]. Multiple studies have shown that cancer patients taking intravenous or oral administration of chemotherapy have shown drug inactivation due to another drug which is also a category of drug resistance or unresponsiveness observed [166–168]. Proton pump inhibitors (PPIs) are the most often prescribed stomach acid-reducing medications, and it is known that many cancer patients take them to treat their gastroesophageal reflux disease and dyspepsia symptoms. Tyrosine kinase inhibitors (TKIs) have poor bioavailability and solubility when taken orally. The uptake of TKIs largely depends on the pH and it decreases as stomach pH is raised by PPIs. Thus, PPIs have a role to play in reduced uptake and efficacy of TKIs class of drugs [169]. On the other hand, uptake of the TKIs is observed to enhance when taken alongside acidic beverages.
Although the gastrointestinal tract may be a site for drug absorption, the liver is the main location for drug biotransformation. Drugs that can activate human cytochrome P450 (CYPs) through higher transcription can lower the blood concentration of the induced enzymes' drug substrates. On the other hand, CYP inhibitors reduce the activity of a prodrug that is converted into a pharmacologically active drug. Additionally, several CYP stimulants or inhibiting agents are also ABC transporter stimulants or inhibiting agents. In summary, DDIs should be crucially considered for cancer patients receiving medications as this leads to drug resistance and decreased effectiveness.
4.2. Factors related to tumors
4.2.1. Intracellular drug concentrations
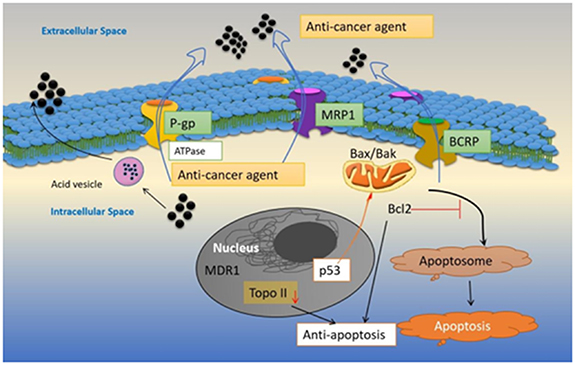
To overcome drug resistance in cancers, escalating therapeutic drug dosage, and repeated administration has been major factor in the efficacy of present cancer therapies. Anticancer medications must be proactive and have high bioavailability in the tumor tissues to be therapeutically effective. Drugs that have targets within the cell must penetrate the plasma membrane to reach tumor cells [170]. Early research on drug resistance suggested that dysfunctional plasma membrane transporter proteins cause the inability of drug accumulation inside cells and this leads to drug resistance [171]. As discussed previously, cancer cells with drug resistance have an active efflux pump that ejects out hydrophobic molecules or metabolites including the drugs through the plasma membrane which leads to decreased intracellular concentration of the drug and low therapeutic effect. One such drug efflux pump is the ABC transporter proteins [172, 173]. Cells exhibit lower intracellular drug concentration during the development of drug resistance and malignant transformation, which is primarily attained by decreased drug inflow, and increased outflow. Since the majority of common anticancer medications are weak bases with pKa between 7.4–8.4 and are hydrophobic, they permeate the cell membrane passively, but the rate of the same drugs is significantly impaired when the extracellular tumor surroundings are acidic because these medications become strongly protonated and pass the cellular membrane considerably less effectively when charged [174]. The decreased drug inflow via influx transporter proteins, which include SLCs, is one of the causes of the reduced intracellular drug levels found in chemo-resistant cancer cells. Additionally, it is widely acknowledged that ABC transporters like P-gp, MDR-associated protein 1 (MRP1), and BCRP are primarily responsible for increased drug efflux. Drug resistance has been linked to the overexpression of proteins of the ABC superfamily, including BCRP (ABCG2), MDR-associated protein 7 (MRP7/ABCC10) and P-gp, MRP1 (ABCC1) (figure 6) [175, 176]. Many neutral and cationic hydrophobic antitumor chemotherapy drugs, such as paclitaxel, DTX, etoposide, and teniposide acquire MDR when P-gp is over-expressed [177]. Patients with non-small cell lung cancer who were administered with paclitaxel had poor response rates when ABCB1 (P-gp) upregulation was present. During up-regulation of MRP1 cancer cells demonstrate MDR towards etoposide, teniposide, vinblastine, vincristine, and DOX [178]. MDR towards methotrexate, DOX, and camptothecin is caused by upregulation in the BCRP gene (ABCG2) [179]. Since the upregulation of ABC transporters causes poor drug responses and outcomes in patients with many types of cancers, so inhibiting these transporter proteins could be an effective strategy to tackle multidrug resistant cancers and improve the therapeutic response in clinics.
