Abstract
A low-cost and minimal-processing-step method is demonstrated to synthesize Yb2O3-ZrO2 system by laser excitation catalyzed solid-state reaction. With 980 nm CW laser irradiating, near-resonant excitation of Yb3+ ions can obviously enhance solid state reaction. As the molar content of Yb2O3 rises from 12.5% to 50%, the lattice parameter of Yb2O3-ZrO2 ceramic increases distinctly. Among them, high crystal quality of Yb2Zr2O7 and Yb0.2Zr0.8O1.9 are synthesized at the laser power of 400 W for 10 s. Two obvious Raman peaks of Yb2Zr2O7 represent increased Raman activity. X-ray photoelectron spectroscopy (XPS) and Impedance spectroscopy demonstrate the huge amount of the oxygen vacancies in Yb0.2Zr0.8O1.9. Yb2Zr2O7 shows lowest thermal conductivity among all the ceramics studied, within the range of 0.497–0.730 W mK−1 from 25 °C to 1200 °C, which indicates a promising thermal barrier material.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Thermal barrier coatings (TBCs) are widely used to improve the thermophysical performance and durability of blades in gas turbines or jet engines. The rapid developments of advanced aircraft engines have stimulated the high demands of TBCs [1, 2]. The most commonly used TBCs materials in industry is 8 wt% Y2O3-stabilized ZrO2 (YSZ), which however, suffered from sintering, corrosion, phase degradation and short-term service life in harsh environment [3–5]. The excellent thermal insulating property and durability make the rare-earth zirconate materials to be intensively investigated as promising candidates for next generation TBCs. Especially, Re2Zr2O7 (Re = rare earth) ceramic materials, with the ordered pyrochlore structure or the disordered defective fluorite structure, are important candidates, which exhibit lower thermal conductivity and excellent stability as compared with YSZ [6, 7]. Furthermore, defect engineering has been proven to be an effective route to further decrease the thermal conductivity by doping at either Re or Zr site [8]. Ponnilavan et al. have reported that titanium substitution in Gd2Zr2O7 could affect the crystal structures of Gd2Zr2O7 by Ti4+ content [9]. Although the structure of Gd2Zr2O7 demonstrates a great flexibility to accommodate a wide range of Ti4+ substitution at its lattice, the dense morphological features deteriorated according to mechanical data. Rejith et al have investigated nanocrystalline La2Zr2O7 and Y2Zr2O7 by adding 1.6 wt% of ZnO or Ce2O3 and the results show that oxygen vacancies play a key role in the structure and conductivity [10]. Mobile oxide ions and conductivity have been raised with ZnO addition whereas decreased with Ce2O3 addition. Although doping can improve the thermophysical performance of TBCs, the structural disorder could cause the phase instability [11]. Li et al have investigated the behavior of Yb2Zr2O7 ceramic in molten V2O5 and provided a better understanding on Yb2Zr2O7 ceramic in the mechanism of thermal exposure and hot corrosion [12]. Yb2Zr2O7 can be synthesized through oxide raw materials at elevated temperatures or even thermal decomposing methods, but exploring suitable synthesizing routes is necessary to improve the performances of TBCs. The rare-earth zirconate ceramics are usually synthesized by solid state reaction in furnace involving a long reaction time and high temperature. The solid-state reaction occurs commonly above 1600 °C for more than 12 h to generate the target products, leading to excessive energy consumption and inhomogeneity [8, 13, 14]. Employing the solid-state reaction at 1600 °C for 12 h, Zhou et al have synthesized novel thermal barrier coating Y4Al2O9 (YAM) ceramic with low thermal conductivity [13]. However, the micro-crystalline and grain boundaries are anomalous and heterogeneous, which is not beneficial to explore the thermophysical mechanism. An efficient synthesis technology of the thermal barrier materials is urgently needed from the perspective of application.
As for conventional methods, electron beam-physical vapor and air plasma spraying have been developed to prepare YSZ or ZrO2-based TBCs for higher efficiency. Recently, good synthetic performance and universality in materials make the laser sintering become a rapid and widely-used prototype technique. At present, laser sintering has been successfully industrialized for a pressure-less, mask-less, and scalable manufacturing route [15, 16], which provides versatile material synthesis by forming three-dimensional net structure in a single operation. The ceramics materials synthesized by laser sintering have brought various applications with vast commercial values. Considering the photo-absorption characteristic of ytterbium (Yb) element, we present a low-cost and minimal-processing-step approach to prepare series of Yb2O3-ZrO2 system by laser sintering technique.
