Abstract
Our research aimed to find a new material that can be an efficient heavy metal free flame retardant for plasticized poly(vinyl chloride) comparable to the conventional flame retardants. One of these extraordinary materials is Oxydtron using as an admixture for concrete. Oxydtron showed unexpected efficiency as a flame retardant agent and an excellent heat stabilizer as well. Limiting oxygen index (LOI), static heat stability, Congo-red, and differential scanning calorimetry (DSC) were carried out. The thermal tests proved that Oxydtron is suitable to improve plasticized poly(vinyl chloride) performance at high temperatures applications in terms of flame retarding and thermal stability. Therefore, the positive result obtained by the addition of Oxydtron is reducing of plasticized poly(vinyl chloride) flammability by 25.23%, and increasing its thermal stability as well.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Most of conventional flame retardants and stabilizers for plastics contain heavy metals or other ingredients e.g. bromine being potentially harmful to the environment or health. Although poly(vinyl chloride) is inherently flame retardant; plasticized poly(vinyl chloride) needs additives improving its flame retardant properties because of the easily burning plasticizers. This is crucially important in the electrical industry and in municipal buildings. Moreover, all poly(vinyl chloride) formulations must contain heat stabilizers because the limited heat stability of poly(vinyl chloride) producing hydrochloric acid during degradation which so called burning of poly(vinyl chloride). There are heavy metal free flame retardants (earth metal hydroxides) but these must be used in high percentages (40–60 pphr) is reducing the mechanical properties and wear resistance of the plastic. Polymers are ideal for use in many important applications due to their distinctive characteristics, but unfortunately there's a main disadvantage with these materials which is a low thermal resistance at elevated temperatures [1–3]. As we know, the use of flame retardants and refractory additives considered the optimal and most important method to improve the effectiveness of flame retarding and thermal resistance of polymers. These unusual additives must be easily processable with the polymers, and should not excessively reduce the performance of other properties, also an essential aspect that these additives should not cause any environmental problem in terms of recycling or disposal [4–11]. The rapid evolution of flame retardation concept (materials and applications) in recent years, makes it necessary to develop the way of thinking in this field as well; where the search for a new and unconventional materials in industry is imperative to balance environmental considerations for the safe usage of non-polluting materials (or at least with limited damage) and to maintain the best possible achievement of new materials compared to traditional materials. The aim is not only to use new engineering materials in the industry, but to be more efficient or at least maintain the same level of performance of the traditional materials currently available which can be termed metaphorically - the sustainability of properties - as with the sustainability of natural materials, otherwise there will be no benefit from the use of such materials [1, 9, 11, 12]. Poly(vinyl chloride) considered as one of the most important polymers and widely used in plastics industry; but unfortunately poly(vinyl chloride) like others polymers has low thermal resistance where this polymer begins to degrade by release of chlorine containing compounds (such as HCl) from its internal structure at high temperatures. In order to solve this problem, it is necessary to use fillers to increase the thermal resistance of the poly(vinyl chloride), which will make the structure of poly(vinyl chloride) more stable at elevated temperatures, and also will raise the resistance against the release of chlorine containing compounds. As mentioned earlier, these additives usually include traditional materials known for their frequent circulation in the industry as flame retardants and refractory materials which have one characteristic in which they can perform their functions, such as their ability to retarding flames, increase heat resistance, or improve mechanical, chemical properties ...etc [13–18]. However, we believe that this will not be sufficient for the future of polymers industry according to the indicators of the development of this industry now; since the material must have more than one advantage to perform its function within the polymer structure, because even with the addition of flame retardant or thermal resistance material, the polymer will be affected after removal of the heat source also not only during exposure and will not return to its first state, and even the substrates of the material not exposed to heat will be affected and change their properties. Therefore, these additives should provide thermal stability for overall structure of the polymer.