Figure 6. Various intracellular mechanisms are adapted by cancer cells during the development of multidrug resistance.
Download figure:
Standard image High-resolution image4.2.2. Role of extracellular vesicles (EVs) in developing drug resistance
EVs have been well studied as a route for the development of MDR in cancers. EVs are phospholipid bilayer-enclosed NPs (25–1000 nm) which are incapable of replication. In the payload of EVs, drug efflux pumps like as P-gp, MRP1, and BCRP has been discovered [180]. It is thought that some of the membranes of the EVs ejected by MDR cells may have these drug transporters oriented inverted, which would facilitate the entry of medicines into the EVs [181]. The ability of EVs to enclose chemotherapy medicines in their payload is undoubted. EVs may expel medications into the extracellular environment once they have been liberated from tumor cells thus the bioavailability of the chemotherapeutic drug in the tumor cells is reduced and the therapeutic or drug response is inhibited [181, 182]. This unique mechanism of EVs-mediated therapeutic resistance is supported by in vitro research on the absorption of chemotherapy medicines within EVs. However, there is not much clinical evidence to support the fact that EVs play a role in the development of drug resistance. Rituximab was administered to lymphoma patients in research that used fewer clinical samples [183]. Rituximab was shown to bind to EVs extracted from the patient's plasma, indicating a reduction in the drug's availability for therapeutic effect. In another clinical research, it was observed that EVs isolated from early-stage breast cancer patients of human epidermal growth factor receptor 2 (Her2) showed reduced absorption of trastuzumab compared to patients with advanced stages of the disease, indicating the role of EVs in reducing the availability of the drug and assisting in the development of chemotherapy resistance and poor outcome [184].
4.3. Disruption of apoptosis, autophagy, and anoikis as mechanisms of drug resistance
The potential of anti-cancer medications to cause cell death is what determines much of its cytotoxicity. Necrosis, autophagy, and apoptosis are the three main mechanisms of cell death, and these processes are primarily identified by the attributes of their morphology, biochemistry, and molecules [185]. Induction of autophagy-associated cell death, activation of pro-apoptotic receptors, ROS generation, DNA damage, and immune cell effector response are a few of the molecular and physiological mechanisms by which anticancer drugs can cause cell death [186]. On the other hand, cancer cells constantly adapt and evolve, giving them the capacity to avoid cell death (figure 6) [187]. The primary regulator of cell survival and the factor that determines how susceptible cancer cells are to apoptosis is the delicate balance between pro-apoptosis and anti-apoptosis proteins. In this context, drug resistance develops during the overexpression of antiapoptotic proteins such as BCL2, MCL-1, and BCL-XL in tumor cells (figure 6) [188, 189]. In patients with bladder cancer, the expression of Bcl2 is considered a marker for poor prognosis in chemotherapy treatment [190]. Patients with estrogen receptor-positive breast cancer and low levels of BAD expression had significantly worse overall survival (OS) and DFS. Resistance to chemotherapy was also linked to death receptor pathways. In metastatic ovarian cancer, upregulation of FAS and TRAIL R1-R2 was linked to a reduced response to treatment [191]. Similarly in acute myeloid leukemia (AML) upregulation of TRAIL is found to be associated with poor therapeutic response in patients [192]. According to research, individuals with colorectal cancer (CRC) who had high TRAILR1 expression had worse DFS, worse OS, and shorter time to recurrence [193]. In AML, upregulation of XIAP, a protein that inhibits apoptosis, has been linked to a poor prognosis [194]. The tumor suppressor gene with the highest rate of mutation in human cancers is the p53 protein. Since p53 induces the expression of death receptors (like DR5, FAS), and inhibits the expression of antiapoptotic proteins (like survivin) and proapoptotic proteins (like BID, BAX), p53 can also contribute to apoptosis resistance (figure 6) [192]. Additionally, most cancer drugs cause cell death by inducing reactive oxygen production which leads to DNA damage and cell death [195]. However, upregulation of GSH, a crucial component of cellular antioxidant defense systems and numerous metabolic processes acts to lower the reactive oxygen production and thus is a major contributor to drug resistance [196].
