Abstract
Pilots have reported experiencing in-flight hypoxic-like symptoms since the inception of high-altitude aviation. As a result, the need to monitor pilots, in-flight, for the onset of hypoxic conditions is of great interest to the aviation community. We propose that exhaled breath is an appropriate non-invasive medium for monitoring pilot hypoxic risk through volatile organic compound (VOC) analysis. To identify changes in the exhaled breath VOCs produced during periods of reduced O2 levels, volunteers were exposed to simulated flight profiles, i.e. sea level for 5 min, O2 levels found at elevated altitudes for 5 min or placebo and 5 min at 100% O2 recovery gas, using a modified flight mask interfaced with a reduced O2 breathing device. During the course of these test events, time series breath samples from the flight mask and pre/post bag samples were collected and analyzed by gas chromatography/mass spectrometry (GC/MS). Seven compounds (pentanal, 4-butyrolactone, 2-pentanone, 2-hexanone, 2-cyclopenten-1-one, 3-methylheptane and 2-heptanone) were found to significantly change in response to hypoxic conditions. Additionally, the isoprene, 2-methyl-1,3-butadiene, was found to increase following the overall exposure profile. This study establishes an experimental means for monitoring changes in VOCs in response to hypoxic conditions, a computational workflow for compound analysis via the Metabolite Differentiation and Discovery Lab and MatLab© software and identifies potential volatile organic compound biomarkers of hypoxia exposure.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
At present, pilots and other US Air Force flight personnel are trained to recognize hypoxic symptoms at onset and react according to their self-assessed state. However, modern jet aircraft may have bursts of low oxygen or sudden loss of cabin pressure at high altitude, which may prevent a pilot from noticing detrimental conditions prior to loss of consciousness. Such incidents pose an immediate threat to pilot, aircraft and civilians on the ground. However, a non-invasive media, applicable to in flight monitoring, for diagnostic biomarker discovery is extremely limited.
The identification volatile organic compounds (VOCs) in exhaled breath, which may be used as disease biomarkers, has been pursued for decades [1]. Due to the inherent non-invasive sampling and the ability to detect several hundred compounds from a single sample, exhaled breath VOC analysis is an attractive alternative to serum or urine for biomarker discovery. Furthermore, several studies have shown that endogenous VOCs can change as a result of physiological changes, stressors or disease states, to the organism [2–6]. These examples strongly suggest the potential of exhaled breath as a non-invasive medium for discovery and identification of volatile biomarkers of hypoxia.
We hypothesize that a pilot's exhaled breath VOCs can be used as a source for diagnostic biomarkers for these hypoxic episodes. Such markers will allow for monitoring a pilot's real time physiological status in flight and lead to warning indicators of symptom onset, potentially triggering flight control system automation if consciousness is lost.
Methods
Human subject recruitment and information
All human subjects for this study were volunteer active duty military of variable rank (table 1). Written consent was obtained from the Wright Patterson Air Force Base Institutional Review Board (IRB: NAMRUD.2013.0003). All subjects were informed of and agreed to, in writing, the experimental set up prior to experiment initiation. An experiment progressed or was terminated at any time at the subject's request.
Table 1. Volunteer test subject metadata for hypoxic exposure simulations. Data shows volunteer deidentified individual data, exposure data and type of sample acquired. Sr. = Senior, LT. = Lieutenant, Sgt. = Sergeant.