In this work, we have manufactured Yb2O3-ZrO2 ceramics by laser excitation which activates self-propagating sintering. With a continuous-wave (CW) laser of 980 nm in wavelength irradiating on specimen, Yb3+ ions can not only act as reagent, but also promote the photon absorption and dramatically lower required laser energy. The homologous solid-solution of Yb2O3-ZrO2 ceramics are synthesized in air environment by controlling the laser power, sintering time and doping concentration of Yb2O3. The crystalline structure, element valence state, thermal conductivities and impedance spectroscopy have been investigated.
2. Experimental procedures
2.1. Sample preparation
Yb2O3 and ZrO2 (Sinopharm Chemical Reagent Co., Ltd) powders were used as raw materials to prepare compound Yb2O3-ZrO2 ceramics. In the preparation process, raw materials worked as reactants without acidizing or dissolving treatment. Appropriate amounts of these materials were precisely weighted according to molar ratios and then were mixed in ZrO2 balls milling container for 12 h, the rotating speed of the ball milling was set to 300 rpm. Then, the mixed powders were transferred into a copper vessel with inner diameter of 13 mm. The thickness of powders were 4 mm.
The laser beam was supplied by a multi-mode diode laser system (DILAS, 980 nm, CW, 1200 W), which was irradiated to the mixed powders via a quartz convex lens (f = 30 mm). The solid-state reaction was occurred in atmospheric environment. The spot size of laser beam was 12 mm in diameter and the irradiating time was set to 10 s, afterwards, clumpy resultant was cooled at room temperature naturally. Later, the as-synthesized lump was ground into powder to study its properties.
2.2. Characterizations
The crystallographic structure identification of specimens was performed by x-ray diffraction (XRD, Rigaku Ultima VI x-ray diffractometer) with Cu-Kα radiation at room temperature. Transmission electron microscopy (TEM, FEI Tecnai G20) imaging was also performed to study the evolution of the lattice parameters varying with different doping concentrations. Raman spectroscopy (Raman, Brucker RFS100/S) with Argon ion laser radiation at 514 nm was performed. X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI) was presented to study the alteration of the bonding environment of elements with a monochromatic Al-Kα source. Complex impedance spectroscopy was measured by using a high-precision LCR meter (Agilent 4990 A) which was operated at frequencies ranging from 10 Hz to 10 MHz.
The thermal diffusivities (α) of specimens were examined on TADLF-2800 using a laser-flash apparatus from 25 °C to 1200 °C with an interval of 100 °C. Before the measurement, a disk with 12.7 mm in diameter and 0.8 mm in thickness was carefully formed under the pressure of 200 MPa for 2 min. Both the front and rear surfaces of specimen were coated with a thin film of graphite for thermal absorption of laser beam energy. The density (ρ) was measured based on Archimedes method. The specific heat capacity (Cp) was calculated from heat capacity values of the constituent elements based on the Neumann-Kopp rule [17]. The thermal conductivities (λ) were given by the following equation:
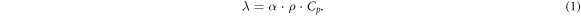
The uncertainty of thermal conductivity was estimated to be 5% with consideration of the uncertainties on the thermal diffusion coefficient (α), density (ρ), and specific heat capacity (Cp). The value of thermal conductivity was corrected by using the following equation [18]:
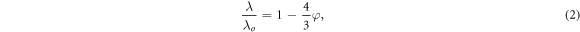
where λo is the actual thermal conductivity and φ is the fractional porosity.