4.3.1. Role of DNA damage repair in developing drug resistance
Any cell must have an effective DNA damage repair mechanism to sustain genomic stability, retain cellular homeostasis, and stop the growth of cancer. Mutations build up when the DDR's regular control is compromised, resulting in carcinogenesis, rapidly evolving tumors, and resistance to various DNA-damaging drugs. Platinum medicines including carboplatin, CP, and oxaliplatin are among the chemotherapy drugs used in the majority of traditional cancer therapies that cause DNA damage [197]. Some drugs, like nitrogen mustard or chloroethylnitrosoureas, create DNA adducts that prevent cancer cells from actively replicating DNA [197]. Replication stress is created when DNA replication fails and DNA damage cannot be repaired, which causes cells to undergo apoptosis. Deregulated activity in DNA damage repair pathways and significantly enhanced capacity of the cell to restore DNA damage and prevent apoptosis is closely linked with the emergence of drug resistance [198]. When a clinical examination was performed for almost 62 susceptible genes in a cohort composed of 15 000 breast cancer patients and normal people, it was observed that almost 57 of the breast cancer patients were found to have lost function mutation in RAD51D which is a DNA damage repair gene [199]. On the other hand, less than 15 healthy people showed a similar mutation in RAD51D thus indicating the role of DDR genes in developing drug resistance [199].
4.3.2. Epigenetic alteration
Alterations in DNA structure are known as epigenetic modifications, which do not involve sequence changes yet are persistently passed down from cell to cell. The epigenetic controls that contribute to treatment resistance in cancer include histone, DNA methylation, microRNA, and chromatin alterations [200]. Several MDR genes, pro-apoptotic genes (APAF-1) [201], including drug transporters (ABCB1) [202], histone modifiers [203], and DNA-repair proteins (MGMT) may have their expression altered after chemotherapy due to epigenetic processes.
One significant epigenetic change seen in many malignancies is DNA methylation which is carried out by DNA methyltransferase [204]. During methylation of the genome, a methyl group is bound to the cytosine residues at the 'CpG islands' thus helping to silence genes by inhibiting gene transcription. The majority of abnormal DNA methylation in cancer is connected to genes that regulate cell proliferation and differentiation, Wingless/Integrated (WNT), Mitogen-activated protein kinase (MAPK), p53, and Vascular endothelial growth factor (VEGF) signaling, or the production of cell cycle inhibitors [205]. Hypermethylation and MDR are highly associated with many malignancies [206]. Different promoter methylation patterns in testicular cancer can be identified from non-seminomas, reflecting particular clinical traits for MDR in patients [207]. Thus, to improve the efficacy of various treatment regimens and combat MDR, therapeutic targeting of epigenetic changes emerges as a crucial and promising method [208].