| Metadata for hypoxic exposure simulations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Gender | Age (yr) | Rank | Previous AF hypoxia training | Smoker | Total altitude exposure duration | Max altitude (O2 equivalent) | Samples type acquired |
| 1 | Male | 27 | Captain | No | No | 5 min | 25 000 ft. | Bag |
| 2 | Male | 30 | Sr. Airman | No | No | 5 min | 25 000 ft. | LESS-P, Bag |
| 3 | Male | 27 | Sr. Airman | Yes | No | 4 min 51 s | 22 000 ft. | LESS-P, Bag |
| 4 | Male | 40 | Captain | Yes | No | 5 min | 25 000 ft. | LESS-P, Bag |
| 5 | Male | 20 | Sr. Airman | No | No | 5 min | 25 000 ft. | Bag |
| 6 | Male | 24 | 2nd LT. | No | No | 3 min 50 s | 25 000 ft. | LESS-P, Bag |
| 7 | Male | 42 | Major | Yes | No | 4 min 21 s | 25 000 ft. | LESS-P, Bag |
| 8 | Male | 29 | Staff Sgt. | No | No | 5 min | 25 000 ft. | Bag |
Experimental setup
Volunteer subjects were placed in a modified flight mask in line with a reduced oxygen breathing device (ROBD) to provide atmospheric oxygen (O2) content for altitudes up to 34 000 feet as described previously by Phillips et al [7] (ROBD-2, Environics, Tolland, CT). Simulated flight profiles consisted of three, five-minute stages in sequential order, sea level (21% O2), exposure: altitude (up to 25 000 feet, 7620 meters, 8% O2) or placebo (sea level, 21% O2), and recovery (100% O2). This profile is similar to the protocol used by pilots in flight when experiencing a hypoxic episode. See supplemental data 1 for a diagram of the experimental setup and ROBD. Throughout the entire experiment, oxygen saturation (SpO2) was read at 1 Hz and tabulated via Datex-Ohmeda finger Oximeter (3900P, GE Healthcare, Buckinghamshire, UK). As a safety precaution, if the measured SpO2 fell below 55%, the test subject was advanced to the 100% O2 recovery gas. Additionally, the test subject, at any point in the exposure, could request to be advanced to 100% O2 recovery gas.
Thermal desorption tubes
Stainless steel Tenax TA thermal desorption (TD) tubes were used in all analyses in this study (Markes International, South Wales, UK). All sorbent tubes were conditioned in a Markes International TC-20 conditioner, as described by the manufacturer, prior to use.
Exhaled breath collection
Exhaled breath was collected via two distinct mechanisms. Immediately prior to and following the simulated flight profile, volunteer subjects (n = 8) filled, following the exhalation protocol, 1 L ALTEF polypropylene bags with the final portion of their exhaled breath (supplemental data 2, Jensen Inert Products, Coral Springs, FL). Breath was removed from the bag via a MultiRAE Pro pump at 270 ml min−1 for 2 min (540 ml total volume) through an inline Tenax TA thermal desorption tube (RAE Systems Inc., San Jose, CA). CO2 (%) was recorded for each bag sample and provided in supplemental data 3 [8, 9]. All sample tubes were capped with brass caps housing PTFE ferrules and stored at 4 °C until analyzed (Markes International, South Wales, UK).
Additionally, exhaled breath was collected serially throughout the course of the experiment (n = 5) using the Logistically-Enabled Sampling System-Portable (LESS-P, Signature Science LLC, Austin, TX). The LESS-P, a portable pump capable of sampling up to 28 TD tubes serially over time with programmable dwell times and flow rates, was placed in line with the modified flight mask. Samples were taken via the LESS-P on individual Tenax TA TD tubes every minute of the exposure at a flow rate of 200 ml min−1. Prior to and during sampling, the flow rate to the LESS-P was verified by a DryCal Defender 510L flow meter (Bios International Corp, Butler, NJ, supplemental data 4). N = 5 test subjects for the LESS-P due to flow rate verification (±2.5% of set value). Following the experiment, all samples were capped with brass/PTFE caps and stored at 4 °C until analyzed.
Gas chromatography mass spectrometry (GC-MS)
All sorbent tubes were thermally desorbed on a Markes International TD-100 thermal desorber and analyzed on a Trace Ultra-ISQ gas chromatograph in line with a single quadraple mass spectrometer (Thermo Scientific, Waltham, MA). Thermal desorption and GC-MS analysis of sample tubes were conducted as described previously [10]. Briefly, thermal desorption was carried out at 310 °C over 10 min. Trap settings were as follows: flow path temperature 160 °C, trap flow rate 50 ml min−1, trap purge time 1 min, trap low temperature of 25 °C, trap high trap temperature 315 °C, trap heating rate 40 °C s−1 and post trap split 3.5 : 1. TO-14 A internal standards, bromocholoromethane, 4-bromofluorobenzene, chlorobenzene-d5, and 1,4-difluorobenzene, were applied automatically to the sorbent tube by the Markes TD-100 prior to thermal desorption (25 ppm, Linde Gas North America, Stewartsville, NJ). GC separations were carried out on a Restek Rxi-624Sil GC column (Bellefonte, PA, 60 m × 0.32 mm ID × 1.80 μm df), with a constant flow of helium (2 ml min−1), over a temperature range of 40 to 240 °C at an increase of 10 °C min−1 with a 20 min hold time at the maximum temperature. Mass spectral analysis was carried out via electron impact ionization at 70 eV with an ion source of 275 °C while scanning over a 35–300 m/z range every 0.154 s. Data was acquired using the Thermo Scientific Trace finder EFS software package (v. 3.0). Raw data file conversion from .RAW to .CDF occurred using the File Conversion tool as part of the Xcalibur software package (v. 3.0, Thermo Scientific).