Oxydtron is one of the non-traditional polymer additive materials that we used in this study. According to its technical data sheet Oxydtron is a nanocement mineral admixture which is added to the concrete mixture to increase the water leakage-, freezing-, heat- and acid /alkali resistance [19]. Those who hear about Oxydtron for the first time assume it to contain nanoparticles as indicated by the term 'nanocement' but this is not true because Oxydtron is a micro particles powder and just the particle's coating layer is consisting modifier materials of nano size (10 to 100 nm); which gives this material special characteristics in addition to its low cost [20, 21]. For further clarification, many admixtures are added to the concrete mixture along with basic components (cement, water, aggregates) which can be added during the mixing process of the basic components in order to improve the properties of the concrete mixture. The improved properties vary according to the type of the admixture, where there are admixtures that improve the workability of the concrete and its durability, or accelerate or slow the cohesion of concrete, another admixture type works on increasing the wear resistance of the concrete surface, and there are admixtures minimizing the amount of water needed,.....etc [22, 23]. Oxydtron contains high-temperature oxides and carbonates that make it one of the alternative options for conventional flame retardants used today [20].
2. Experimental part
2.1. Materials
The percentages of following primary materials used in this study illustrated in table 1. The description of these materials as follows:
- 1.Suspension type poly(vinyl chloride) S-5070, K.value 70 (under trademark Ongrovil®) produced and supplied by BorsodChemZrt., Hungary.
- 2.Plasticizer DOP (Di-Octyl Phthalate) supplied by DEZA, a. s. CO., Valašské Meziříčí, Czech Republic.
- 3.Calcium-Zinc based stabilizer (under trademark Newstab-50) which supplied by Betaquímica CO., Barcelona, Spain.
- 4.Wax-E (under trademark Licowax®E) supplied by Clariant International Ltd, Muttenz Switzerland.
- 5.Oxydtron (type A) supplied by Bioekotech Hungary Kft.
Table 1. Materials used and their percentages.
| Component type | Trade name | Quantity | Unit |
|---|---|---|---|
| poly(vinyl chloride) | S-5070 | 100 | pphr |
| plasticizer | DOP | 70 | pphr |
| Solid Ca–Zn primary stabilizer | Newstab-50 | 1.5 | pphr |
| External lubricant | Wax E | 0.3 | pphr |
| Flame retardant additive | Oxydtron | 1, 3 and 5 | wt% |
2.2. Mixing procedure
All the raw materials illustrated in table1 (except Oxydtron) had been mixed together using a mechanical mixer type Mischtechnik MTI 10 to formation poly(vinyl chloride) basic formulation mixture. After the mixing process starts, the temperature gradually increases from room temperature to 150 °C due to shearing between the materials particles by speed increasing from 600 rpm to 2700 rpm. The mixture stays at 150 °C and 2700 rpm for 15 min to allow the plasticizer to fully penetrate into particles of poly(vinyl chloride) in order to obtain a homogenized structure and optimum characteristics after fabricating. The mixing speed is then reduced to 600 rpm leading also to a temperature drop to 45 °C and the mixture remains at this temperature and speed until the mixing process is completed. Mixing process takes about 40 min to complete. After the main mixing process, the Oxydtron is added in weight fraction (1, 3, and 5 wt%) to the poly(vinyl chloride) basic formulation mixture and mixed in a small electric mixer until the Oxydtron particles are evenly distributed and homogenized into sample powder mixtures.
2.3. Sample preparation
All samples have been prepared according to ISO standards and all preparation steps have been completed at BorsodChem Zrt., Hungary as follows:
- 1.Static heat stability samples: these samples were prepared by using laboratory roll mill type SCHWABENTHAN, with 170 °C temperature, time 5 min, rolling speeds 21 rpm (front roller) and 24 rpm (back roller). The samples produced according to EN ISO 305:1990 standard as sheets with 1 mm thickness [24].
- 2.Limiting oxygen index samples: L.O.I samples were fabricated according to ISO 4589–2 standard [25] by using an extrusion machine type GÖTTFERT with extruder rod diameter equal to Ø 20 at 170 °C temperature and 60 rpm speed at uniform conditions (pressure, temperature, and compression).
- 3.Congo-red test: the congo-red test samples were prepared according to ISO 182–1:1990(E) standard [26] by using extrusion machine type SCHLOEMANN as disk shape particles with 3 mm diameter and 2 mm thickness at uniform conditions (pressure, temperature, and compression).