5. Therapeutic approaches of NP-mediated PDT in multi-drug resistant cancers
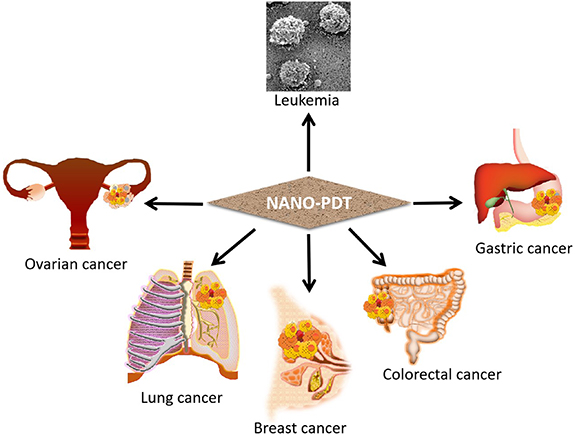
In contemporary years, several studies have investigated NP-based PDT for promoting cancer treatment and therapy. MDR, the primary process through which several malignancies acquire chemotherapeutic drug resistance, is an important cause of the ineffectiveness of several kinds of chemotherapy. It impacts those suffering from a range of cancers, including breast cancer, lung cancer, blood malignancies, oral cancer, etc [209]. The PDT technique lessens the need for extensive surgery and speeds up recovery; furthermore, it is capable of being performed without accumulating adverse effects and may be used in conjunction with conventional therapies [8]. PDT is also effective against several drug-resistant malignancies, such as nasopharyngeal carcinoma [210], breast cancer [211], uterine sarcoma [212], and gastric cancer [213] as shown in (figure 7). In the next section, the mode of action of various NP-based photodynamic therapies for MDR malignancies will be discussed.
Figure 7. Recent applications of nanomaterial-based photodynamic therapy in various types of multidrug resistant cancers.
Download figure:
Standard image High-resolution image5.1. Lung cancer
MDR and malignant cell metastasis are two of the primary reasons why cancer treatments fail. Drug-selected MDR may emerge following chemotherapeutic medication and metastasis-associated MDR may develop resistance to drugs through cellular adaptation to microenvironmental shifts during metastasis. The expanding body of research suggests that cancer patients who have not received chemotherapy treatments may develop drug resistance and that the emergence of resistance to drugs is associated with the progression of cancer metastasis. It has been observed that 76%–79% of chemotherapy-naive individuals with the third or fourth stage of non-small cell lung carcinoma were not able to become responsive to paclitaxel (Taxol) [214]. The metastasis-associated paclitaxel-resistant human lung cancer cell line H460 was demonstrated to behave similarly to circulating tumor cells in non-adherent and low-attachment culture conditions by functioning as floating cells [215]. Because of this, an in vitro model can be used to research how the development of MDR in a solid cancerous tumor is related to cancer spread. This study concluded that MDR cancer cells are caused by inappropriate medication selection and non-adherent culture conditions.
Small-sized pTHPP-PLHNPs were synthesized using a self-assembled nanoprecipitation technique. In an in vitro study, it was found that the formulated hybrid NPs exert photocytotoxicity of pTHPP by accumulating within the tumor cells, which generate superoxide anions and induce apoptosis in both A549 parental cells and its MDR variant irradiated with light of wavelength 653 nm for 12 min 45 s, at a light dose of 6 J cm−2, by using an LED lamp, with no effect on P-gp overexpression in the MDR variant [216]. Moreover, these hybrid NPs (THPP-PLHNPs/PDT) can effectively eliminate MDR-floating lung cancer cells from circulation associated with metastasis. The key factor of pTHPP-PLHNPs/PDT was the targeted drug delivery capability based on the PLGA-lipid hybrid NPs, and this capability may also be advantageous for delivering non-PDT medicines. The pTHPP-PLHNPs nano platform is a promising strategy for the PDT of MDR human lung carcinoma [216]. Another research group came up with a unique drug delivery approach (PTX/CQE) using paclitaxel (PTX), quercetin (QT), and chlorine e6 (Ce6) as a chemo-photodynamic treatment combination to stop breast cancer from spreading to the lungs and becoming resistant to multiple drugs. This nano platform can easily accumulate within tumors and shows good permeability and retention (EPR) effects. It was found that upon NIR light irradiation, PTX/CQE NPs can increase intracellular ROS production, which leads to decreased mitochondrial membrane potential followed by apoptosis [217]. In a unique plasmonic CuO/Cu2O tapered nanocube-based theranostic nanomedicine, Shanmugam et al demonstrated that the nanomedicine may function as both a NIR fluorescence (NIRF) imaging agent and a MRI agent in the biological window II (1000–1500 nm) [218]. The MDR lung cancers are successfully destroyed by these nanomedicines, which effectively create singlet oxygen (1O2) and mediate a photodynamic therapeutic effect by absorbing NIR light at 1550 nm wavelength. A human small-cell lung cancer cell line called H69AR is a drug-resistant variation of the cell line. According to Shanmugam and colleagues, plasmonic CuO/Cu2O TNCs-based nanomedicine does not cause drug resistance to develop in H69AR MDR-lung cancer cells [218].