Metabolite differentiation and discovery laboratory peak registration
Data pre-processing (i.e. baseline correction, registration and alignment) was performed, using the Metabolite Differentiation and Discovery Lab software developed by our group (v. July 2014), with the parameters provided in supplemental data 5 [11, 12]. All pre-processed peak sets were exported for statistical analysis.
Statistical analysis
All statistical analyses were performed within MatLab© software environment (v. R2013a, MathWorks, Natick, MA). Quantile normalization was applied to LESS-P samples to correct for sample-to-sample total intensity variations that may be associated with collection volume differences. However, the quantile normalization applied to the time series data suppressed the variation in the most highly abundant exhaled breath compounds e.g. acetone, isoprene. After normalization, a log2 transformation was applied to force Gaussian distribution behavior. Samples within a level (5 min sea level, 5 min elevated altitude, 5 min recovery oxygen) were averaged per subject, and finally, the log2 ratio between hypoxia and placebo was assessed using a linear statistical model or analysis of variance (1,279 total fragment ions) and ranked by p-value.
The ALTEF bag sample collection volume was well controlled, and hence, the data was not normalized. In the bag sample analysis, a Spearman rank correlation was applied to order VOCs with regard to minimum SpO2.
For all analyses, compounds were tentatively identified via mass spectral comparison to the NIST 11 Mass Spectral Library (v. 2.0, National Institute of Standards and Technology, Gaithersburg, MD). Following statistical analyses, tentatively identified compounds were confirmed (chromatographic retention time and mass spectra) through comparison with purchased neat standards. All standards (pentanal, 4-butyrolactone, 2-pentanone, 2-hexanone, 2-cyclopenten-1-one, and 3-methylheptane and 2-heptanone) were purchased from ChemService Inc. (West Chester, PA).
Results
Assessment of respiratory hypoxic stress
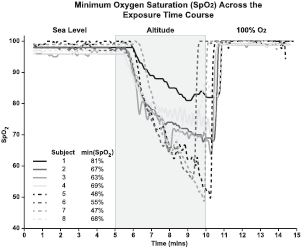
To monitor the individual subject hypoxic state, the SpO2 levels of each individual were measured and time series traces for each test subject are depicted in figure 1. The eight individuals demonstrate a minimum SpO2 of 47–81% occurring at 3–5 min of the restricted breathing. Variability between the minimum SpO2 achieved by individual subjects on the ROBD system has been observed previously [7]. The differences, attributed to individual subject variability and oxygen delivery inconsistencies, result in fluctuating levels of hypoxia for each test subject. The metadata, such as gender, age, rank and smoking status, for each test subject and the simulated exposure parameters are provided in table 1. These results illustrate the variability of individuals in achieving a uniform minimum SpO2.
Figure 1. Minimum oxygen saturation (SpO2) across the exposure time course. Data shows the time course SpO2 traces and minimum observed SpO2 values for each test subject (n = 8). The results demonstrate the variability in the minimum SpO2 achieved by each subject in response to altitude exposure.