- 4.Differential scanning calorimetry test: by following the ISO 11357–2 standard [27] a 10 milligrams weight sample from poly(vinyl chloride) and Oxydtron additives were fabricated.
2.4. Tests
The tests were completed at BorsodChem Zrt. (Static heat stability, L.O.I, Congo-red, DSC, FTIR, and Color change) and University of Miskolc (SEM, Oxides analysis, and Particle size distribution analysis). The devices used are periodically calibrated by the company and the university in order to provide a maximum test accurate.
- 1.Static heat stability test has been completed by using a Stabilemetr PVC 03 device (heat stability testing oven method) found at BorsodChem Zrt., Hungary; Laboratory of Vinyl Technology. This test finished within 2 h at 190 °C, where the samples move out of the device with velocity 2 mm min−1.
- 2.Limiting oxygen index test: L.O.I test was done by using a Stanton Redcroft FTA flammability unit at BorsodChem Zrt., Hungary; Laboratory of Vinyl Technology. In L.O.I test; the poly(vinyl chloride) sample is placed into a glass cylinder opened from the top and connected from the bottom by the device's air tunnel. When the test begins, the device injects a mixture of oxygen and nitrogen gas into glass cylinder containing the poly(vinyl chloride) sample for developing and speeding up the ignition process.
- 3.Congo-red test: was done by using a Julabo MC-12 circulator oil bath at 200 °C. Congo-red test device found also at BorsodChem Zrt., Hungary; Laboratory of Vinyl Technology. In congo-red test, the poly(vinyl chloride) sample in particle form is placed into glass test tube with a quantity reached to 50 mm. Then the glass tube is placed inside the device; and the poly(vinyl chloride) sample is completely immersed in the oil bath (50 mm). The test begins at a uniform and stable temperature (200 °C) as mentioned, until the color of the paper changes due to increased poly(vinyl chloride) degradation and HCl release, where the beginning and ending time of discoloration is recorded.
- 4.Differential scanning calorimetry test: the Mettler Toledo DSC823e instrument was used to complete the DSC test to measure the gelation grade (G.G) using a heating profile of 30 °C to 240 °C with a heating rate 20 °C min−1. The instrument found at BorsodChem Zrt., Hungary.
- 5.Color change measurement: datacolor spectraflash SF 300 spectrophotometer was used to analyze the color change for the samples of static heat stability test. The spectrophotometer is found at BorsodChem Zrt., Hungary. This test was done according to ISO 11664–1standard [28].
- 6.Scanning electron microscopy SEM: was used to analyze the chemical composition and structure analysis of Oxydtron as shown in figure 1 and table 2. This test was carried out using a Carl Zeiss EVO MA10 SEM at the institute of physical metallurgy, metal forming and nanotechnology, University of Miskolc, Hungary.
- 7.Oxides analysis: during the experiments an axially viewed ICP-AES spectrometer, made by Varian Inc. was used. The results of the analysis are listed in table 3. From this analysis we can see that Oxydtron consists of some mineral components such as calcium oxide, silicates and aluminates; so these components play important roles during the preparation processing of poly(vinyl chloride). ICP-AES spectrometer is at the institute of chemistry, University of Miskolc, Hungary.
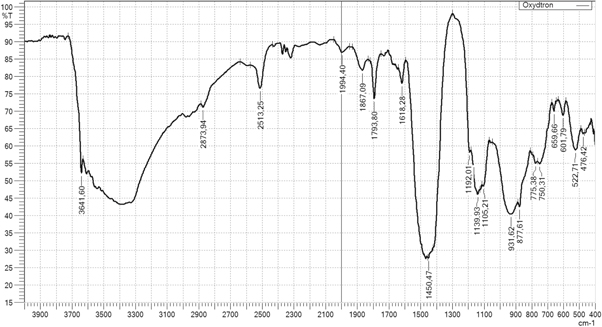
- 8.Fourier transform infrared (FTIR) analysis: a Shimadzu IRTracer-100 device was used for samples analysis. FTIR device is found at BorsodChem Zrt., Hungary. The analysis of chemical compounds of the Oxydtron by FTIR shown in figure 2 and table 4. We can see many active groups in this analysis which represents aldehyde compounds, ketone, amines, polyamides, and alcohols or aromatic or phenolic compounds. All of these compounds can form double and tie triple bonds and aromatic rings with poly(vinyl chloride) causing a stronger material.