3. Results and discussion
3.1. Laser excitation activated self-propagating sintering of Yb2O3-ZrO2 system
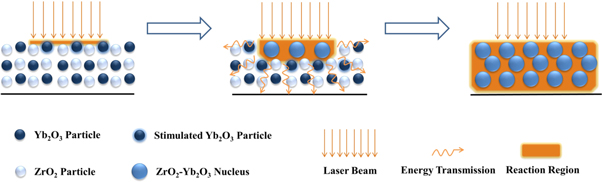
Figure 1 shows the mechanism of laser excitation activated self-propagating sintering of Yb2O3-ZrO2 system. Raw materials (Yb2O3 and ZrO2 powders) are mixed adequately and tabled on a copper vessel. A CW diode laser at 980 nm irradiated onto the surface of the raw materials. This mechanism makes good use of Yb3+ photon absorption at 976 nm. With 980 nm CW laser irradiating, solid state reaction could be enhanced obviously by near-resonant excitation of Yb3+ in self-propagating sintering process [19]. The Yb3+ ions in Yb2O3 are stimulated to excited level by absorbing laser photons and heats the raw materials simultaneously. The stimulated Yb2O3 particles reacted with ZrO2 to recombine Yb2O3-ZrO2 system nuclei. Moreover, this process enhances laser absorption and energy delivery to heat unreacted powder, leading to self-propagating sintering compounds finally. The technique takes full advantage of Yb3+ photon absorption, which significantly lowers the laser power intensity in process of laser sintering.
Figure 1. Mechanism of laser excitation activated solid-state reaction and self-propagating sintering.
Download figure:
Standard image High-resolution image3.2. Phases and structures of Yb2O3-ZrO2 system
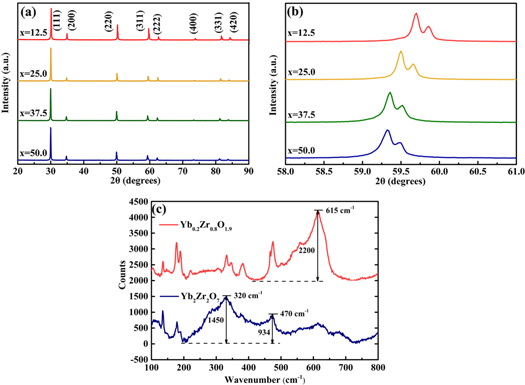
We obtained resultants of Yb2O3-ZrO2 systems synthesized by laser excitation, the laser beam power ranges from 100 to 1200 W. As the laser power increases, the clumpy resultant become more and more transparent. When the power reaches 800 W, the almost transparent crystal can be obtained. At higher laser power, however, the clumpy resultant would crack at it cools at room temperature naturally. The as-synthesized lump was ground into powder to study its properties. Figure 2(a) shows the XRD patterns of Yb2O3-ZrO2 system sintered by 12 mm diameter laser beam at 800 W for 10 s. The series of Yb2O3-ZrO2 system were fabricated with different Yb2O3 concentrations of 12.5–50 mol% in the raw materials. It can be observed that all Yb2O3-ZrO2 ceramics are single-phase structure without unrelated peaks. Almost every peak of specimens in XRD patterns agrees with standard peak of Yb0.2Zr0.8O1.9 (JCPDS file NO. 78–1309) and Yb2Zr2O7 (JCPDS file number 78–1300) when x is 12.5 and 50, respectively. Yb2O3-ZrO2 system exhibit a defected fluorite-type structure by the ionic radius ratio of r(Yb3+)/r(Zr4+) below 1.26, which is consistent with the XRD of Yb2O3 concentrations range of 12.5–50 mol% [20, 21]. These well-defined peaks of all synthesized specimens clearly indicate their good crystallinity. Figure 2(b) shows the XRD patterns of Yb2O3-ZrO2 system ceramics scanning from 58° to 61° in detail. The peak is inclined to a lower degree as increasing x mol% of Yb2O3 in Yb2O3-ZrO2 system, which means a distinct increase of lattice parameter. It is in good agreement with Vegard's rule.
Figure 2. XRD patterns of x mol%Yb2O3:ZrO2, x = 12.5, 25, 37.5, 50: (a) in the 2θ range of 20° to 90°; (b) a single peak in a 2θ range of 58° to 61°. (c) Raman spectra of Yb0.2Zr0.8O1.9 and Yb2Zr2O7.
Download figure:
Standard image High-resolution imageThe samples were further characterized by Raman spectroscopy as it is locally sensitive to lattice defects. Figure 2(c) exhibits the Raman spectra of Yb0.2Zr0.8O1.9 and Yb2Zr2O7. Interestingly, there is a distinct peak at 615 cm−1 of Yb0.2Zr0.8O1.9 which indicates the possibility of huge oxygen vacancies on the surface. Oxygen vacancies are easily formed by disturbed Zr-O bonds and Yb-O bonds for rapid temperature changes, which occurred in the process of drastic laser sintering [22]. According to factor group analysis, Ln2Zr2O7 oxides with pyrochlore structure give six Raman active modes of vibrations, whereas Ln2Zr2O7 almost cannot found Raman peak if it adopts disordered fluorite structure [8]. In principle, Yb2Zr2O7 has disordered fluorite structure. However, there are two obvious peaks observed at 320 and 470 cm−1 as shown in figure 2(c), which means increased Raman activity. The changes of Raman active modes could be attributed to crystal structure transformation from disordered fluorite structure to pyrochlore structure.