5.2. Breast cancer
The world's deadliest disease for women is breast cancer. Breast malignancies were typically treated with chemotherapy, radiation, and surgical excision, but the main difficulty was to boost medication concentration in breast cancer cells while minimizing patient toxicity and adverse effects. About 90% of cancer patients experienced chemotherapy failure due to MDR of the medications, which hampered the advancement of chemotherapy. So, to decrease drug doses and overcome resistance to chemotherapeutic drugs, a novel, promising nanotechnology-based platform is developed. NP-mediated PDT is one of the outstanding applications to treat MDR breast cancers. Zeng et al have applied combined chemotherapy and NIR-triggered PDT to treat MDR breast tumors both in vitro and in vivo. They have synthesized folic acid (FA)-conjugated NaYF4:Yb/Tm-TiO2 nanocomposites loaded with the chemotherapeutic agent DOX [219]. This FA-targeting nanocarrier can easily be uptake by cells and can accumulate DOX within drug-sensitive MCF-7 and resistant MCF-7/ADR cells. As a result, the viability of MCF-7/ADR cells decreased by 53.5%, and the size of MCF-7/ADR tumors was inhibited up to 90.33%, compared with free DOX when a combination of chemotherapy and PDT was applied [219]. Therefore, it is evident that combined chemotherapy and PDT of FA-NPs-DOX nanocomposites under the irradiation of a 980 nm laser can defeat the MDR of breast tumors [219]. A breast cancer resistance protein (ABCG2) was discovered to be a PS called BPD. In light of this knowledge, porphyrin-lipid nanovesicles were developed as a novel method to increase PDT in three breast cancer cell lines by evading P-gp MRP1 and ABCG2-mediated efflux of BPD because researchers wanted to investigate how the P-gp and MRP1 facilitates the transport of BPD. So three breast cancer cell lines were developed as MCF-7 TX400 subline with P-gp overexpression, MCF-7 MX100 subline with ABCG2 overexpression, and MCF-7/VP subline with MRP1-overexpression which were chosen to receive 400 ng ml−1 paclitaxel, 100 nM mitoxantrone (MTX), and 4 µM etoposide respectively [220]. Interestingly it was observed that cells overexpressing P-gp and ABCG2 were involved in BPD transport only but cells overexpressing MRP1 have no role in BPD transport [220]. From this study, it was concluded that P-gp and ABCG2, but not MRP1, are capable of transporting BPD PSs with ease. Cancer cells are shielded against PDT with BPD by ABCG2 and P-gp. Lipidation promotes intracellular BPD retention for increased PDT effectiveness and conceals BPD to reduce P-gp and ABCG2-mediated efflux [220]. In another study, Rezaivala et al synthesized theranostic MNPs coated with spiky AuNPs (Au–CoFe2O4) [221]. Its star-shaped Au shell acts as a PS in PTT, and for combined chemotherapy with MTX, the chemotherapeutic agent is loaded in the core of the nanoshell [221]. These nanoshells were evaluated in the aggressive breast cancer cell line MDA-MB-231 for their cytotoxicity. It was found that cell death is mediated by the overlap of the absorption spectrum of the nanostructure and the emission spectrum of the light source [221]. Yu et al developed a strategy to combat MDR breast cancer [222]. They developed a new NIR-triggered co-release system based on the chemotherapeutic agent DOX and PS ICG on AuNCs through combined chemo, photothermal/PDT. MCF-7/ADR is an MDR human breast cancer cell. This nanosized co-release system kills MCF7/ADR cells by generating ROS and inducing apoptosis [222].