Download figure:
Standard image High-resolution imageHypoxia VOC biomarker identification from exhaled breath
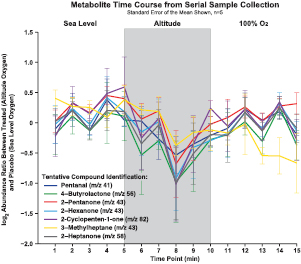
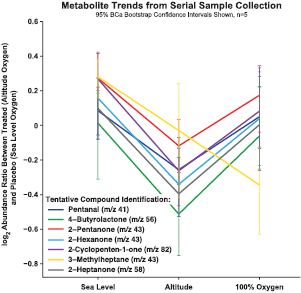
In order to account for oxygen delivery differences, a subject's minimum SpO2 value was used as a primary endpoint for discovering interesting metabolites in addition to gross differential changes between placebo (sea level oxygen) and treated (altitude oxygen). Figure 2 depicts the paired log2 abundance ratio (altitude/placebo) from the GC-MS data for seven VOCs, identified to significantly change, at each 1 min sample, over the time course of the exposure (n = 5). These results illustrate a decrease in the VOC's abundance ratio at the 8–9 min sample corresponding to the time point of minimum SpO2. Additionally when the mean paired log2 abundance ratios, between placebo (sea level oxygen) and treated (altitude oxygen), of the seven compounds were calculated across each exposure level or segment (sea level 1–5 min, exposure 6–10 min and 100% O2 11–15 min) and assessed using a linear statistical model, a significant decrease in the seven compounds was observed at altitude oxygen (figure 3). The data shown in figure 3 are the unique compounds with a p < 0.05. The compounds, in figures 2 and 3, were tentatively identified as pentanal, 4-butyrolactone, 2-pentanone, 2-hexanone, 2-cyclopenten-1-one, 3-methylheptane and 2-heptanone. Furthermore, all compounds, except that identified as 3-methylheptane, show a return to sea level log2 abundance ratio, placebo (sea level oxygen) and treated (altitude oxygen), during the 100% O2 time points suggesting a recovery from hypoxic stress (figures 2 and 3). Collectively through time series sampling, the seven VOCs listed above were identified with a mean difference observed between sea level, elevated altitude and/or recovery oxygen phase.
Figure 2. Metabolite log2 abundance ratio across the exposure time course. Data depicts traces of select metabolites log2 abundance ratio (treated, altitude oxygen, divided by placebo, sea level oxygen) across the exposure time course (n = 5). Error bars signify the standard error of the mean. The results show a decreased log2 abundance ratio when subjects were exposed to altitude oxygen. Compounds tentatively identified via spectral comparison with the NIST11 library.
Download figure:
Standard image High-resolution imageFigure 3. Metabolite trends across the exposure time course. Data illustrates the average log2 abundance ratio (treated, altitude oxygen, divided by placebo, sea level oxygen) for each level (sea level, altitude and 100% O2, n = 5). Error bars signify the 95% confidence interval. A significant decrease in the select metabolites was observed when a one-way ANOVA was applied to individual levels across all subjects. The results demonstrate the ability of the workflow to identify significant changes in VOCs in response to altitude oxygen exposure. Compounds tentatively identified via spectral comparison with the NIST11 library.
Download figure:
Standard image High-resolution imageConformation of hypoxia VOC biomarker identifications
To confirm the identity of the compounds shown to change in response to hypoxic stress (figures 2 and 3), neat standards were purchased and analyzed for chromatographic retention time and mass spectra. Representative total ion chromatograms and spectra from both experimental and standard samples for each compound (pentanal, 4-butyrolactone, 2-pentanone, 2-hexanone, 2-cyclopenten-1-one, and 3-methylheptane and 2-heptanone) are provided in supplemental data 6. A summary of the experimental and standard data for all seven candidate compounds is provided in table 2). These results show identification and confirmation of a group of seven candidate VOC biomarkers of hypoxia.
Table 2. Summary of the identification confirmation experiment for the tentatively identified compounds. Data shows the retention time and major ions associated with experimental and standard samples. These results confirm the identification of seven potential hypoxia VOC biomarkers.