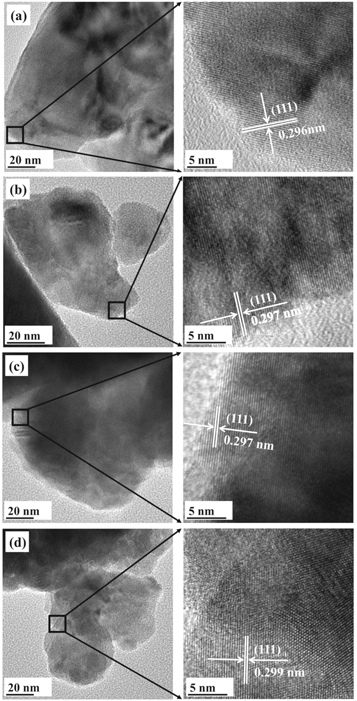
Figure 3 clearly demonstrates well-defined crystalline lattice structure of Yb2O3-ZrO2 system ceramics with marked interplanar spacing of the [111]. It can be seen that the spacing shifted slightly from 0.296 to 0.299 nm with the increase of Yb2O3 ratios which means crystal lattice parameter increases. It is in accordance with the results of XRD. Respectively, the interplanar spacings of figures 3(a) and (d) are in accord with standard spacing of Yb0.2Zr0.8O1.9 and Yb2Zr2O7. Accordingly, it can be verified that Yb2O3 and ZrO2 powders generate Yb2O3-ZrO2 ceramics effectively by laser sintering.
Figure 3. TEM images of x mol%Yb2O3:ZrO2 ceramics: (a) x = 12.5; (b) x = 25; (c) x = 37.5; (d) x = 50.0.
Download figure:
Standard image High-resolution image3.3. Synthesis conditions and properties of Yb0.2Zr0.8O1.9 and Yb2Zr2O7
Figure 4(a) represents the XRD patterns of resultants with 12.5 mol%Yb2O3 to ZrO2 powders sintered by laser beam power at 100, 200, 300, 400, 500 and 1200 W for 10 s respectively. At 100 W, there were a bit of Yb0.2Zr0.8O1.9 among those unreactive Yb2O3 and m-ZrO2 after laser sintering. More ingredients were involved in the synthetic process to synthesize Yb0.2Zr0.8O1.9 with higher laser beam power. Nearly pure Yb0.2Zr0.8O1.9 was generated when the laser power was up to 400 W. In addition, in order to investigate the influence of further high laser power on the reaction products, the laser power was increased up to 1200 W with an interval of 200 W. The XRD patterns of resultants sintered at laser power range from 400 W to 1200 W are quite similar to that at 400 W. This also indicates the excellent stabilities of synthesized Yb2O3-ZrO2 system ceramics. Figure 4(b) represents the XRD patterns of resultants with 50 mol%Yb2O3:ZrO2 powders which are sintered at 100, 200, 300, 400, 500 and 1200 W for 10 s. Similarly, a small amount of Yb2Zr2O7 were found among those unreactive Yb2O3 and m-ZrO2 after laser sintering at 100 W. As increasing laser power, Yb2Zr2O7 of increased phase purity was produced. Finally, pure Yb2Zr2O7 was generated when the laser power reached 400 W. The XRD patterns of resultants almost keep unchangeable as laser power worked at the region from 400 W to 1200 W.
Figure 4. XRD patterns of resultants sintered by laser beam at 100, 200, 300, 400, 500 and 1200 W respectively: (a) 12.5 mol%Yb2O3:ZrO2; (b) 50.0 mol%Yb2O3:ZrO2.