- 9.Particle size distribution analysis: HORIBA LA-950V2 laser particle size analyzer was used for analyze particle size distribution gradient as shown in figure 3.
Figure 1. SEM-energy dispersive x-ray microanalysis for Oxydtron.
Download figure:
Standard image High-resolution imageTable 2. Chemical composition of Oxydtron analysed by EDAX- SEM.
| Element | Wt% | At% | Net Inte. | BkgdInte. | Inte. Error | P/B |
|---|---|---|---|---|---|---|
| C | 7.57 | 14.33 | 22.07 | 1.00 | 4.06 | 22.07 |
| O | 34.78 | 49.43 | 121.40 | 1.50 | 1.68 | 80.93 |
| Na | 0.64 | 0.63 | 6.53 | 3.87 | 10.56 | 1.69 |
| Mg | 0.77 | 0.72 | 11.23 | 5.73 | 7.74 | 1.96 |
| Al | 2.09 | 1.76 | 36.63 | 7.03 | 3.55 | 5.21 |
| Si | 10.45 | 8.46 | 195.23 | 9.40 | 1.37 | 20.77 |
| S | 1.48 | 1.05 | 27.37 | 9.90 | 4.58 | 2.76 |
| K | 1.08 | 0.63 | 18.13 | 8.00 | 5.88 | 2.27 |
| Ca | 38.85 | 22.04 | 563.83 | 7.70 | 0.78 | 73.23 |
| Ti | 0.17 | 0.08 | 1.70 | 6.00 | 39.75 | 0.28 |
| Fe | 2.12 | 0.86 | 12.63 | 3.67 | 6.46 | 3.45 |
Table 3. The approximate chemical composition of Oxydtron (expressed as oxides) by ICP-AES method.
| Oxides | Ratio, wt% |
|---|---|
| Al2O3 | 4.47 |
| CaO | 58.0 |
| Cr2O3 | 0.006 |
| Fe2O3 | 2.67 |
| K2O | 0.78 |
| MgO | 1.20 |
| Mn2O3 | 0.05 |
| Na2O | 0.31 |
| SO3 | 2.50 |
| SiO2 | 21.44 |
| TiO2 | 0.274 |
| ZnO | 0.138 |
Figure 2. FTIR analysis for Oxydtron.
Download figure:
Standard image High-resolution imageTable 4. The IR active groups and positive numbers for Oxydtron powder.
| Active group | Wave number, cm−1 |
|---|---|
| OH | (3641.60) |
| CH | (2873.94) |
| C–H | (2513.25) |
| C=O | (1994.40–1793.80) |
| C=C | (1618.28 ) |
| −CH2 | ( 1450.47) |
| C–O–H or C–O–R | ( 1192.01–1105.21) |
| =CH | (931.62–659.66) |
Figure 3. Particle size distribution analysis for Oxydtron.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Flame retardancy test
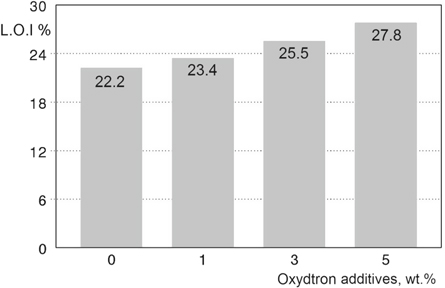
The limiting oxygen index test results for plasticized poly(vinyl chloride) containing Oxydtron are shown in figure 4. From the first bar in this figure we can see that the poly(vinyl chloride) basic formulation has a low L.O.I value (22.2%) even though it is considered high compared to other polymers, and this behaviour is normal due to the low thermal resistance of polymers in general so they ignite rapidly when exposed to fire [29]. Therefore, the simple and optimum solution to improve the flame retarding and thermal resistance of polymers is adding additives. These additives can be used as fillers or coating layers depending on the application for which the material is used [30, 31]. This improvement in flame retardation can be clearly seen in the second bar where the value of L.O.I of poly(vinyl chloride) was increased to 23.4% after adding 1 wt% Oxydtron. The flame retardation of poly(vinyl chloride) is increasingly rising with increasing of Oxydtron percentage additives, where we can note from the third bar that L.O.I for poly(vinyl chloride) reached to 25.5% after adding 3% Oxydtron; and the optimum L.O.I value was obtained by 5% Oxydtron addition (27.8%) as shown in fourth bar; where the flame retardation improvement rate reached to 25.22% more than poly(vinyl chloride) basic formulation. This behavior of flame retarding can be explained by understanding the working mechanism of Oxydtron as flame retardant which does not depend on the changes that occur in its structure when exposed to high temperatures (as with conventional flame retardants) but