5.3. Gastric cancer
The fifth-most frequent cancer in the world is gastric cancer, also referred to as stomach cancer. It seriously endangers people's health. The primary adjuvant therapy for gastric cancer is chemotherapy followed by surgery. MDR is a major concern in postoperative chemotherapy for gastric cancer and has a detrimental impact on therapeutic outcomes. According to studies, ROS generation is an efficient way to treat tumors, and the TME is directly associated with the MDR mechanism. GRPR78 is an MDR-related protein overexpressed in stomach cancer. Through its attachment to GRP78, the extracellular absorption peptide GMBP1 is capable of targeting MDR gastric cancer cells [223]. MRI was used by Zhan et al to observe MDR gastric cancer progression in vivo. They synthesized Mn3O4 nanoplates and conjugated them with GMBP1 peptide to form Mn3O4@PEG-GMBP1 NPs [223]. Ex vivo, in vitro, and in vivo MRI experiments were performed in SGC7901/ADR tumor-bearing nude mice, as well as blocking studies, to confirm the selectivity of the resultant Mn3O4@PEG-GMBP1 NPs to MDR gastric cancer cells [223]. To determine the cytotoxic parameters of these nanoplates, histological analyses, in vitro and in vivo toxicity tests, and other experiments were conducted. Therefore, it was concluded from these findings that Mn3O4@PEG-GMBP1 NPs may act as a good MR contrast agent in vivo for monitoring MDR in gastric cancer [223]. In another study, GMBP1-modified pH/H2O2/GSH-responsive Mn3O4 multifunctional self-enhanced nano platform was developed [224]. To improve their biocompatibility within the tumor, Mn3O4 NPs were coated with polydopamine (PDA) to form Mn3O4@PDA NPs. This nano platform efficiently delivers Fenton-like Mn2+, shows GSH depletion properties, and combines chemo-photo thermal and photodynamic properties for in vivo instantaneous monitoring of MDR. This nano platform has potential for the medical treatment of MDR gastric/stomach cancer [224].
5.4. Other cancers
Barth et al constructed a nontoxic and nonaggregating nanocomposite for in vivo PDT of multi-drug resistant leukemia [225]. They synthesized calcium phosphosilicate NPs and encapsulated them with NIR-fluorescent dye ICG as PS. These nanocomposites generate ROS and induce apoptosis in leukemic cells upon 0.2 J cm−2 NIR light exposure [225].
In colorectal malignancies, MDR is extremely prevalent. High patient mortality is caused by it. One study combined the photothermal characteristics of AuNRs and the photodynamic features of the PS verteporfin to create a less invasive method to treat multi-drug resistant CRC. Gellan gum (GG) and lipoic acid are both naturally biodegradable compounds. To create a stable colloidal dispersion, AuNRs were encapsulated with lipoic acid and GG (AuNRs_LA, GG), then loaded with verteporfin (AuNRs_LA, GG/Vert). By combining PTT with PDT, this construct exhibits cytotoxicity against the human colon carcinoma cell line (HCT116) as well as against HCT116 mouse xenograft models [226].
One of the main issues with effective CRC chemotherapy is MDR. A therapeutic approach based on porphyrin/camptothecin/floxuridine triad microbubbles (PCF-MBs) has been developed by Chen et al [227]. This therapeutic composite features no early release, a large drug-carrying ability, and extremely stable combined delivery of medications. It was interestingly discovered that HT-29 cancer tumor growth rate was suppressed up to 90% when ultrasound and laser irradiation were coupled with PCF-MBs. Therefore, a PCF-MB-based therapy approach to diminish multi-drug resistance in CRC is particularly promising [227].