Download figure:
Standard image High-resolution imageFurthermore, XPS was investigated to study the bonding energy (BE) of O, Zr and Yb peaks on the surface. Figure 5 shows the high resolution XPS spectra of Yb0.2Zr0.8O1.9 and Yb2Zr2O7 solid solutions synthesized at 800 W, respectively, which is characterized by Yb 4p, Zr 3d and O 1 s profiles. The XPS survey spectra of Yb0.2Zr0.8O1.9 and Yb2Zr2O7 are shown in figure 5(a).
Figure 5. High resolution XPS spectra of Yb0.2Zr0.8O1.9 and Yb2Zr2O7: (a) Survey; (b) Yb 4p; (c) Zr 3d; (d) O 1s.
Download figure:
Standard image High-resolution imageFigure 5(b) shows the high resolution Yb 4p XPS spectra of Yb0.2Zr0.8O1.9 and Yb2Zr2O7. Both of them are resolved into two distinguishable peaks corresponding to 4p3/2 and 4p1/2, which are centered at 332.8 and 346.5 eV respectively. Similarly, as shown in figure 5(c), the 3d5/2 and 3d3/2 of Zr were observed at 182.1 and 184.5 eV in Yb0.2Zr0.8O1.9 as well as at 181.9 and 184.3 eV in Yb2Zr2O7. By contrast, the high resolution Zr 3d XPS spectrum of Yb2Zr2O7 shows a slight shift towards lower energy. The Zr4+ cation contains eight oxygen neighbors in fluorite structure cell; whereas Zr4+ is coordinated by six oxygen ions in pyrochlore structure, indicating a more stable composition [23]. The BE in synthetic Yb2Zr2O7 is lower than in Yb0.2Zr0.8O1.9, which could be ascribed to crystal structure transformation from disordered fluorite structure to pyrochlore structure. Figure 5(d) shows the high resolution XPS spectra of O 1s electron of Yb0.2Zr0.8O1.9 and Yb2Zr2O7. Interestingly, two peaks of Yb0.2Zr0.8O1.9 are observed at 529.8 and 531.0 eV corresponding to the lattice oxygen and oxygen vacancy, respectively. Similarly, two peaks center at 529.7 and 531.4 eV of Yb2Zr2O7 ceramic corresponding to two different oxygen states respectively. It can be also observed that the amount of the oxygen vacancies in Yb0.2Zr0.8O1.9 is much higher than that in Yb2Zr2O7. The relative amount of the oxygen vacancies is calculated to be 55.3% in Yb0.2Zr0.8O1.9 and 40.2% in Yb2Zr2O7, which is consistent with the huge oxygen vacancies conjecture based on Raman observation.
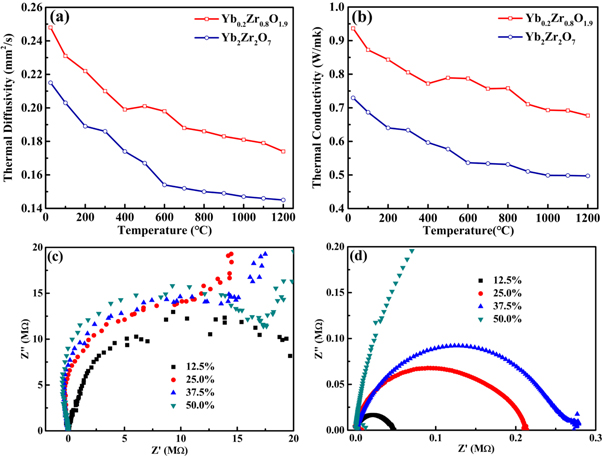
The variations in thermal diffusivity with temperature for Yb0.2Zr0.8O1.9 and Yb2Zr2O7 ceramics are plotted in figure 6(a). The values of thermal diffusivity are the average arithmetic means of three measurements. Distinctly, the thermal diffusivities of Yb0.2Zr0.8O1.9 and Yb2Zr2O7 ceramic generally decrease with the increase of temperature from 25 °C to 1200 °C. The thermal diffusivity of Yb0.2Zr0.8O1.9 ceramic is in the range of 0.174–0.248 mm2/s from 25 °C to 1200 °C, and the thermal diffusivity of Yb2Zr2O7 ceramic is in the range of 0.145–0.215 mm2/s.
Figure 6. Thermal properties of Yb0.2Zr0.8O1.9 and Yb2Zr2O7 ceramics are plotted as a function of temperature: (a) Thermal diffusivities; (b) Thermal conductivities. Complex impedance plots for x mol%Yb2O3:ZrO2, x = 12.5, 25, 37.5, and 50, measured at (c) 25 °C and (d) 500 °C, respectively.