depends on creating additional stabilizing synergistic action with the original stabilizer as were discovered that the Oxydtron is an excellent polymer stabilizer and proved by the thermal tests. This additional and unexpected stabilizing synergistic behavior is due to Oxydtron's composition which consists of many compounds such as calcium carbonate (CaCO3) which is considered as a universal stabilizer for poly(vinyl chloride) and also silicon dioxide (SiO2) in addition to many other compounds. These oxides and carbonates will act as a synergist agent with the original stabilizer leading to increase the thermal stability of poly(vinyl chloride); where the trio system materials 'Original stabilizer-Silicon dioxide-Calcium carbonate' will effectively form a physical barrier against degradation of poly(vinyl chloride) structure. On the other hand, calcium carbonate is a flame retardant agent, so its presence within the unique structure of Oxydtron helps to increase the flame resistance of poly(vinyl chloride). This stabilizing mechanism is not achievable with traditional flame retardants, so without flame, it cannot act as a flame retardant, instead that these flame retardants will work only as fillers that can change the thermal resistance of poly(vinyl chloride) slightly.
Figure 4. Limiting oxygen index (L.O.I) test results for plasticized poly(vinyl chloride) containing Oxydtron.
Download figure:
Standard image High-resolution image3.2. Static heat stability test
The additional stabilizing synergistic action with the original stabilizer can be observed clearly from the results of the thermal stability test shown in figure 5 which represent the behavior of plasticized poly(vinyl chloride) containing Oxydtron under static heat stability test at 190 °C. It can be seen from this figure below that the color of poly(vinyl chloride) will change very rapidly with different gradients from transparent to dark depending on the time period of exposure to heat. This change is very rapid because of the low thermal resistance of poly(vinyl chloride); where the color change started from the transparent appearance to pale yellow, and then turns yellowish orange, and then turn to yellowish red, then change the appearance to pale red, then to dark red, and then brown, until it becomes completely dark (black) at the end of the test [16, 32–34]. In general, it is not possible to distinguish between all these chromatic gradients with human eyes only, so a spectrophotometer device was used for this purpose [16, 35]. When comparing the color gradation between the samples before and after Oxydtron additions we will note that static heat stability of Plasticized poly(vinyl chloride) containing Oxydtron better than poly(vinyl chloride) alone. And this means that the Oxydtron is a perfect stabilizer for poly(vinyl chloride) as we mentioned above; where the Oxydtron will decrease the tendency of poly(vinyl chloride) discoloration. Also using of Oxydtron as a filler will improve the thermal resistance of poly(vinyl chloride) and increase the efficiency of original stabilizer, it is like forming a kind of synergistic action between them, which is reflected in the reduction of the degradation process. The discoloration resistance of poly(vinyl chloride) will increase by increasing the percentage of Oxydtron additives.
Figure 5. Static heat stability test at 190 °C for plasticized poly(vinyl chloride) containing Oxydtron. OX symbol refers to Oxydtron.