| Compound | Experimental RT (min) | Major experimental ions (m/z) | Standard RT (min) |
Major Standard Ions (m/z) | Confirmed |
|---|---|---|---|---|---|
| Isoprene | 5.23 | 39, 53, 67, 68 | 5.15 | 39, 53, 67, 68 | Yes |
| 2-Pentanone | 9.11 | 43, 58, 71, 86 | 9.00 | 43, 58, 71, 86 | Yes |
| Pentanal | 9.27 | 41, 44, 57, 58 | 9.17 | 41, 44, 57, 58 | Yes |
| 3-Methylheptane | 10.06 | 41, 43, 56, 57, 84, 85 | 9.98 | 41, 43, 56, 57, 84, 85 | Yes |
| 2-Hexanone | 11.24 | 43, 58, 71, 85, 100 | 11.14 | 43, 58, 71, 85, 100 | Yes |
| 2-Cyclopenten-1-one | 12.72 | 39, 53, 54, 82 | 12.62 | 39, 53, 54, 82 | Yes |
| 2-Heptanone | 13.26 | 43, 58, 71, 85, 99, 114 | 13.15 | 43, 58, 71, 85, 99, 114 | Yes |
| 4-Butyrolactone | 15.03 | 41, 42, 58, 86 | 14.92 | 41, 42, 58, 86 | Yes |
| Chlorobenzene-d5 |
12.35 | 40, 52, 54, 82, 117 | 12.25 | 40, 52, 54, 82, 117 | Yes |
aNote: all standard RT are shifted (~0.1 min) due to new column installation prior to standard analysis.
A 0.1 min retention time shift was observed between the experimental and standard samples in the identification confirmation experiment. The difference in retention time can be attributed to the installation of a new analytical column between the two analyses, as shown by the shift in retention time of internal standard chlorobenzene-d5 (supplemental data 6, table 2). Collectively, these data confirm the tentative identifications from our NIST library searches although a change in retention time of 0.1 min was observed.
Exhaled breath biomarkers of an exposure event
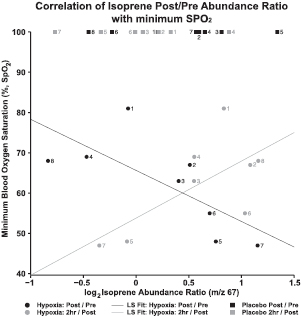
Although application of bag sampling to finding hypoxic biomarkers is difficult due to the 100% O2 recovery time in our experimental design, we applied the use of bag sampling and our data analysis workflow to assess the changes in exhaled breath VOCs prior to and following the entire exposure regimen (supplemental data 1). To evaluate the bag data, the log2 abundance ratios (post exposure bag/pre exposure bag) were calculated and ordinated by Spearman rank correlation with minimum SpO2 achieved (hypoxia samples). Figure 4 depicts nine tentatively identified VOCs; while benzaldehyde and 2-butenal maybe derived from human metabolism, the remaining appear to be environmentally derived. These compounds were significantly correlated with minimum SpO2 (p < 0.01, n = 8). Interestingly, 55.6% of the compounds detected (>0.4 Spearman rho; n = 8) were positively correlated with minimum SpO2 suggesting more severely oxygen-deprived subjects showed broad VOC dilution. In addition, further analysis identified isoprene (2-methyl-1,3-butadiene, supplemental data 6) as the only compound that increased in more severely oxygen-deprived subjects, which is illustrated by the linear fit of the data (black line) having a negative slope (r2 = 0.5398, figure 5). To assess for recovery after 2 h, the 2 h post exposure bag/post exposure bag log2 abundance ratio of isoprene for the altitude oxygen treated subjects shows a decrease as the minimum SpO2 decreases e.g. the linear fit of the data (grey line) has a positive slope (r2 = 0.4604). Placebo (sea level oxygen) do not show a difference in either post/pre or 2 h post/post log2 abundance ratios. Collectively, these results suggest changes in isoprene log2 abundance ratio (post/pre) may be indicative of an overall hypoxia exposure event.
Figure 4. Select metabolite trends across the entire exposure time course. (A) Treated (altitude oxygen) bag pre/post log2 abundance ratios for 10 compounds significantly correlated with minimum SpO2 (p < 0.01, n = 8). (B) Placebo (sea level oxygen) bag pre/post log2 abundance ratios for the same 10 compounds across individuals. The data shows a decrease in the bag pre/post log2 abundance ratios in the treated (altitude oxygen) samples as minimum SpO2 decreases. Compounds tentatively identified via spectral comparison with the NIST11 library.