AOT is an anions-based surfactant utilized as an excipient for intramuscular, topical, and oral medications. Alginate is a type of polysaccharide made from marine weeds that is widely utilized in tissue engineering and drug administration. According to Khdair et al in an in vitro investigation, chemotherapy and PDT together with AOT-alginate NPs can easily accumulate inside tumor cells and kill multi-drug resistant ovarian cancer cells NCI/ADR-RES when irradiated with 50 J cm−2 dose of non-coherent light at 665 nm. In a different study, Busa et al devised a plan to combine chemotherapy and PDT to treat the MDR human uterine sarcoma cell line MES-SA/DX5 [228]. MSNPs, curcumin, and CP were used to create the nanocomposite [228]. IBN-1 NPs were first created with Cur embedded in them, and then the prodrug of CP was added to the carboxylate-modified surface (IBN-1-Cur-CP). In NP form, the Pluronic F127 triblock copolymer functions as a p-gp inhibitor. Excellent structural qualities can be found in these hybrid nanocomposites. They effectively transport Cur and CP molecules into the cytosol by endocytosis and generate large levels of cellular ROS after being irradiated with NIR radiation at 450 nm (15 mW cm−2) for 5 min [228].
To transport PDT chemicals to the appropriate locations in the mitochondria and endoplasmic reticulum, a tumor-targeting nano platform was created. Following the initial synthesis and functionalization of molybdenum disulfide nanoflakes (MoS2) using glucose-modified hyperbranched polyglycerol (hPG), organelle targeting PDT drugs were then loaded [229]. The enhanced concentration of PDT agents within MDR cells and correct subcellular localization of these new nano platform are visible. A HeLa MDR tumor mouse model experiences tumor reduction after NIR exposure due to intracellular ROS production. Apoptosis is followed by the release of cytochrome C when ROS-induced mitochondrial damage occurs [229]. Furthermore, ATP synthesis was also lowered as a result of mitochondrial damage, which led to the reversal of MDR. Therefore targeting tumors in subcellular organelles with the use of this nano platform's NIR-responsive characteristics is a promising method for MDR cancer treatment [229].
6. Conclusion
The four main types of cancer treatment, such as chemotherapy, radiation therapy, immunotherapy, and surgery, have been extensively employed to treat several kinds of tumors successfully. However, there are some issues, like the radioactive damage caused by radiotherapy, the toxic side effects, drug tolerance of chemotherapy, the ineffectiveness of immunotherapy, and the recurrence of malignancies, which have diminished the therapeutic effects of cancer treatment and decreased patient quality of life. Therefore, a novel strategy must be developed to reduce these issues. PDT, a non-invasive approach to treat tumors, is more focused on its target and less harmful to surrounding healthy tissues. The primary mechanisms of PDT are to exert toxicity directly to the cancer cells, injury to tumor blood vessels, and anti-tumor immunological responses. However, PDT has several drawbacks. Only superficial lesions can be treated with it; solid, bulky, or deeply buried tumors cannot. This is because light cannot pass into our body's deep tissues, making whole-body irradiation with the PDT approach impossible. Additionally, the organic PSs used in PDT have a complex structure and do not display a high level of selectivity for cancer cells. Enzymes can quickly break it down, and it is pretty expensive. To address the shortcomings of this traditional PS, a highly promising PDT mediated by nanomaterials has been created. Nanomedicine's ability to perform several functions, transport PS molecules to particular regions, and modify photothermal conversion enables it to get around the limitations of conventional cancer treatments. In this study, we have covered the PDT with NPs used to treat malignancies that are resistant to many drugs. Additionally, according to our study, combining PDT with nanomaterials may increase the anti-tumor effects, reduce side effects, and improve quality of life, which could result in a significant survival benefit.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare no conflict of interest.