Download figure:
Standard image High-resolution image3.3. Color change measurement
Figure 6 shows the yellowing behavior (Δb*) of poly(vinyl chloride) with different percentages of Oxydtron. From this figure we note that poly(vinyl chloride)'s tendency to yellowing is very high and it is a normal behavior due to low thermal stability at elevated temperatures for poly(vinyl chloride) and other polymers in general [36, 37]. The yellowish change is increased by increasing the period of exposure to heat until the yellowing degree starts to decrease and color gradients are shifted towards the darker appearance and then the color becomes completely black. At this point, it can be said that the total breakdown of poly(vinyl chloride) has occurred [38–40]. After adding Oxydtron to poly(vinyl chloride), the yellowing is greatly reduced and depends on the percentage of Oxydtron additive to reach a peak of 5% Oxydtron; in addition the transformation period to dark color has been extended. This means that poly(vinyl chloride)'s tendency to degrade is greatly reduced.
Figure 6. Detecting the yellowish changes of plasticized poly(vinyl chloride) containing Oxydtron for static heat stability samples tested at 190 °C. OX symbol refers to Oxydtron.
Download figure:
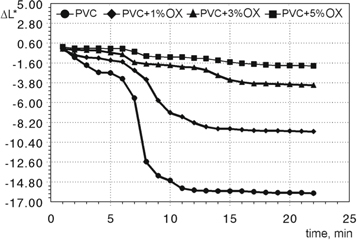
Standard image High-resolution imageFigure 7 represents the lightness changes (ΔL*) of plasticized poly(vinyl chloride) as a function of Oxydtron additives. We can observe from this figure, the lightness appearance of poly(vinyl chloride) begins to deteriorate which can be seen from the sharp decline of the curve in a short time towards more darker appearance until becomes fully black by constantly exposed to high temperatures. This is considered as a normal behavior for poly(vinyl chloride) due to low thermal resistance [37]. But this state will be changed after adding Oxydtron, where the thermal resistance of poly(vinyl chloride) will improve, enabling it to maintain a better light appearance for longer time at the same temperature range and period, where the lightness appearance decreases gradually and not sharply until an optimal lightness curve is obtained at 5% Oxydtron.
Figure 7. Lightness changes of plasticized poly(vinyl chloride) as a function of Oxydtron additives for static heat stability samples tested at 190 °C. OX symbol refers to Oxydtron.
Download figure:
Standard image High-resolution image3.4. Congo-red test
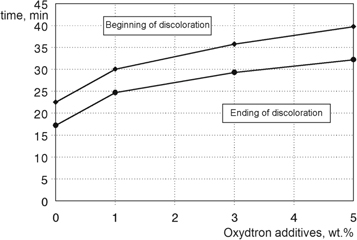
We can be noticed from figure 8 which represents the congo-red test for plasticized poly(vinyl chloride) containing Oxydtron that the time required for beginning and ending of the color change process measured by this test is short; because the thermal stability of poly(vinyl chloride) is low [41, 42]. But after adding Oxydtron the stability of poly(vinyl chloride) has been improved, where this behavior is the outcome of the synergistic action of Oxydtron with Newstab stabilizer which is calcium-zinc based stabilizer as mentioned and this synergistic action will increase the stability of poly(vinyl chloride) against discoloration and reduced the chlorine release. As a result for this behavior the time period for discoloration will extend where the time period for the beginning and ending of the color change for test paper in case of plasticized poly(vinyl chloride) containing Oxydtron will be longer than plasticized poly(vinyl chloride) of basic formulation. This means the release of chlorine has been reduced as a result of the thermal stability improvement of poly(vinyl chloride) structure, which will reduce the degradation rate as a final result. The optimum result obtained by congo-red test was with 5% Oxydtron addition where the time was 86.4% longer than poly(vinyl chloride) of basic formulation.
Figure 8. Beginning and ending of discoloration in congo-red test for plasticized poly(vinyl chloride) containing Oxydtron at 200 °C.