Download figure:
Standard image High-resolution imageFigure 5. Correlation of bag isoprene pre/post log2 abundance ratios with minimum SpO2. Data shows an increase in the isoprene log2 abundance ratio (post/pre) for the treated (altitude oxygen) subjects as minimum SpO2 decreases. Illustrated by the linear fit of the data having a negative slope (r2 = 0.5398, black line). Conversely, the 2 h post/post log2 abundance ratio of isoprene for the altitude oxygen treated subjects shows a decrease as the minimum SpO2 decreases. Illustrated by the linear fit of the data having a positive slope (r2 = 0.4604, grey line). Placebo (sea level oxygen) do not show a difference in either post/pre or 2 h post/post log2 abundance ratios. The numbers beside the data points indicate individual subject number. Data suggests an increase in isoprene log2 abundance ratio maybe indicative of a hypoxic event.
Download figure:
Standard image High-resolution imageDiscussion
Reports of in-flight hypoxic-like symptoms have been made since inception of modern high altitude aviation. These events pose an immediate threat to the safety of those in the air and on the ground. In this manuscript, a novel non-invasive approach was applied to identify compounds in exhaled breath to monitor pilot health. From this methodology, we have identified seven VOCs that decline in response to hypoxic respiratory stress. Furthermore, isoprene was shown increase following a hypoxic exposure profile. Collectively, these data provide direct support for our ultimate goal of real-time monitoring of pilots for hypoxic risk.
While others have reported biomarkers of hypoxic stress in other organisms, such as pigs, the results presented here suggest the first potential biomarkers of hypoxic stress in humans in a flight measurable human effluvia, exhaled breath [13]. The time series data identified six endogenous compounds, which are associated with hypoxic exposure: These oxygenated compounds may be formed via mechanisms associated with oxidative stress-related metabolism. Previous studies have demonstrated the formation of carbonyl products during stressful exercise [14, 15]. In these studies, both 2-butenal and pentanal are shown to be elevated by oxidative stress-related reactive oxygen species acting upon unsaturated fatty acids. Similarly, linear ketones such as 2-pentanone, 2-hexanone and 2-heptanone may be the result of beta-oxidation of unsaturated fatty acids found in the lungs of subjects during the induced hypoxic stress [16]. Buytrolactone is derived from 4-hydroxy-butyric acid and is structurally related to other ketone bodies derived from fatty acid metabolism during hypoglycemic conditions. Whether such conditions exist within the lungs under hypoxic conditions is not known [17]. In addition to oxygenated compounds discussed above, we also demonstrated a significant increase in isoprene levels during sever hypoxic conditions. This compound has been suggested as a biomarker for several conditions such as exercise-related respiratory flow, cholesterol biosynthesis and inflammatory bowel disease [18–20]. However, confirmatory data for some of these are lacking (see Ueta et al and Kwak et al) [21, 22]. As stated above, benzaldehyde may be derived from metabolic processes or from diet or personal products (a commonly used flavoring and fragrance ingredient) [23–25]. Metabolism of phenylalanine by Streptomyces bacteria, which are present in the oral cavity, may provide an endogenous source of benzaldehyde [26, 27]. However, further mechanistic studies will be necessary to identify the true source of the VOCs identified and why we observe a reduction in VOCs under the onset of hypoxia.
While the data shown here has provided positive data for suggested hypoxia biomarkers in humans, experimental design modifications that balance time resolution and GC/MS sensitivity are needed for further studies. For example, the maximum flow rate of the LESS-P time-series sampler used in these experiments is 200 ml min−1. As a result, samples were collected for 1 min each (i.e. 200 ml) to ensure the highest experimental resolution. While 200 ml is minimally adequate for GC/MS analysis, a larger volume, achievable by a higher sampling flow rate or longer sampling time, will yield superior results by improving the dynamic range allowing for deeper analysis into the breath metabolome.
Conclusion
In summary, we have identified and confirmed several endogenous, oxygenated compounds, which are associated with hypoxic exposure. Additionally, isoprene was correlated with hypoxic severity after receiving recovery oxygen. This small set of volatile organic compounds that show a differential response due to hypoxic exposure may potentially be applied as a predictive biomarker. However, further studies with both methodological improvements and greater number of subjects will be required to validate these preliminary data.
Acknowledgments
Support for this work was provided by a subcontract from UES Inc. under United States Air Force contract FA8650-14-D-6516. Opinions, interpretations, conclusions and recommendations are those of the authors and not necessarily endorsed by the United States Government. The authors would like to thank Dr. Jae Kwak and Gregory Sudberry for their help and scholarly input for this manuscript.