Download figure:
Standard image High-resolution imageThe thermal conductivities of Yb0.2Zr0.8O1.9 and Yb2Zr2O7 calculated with formula (1) and (2) are plotted as a function of temperature, as shown in figure 6(b). The values in this figure have been corrected to 100% theoretical density. The thermal conductivity of Yb0.2Zr0.8O1.9 gradually decreases with the increase of temperature up to 1200 °C due to the lattice thermal conduction. The thermal conductivity of Yb0.2Zr0.8O1.9 in this study is calculated in the range of 0.677–0.936 W/mK from 25 °C to 1200 °C. Yb2Zr2O7 solid solutions exhibit reduced thermal conductivity over the entire temperature range, within the range of 0.497–0.730 W mK−1 from 25 °C to 1200 °C. The thermal conductivity of Yb2Zr2O7 synthesized by laser sintering is obviously lower than those by conventional solid-state reaction method [24–26]. The much lower thermal conductivity of Yb2Zr2O7 may be ascribed to the scattering of the phonons by oxygen vacancies [14] and the crystal structure transformation from disordered fluorite structure to pyrochlore structure [8].
Figures 6(c) and (d) show the complex impedance spectrum (Z'' versus Z', Nyquist plot) of Yb2O3:ZrO2 with different Yb2O3 contents at 25 °C and 500 °C, respectively. The semicircular arc with an intercept on the real axis corresponds to the grain boundary resistance. As shown in figure 6(c), the grain boundary resistance increases when Yb2O3 content increases from 12.5 mol% to 50 mol%. This indicates that the defects concentration (i.e., oxygen vacancies) in Yb2Zr2O7 is lower than those in Yb0.2Zr0.8O1.9. When Yb2O3 is doped in ZrO2, Yb3+ substitution of Zr4+ to maintain the electroneutrality of the lattice. At the lower doping amount of Yb2O3, the Yb2O3-ZrO2 system has more defects and lattice distortions caused by Yb intervening, which gives rise to oxygen vacancies or even interstitial ions accumulating in the grain boundaries. Thus, the resistances of grain boundaries are larger when Yb2O3 content is below 50 mol%. Once Yb increased up to the 1:1 ratio of Yb:Zr and the stable phase Yb2Zr2O7 formed, it can greatly reduce the lattice defects. Therefore, Yb2Zr2O7 further decrease oxygen vacancies due to more Yb3+ ions. However, the resistance values still maintain 106 Ω, insulating state. As shown in figure 6(c), the grain boundary resistance decreases for all the Yb2O3:ZrO2 specimens at 500 °C, which reveals increase in the dc conductivity at higher temperature. This could be ascribed to enhanced space charge polarization in ceramics accompanying with temperature rising. The resistance of grain boundary changes abruptly for the samples with different Yb2O3 content, whereas the Yb2Zr2O7 still maintains the larger resistance value. This also confirmed that Yb2Zr2O7 has good crystalline structure and there should be oxygen vacancies existing in Yb0.2Zr0.8O1.9.
4. Conclusions
Yb2O3-ZrO2 system ceramics are synthesized by laser excitation activated self-propagating sintering method. The crystal structure and micro-morphologies have been investigated according to different Yb content and laser power. As the increasing concentration of ytterbium cations in Yb2O3-ZrO2 system solid solution, the size of ceramic lattice slightly increases. Combining with the analysis of element valence, Raman vibration modes, thermal conductivities and grain boundaries resistance, the two type resultants of Yb2Zr2O7 and Yb0.2Zr0.8O1.9 demonstrate different properties and the micro-mechanism has been discussed. Oxygen vacancies have been considered as an important factor influencing the thermo-physical properties. The thermal conductivity of Yb2Zr2O7 ceramics gradually decreases with the increase of temperature up to 1200 °C, within the range of 0.497–0.730 W mK−1, which is lower than that of Yb0.2Zr0.8O1.9. The method of laser excitation can provide a promising solution for synthesizing Yb2O3-ZrO2 system ceramics in TBCs applications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (11974112, 11704127, 61875243, and 11804100).
Data availability
The raw date/processed required to reproduce these findings are available from the corresponding author upon reasonable request.