Download figure:
Standard image High-resolution image3.5. Differential scanning calorimetry test
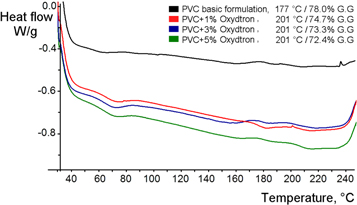
Figure 9 represents the DSC measurements for plasticized poly(vinyl chloride) containing Oxydtron in a temperature range of 30 °C–240 °C in order to investigate the gelation behavior before and after adding Oxydtron additives. From this figure we can observe that Oxydtron shifted gelation grade (fusion degree) towards higher temperatures than poly(vinyl chloride) of basic formulation, where the temperature has been rising to 201 after 1% Oxydtron addition instead 177 °C in case of poly(vinyl chloride) alone; in the same time the gelation grade dropped from 78% for poly(vinyl chloride) to 74.7% for the same percentage and operation conditions of Oxydtron. Although of the increase in the percentage of Oxydtron additives, the temperature remains stable at 201 °C with continued decrease of the gelation grade, where it dropped to 73.3% at 3% Oxydtron and then reached a maximum reduction at 5% Oxydtron with a value of 72.4% where the gelation grade was 8% less than poly(vinyl chloride) basic formulation. The explanation for this behaviour is due to Oxydtron composition which consists of many compounds, including calcium compounds as we have mentioned above, where the calcium compounds used as stabilizers for poly(vinyl chloride). As a fact; the stabilizers especially calcium compounds reduce the gelation grade [43] and since the original stabilizer is also calcium-zinc based, so this stabilizer will create an additional stabilizing synergistic action with Oxydtron compounds which will reduce the gelation grade and at the same time this additional synergistic action will increase the gelation temperature.
Figure 9. Gelation behaviour for Oxydtron containing plasticized poly(vinyl chloride) measured by DSC at 30 °C–240 °C temperature range.
Download figure:
Standard image High-resolution image4. Conclusions
- 1.Oxydtron have the ability to create stabilizing-flame retarding synergistic effects which are proved by the results of thermal tests where the unique Oxydtron composition plays a major role in this characteristic and unexpected behavior for this material.
- 2.All the results obtained were not found in literature before.
- 3.All properties of plasticized poly(vinyl chloride) studied in this study were improved after the addition of Oxydtron; and the improvement ratio increases steadily with increasing the percentage of Oxydtron additives.
- 4.The value of L.O.I improvement after the 5% Oxydtron addition is 27.8% more that of poly(vinyl chloride) of basic formulation.
- 5.The discoloration resistance of plasticized poly(vinyl chloride) increased by increasing the percentage of Oxydtron additives; where the yellowish changes have been decreased accompanied with expansion of transformation time from transparent appearance to fully dark color; also replaced the sharp reduction path of lightness changes with a gradual decreasing path after Oxydtron addition, making plasticized poly(vinyl chloride) able to maintain a lighter appearance for longer time under the same test conditions.
- 6.Extended the time period between beginning and ending of the color change in congo-red test in case on plasticized poly(vinyl chloride) containing Oxydtron to 86.4% longer than poly(vinyl chloride) of basic formulation, which means the chlorine release rate has also been decreased.
- 7.The gelation grade decreased after adding Oxydtron where it was 8% less that of poly(vinyl chloride) of basic formulation due to the presence of calcium compounds in the Oxydtron composition, which works in two ways; first it improves the thermal stability of plasticized poly(vinyl chloride) and; second decreased the gelation grade. But the gelation temperature was shifted to a higher value than poly(vinyl chloride) of basic formulation because of the stabilizing action of Oxydtron additives.
- 8.FTIR analysis detected many active groups within the Oxydtron's crystalline structure which gives it more affinity with plasticized poly(vinyl chloride).
Acknowledgments
We would like to thank Krisztina Tózsa, Barnabás Buzellák, and Irén Buzellákné Pető at the BorsodChem Zrt., Hungary; Árpád Kovacs at SEM laboratory, Faculty of materials science and engineering, University of Miskolc, Hungary; Assoc. Prof. Dr Tamás Szabó, Institute of ceramic and polymer engineering, University of Miskolc, Hungary; and Tamás Kurusta, Faculty of earth science and engineering, University of Miskolc, Hungary who helped us to complete this research.