Abstract
High-latitude and high-altitude ecosystems store large amounts of carbon (C) and play a vital role in the global C cycle. Soil respiration (RS) in these ecosystems is believed to be extremely sensitive to climate warming and could potentially trigger positive C-climate feedback. However, this evidence is largely derived from wet ecosystems, with limited observations from dry ecosystems. Here, we explored the responses of RS, autotrophic (RA), and heterotrophic (RH) respiration under experimental warming in a dry ecosystem, an alpine steppe on the Tibetan Plateau. We assessed the effects of soil temperature and moisture dynamics on RS, RA, and RH and performed a meta-analysis to examine whether the warming effects observed were similar to those reported in wet ecosystems, including Tibetan alpine meadow and arctic ecosystem. Experimental warming did not alter RS, RA, and RH in this alpine steppe, likely because decreased soil moisture constrained positive warming effects. In contrast, the meta-analysis revealed that RS exhibited a significant increase under experimental warming in both the Tibetan alpine meadow and arctic wet tundra. These results demonstrate that RS exhibits different responses to climate warming between dry and wet ecosystems, suggesting potential more complex C-climate feedback in cold regions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Soil respiration (RS), the second largest C flux in terrestrial ecosystems, is an important regulator of atmospheric CO2 concentrations (Bond-Lamberty and Thomson 2010, Melillo et al 2017, Bond-Lamberty et al 2018). The annual RS is 91 Pg C (1 Pg = 1015 g), which is approximately ten times larger than the anthropogenic C release (Bond-Lamberty and Thomson 2010, Metcalfe et al 2011, Hashimoto et al 2015). Over the past decades, RS has exhibited an increasing trend because of continuous climate warming (Bond-Lamberty et al 2018). Compared with other regions, temperature increase in high-altitude and high-latitude ecosystems is twice the rate of the global average (IPCC 2013). Considering the large C storage in these regions (Hugelius et al 2014, Ding et al 2016), the faster climate warming may induce large C loss from these cold ecosystems to the atmosphere through RS and trigger positive C-climate feedback (Schuur et al 2015). RS generally consists of autotrophic respiration (RA), produced by plant roots, and heterotrophic respiration (RH) associated with soil organic matter (SOM) decomposition by soil microbes and fauna (Luo and Zhou 2006). Both components make near equal contribution to RS (Bond-Lamberty et al 2018), but climate warming may cause distinct changes in RA and RH (Wang et al 2014). More importantly, increased RA may be balanced by plant production, but RH is not (Hicks Pries et al 2013, 2015), which could then make these two components generate different contribution to the potential positive C-climate feedback. Therefore, a comprehensive understanding of the responses of RS and its autotrophic and heterotrophic parts to climate warming in cold regions is crucial for an accurate prediction of the direction and magnitude on feedbacks in the terrestrial C cycle.
Previous studies have explored the responses of RS and its components to experimental warming in different biomes (Zhou et al 2007, Dorrepaal et al 2009, Hicks Pries et al 2015, Melillo et al 2017). By integrating these site-level studies, meta-analyses have been performed to obtain comprehensive response tendencies on a global scale (Rustad et al 2001, Wang et al 2014, Carey et al 2016). These meta-analyses revealed that experimental warming stimulated RS below a threshold of 25 °C (Carey et al 2016), and also enhanced RH, but had little impact on RA (Wang et al 2014). Although responses of RS and its components under climate warming are better understood, their attention has mainly focused on temperate and tropical ecosystems (Wang et al 2014, Carey et al 2016). Actually, climate warming is expected to result in a more drastic RS change in cold regions compared with temperate and tropical regions (Jahn et al 2010). However, current studies on warming effects on RS in high-latitude and high-altitude ecosystems are mainly derived from wet ecosystems, such as wet tundra in arctic regions (Oberbauer et al 2007, Dorrepaal et al 2009, Hicks Pries et al 2015, Natali et al 2015) and alpine meadow on the Tibetan Plateau (Lin et al 2011, Shi et al 2012, Fu et al 2013, Xue et al 2015), with limited evidence from dry ecosystems. Compared with wet ecosystems, warming effects on RS in the dry ecosystems may be regulated by soil water availability due to its limitation on root and microbial growth and activity (Sheik et al 2011, Suseela and Dukes 2013). Thus, whether warming effects on RS in cold and dry ecosystems are similar to those in cold and wet ecosystems require further investigation.
The purpose of our study was to explore the mechanisms by which RS and its components respond to experimental warming in cold and dry ecosystems, and whether these differ from those in wet ecosystems. Specifically, based on a warming experiment in a Tibetan alpine steppe (a typical cold and dry ecosystem), we conducted a three-year field measurement on RS, RA, and RH. We then investigated how changes in soil moisture regulated warming effects on RS and its components, and performed a meta-analysis to examine whether the finding in our dry alpine steppe differed from that in those cold and wet ecosystems including Tibetan alpine meadow and arctic tundra.
2. Materials and methods
2.1. Field experiment in Tibetan alpine steppe
2.1.1. Experimental site and design
The field experiment was conducted in a typical alpine steppe in Gangca county, Qinghai Province, China (37.30° N, 100.25° E, elevation 3290 m). The mean annual temperature and precipitation at the study site are 0.08 °C and 387 mm, respectively. This experiment site has experienced continuous climate warming over the past three decades, with a mean rate of 0.04 °C per year (Li et al 2019). Dominant plant species of the study site include Stipa purpurea as well as Carex stenophylloides, and the related soil type is Haplic Calcisol (Li et al 2019, Zhang et al 2019).
In May 2013, an enclosure in the study area was established to avoid disturbance from grazing and human activities. The field manipulation contained 10 blocks, with two plots within every block assigned to control and warming treatments. Hexagonal open-top chambers (OTC) were applied to achieve passive warming (Li et al 2019). The size of OTCs was 80 cm along for the top edge, 120 cm along for the bottom edge, and 50 cm height. The transparent material was made of clear acrylic sheeting 2 mm thick and light transmission of over 92%. The OTCs were set out all year round in the field. From May 2014, soil temperature and moisture were monitored simultaneously at a depth of 5 cm in each plot using EM50 data collectors (Decagon, Washington, DC, USA) at 30 min intervals.
2.1.2. Soil respiration measurements
To explore the response of RS and its components to experimental warming, RS, RH, and RA fluxes were measured based on the deep-collar method used in previous studies (Dorrepaal et al 2009, Hasselquist et al 2012, Suseela and Dukes 2013, Peng et al 2017a). Specifically, RS and RH were measured from 09:00 am to 12:00 pm (local time) every 10 days during the growing seasons of 2014–2016, using a detector of LI-8100 soil CO2 flux chamber (LI-COR, Lincoln, Nebraska, USA). In each plot, RS measurements were taken using a PVC collar (20 cm in diameter and 5 cm tall) installed 3 cm into the soil. Meanwhile, RH measurements were taken using a PVC collar (20 cm in diameter and 62 cm tall) which was installed 60 cm into the soil to exclude roots. To eliminate aboveground respiration, small living plants in collars were weeded at least one day before each measurement. RA was got from calculating the difference between RS and RH.
2.1.3. Data analysis of the field measurements
Data from the field measurements were analysed using a repeated measures ANOVA to examine the effects of warming, sampling times and their interactions on RS, RH and RA. Paired t-tests were performed to determine the differences in seasonal average of soil temperature, moisture and RS, RH and RA between warming and control treatments. To explore the warming effects on temperature sensitivity of RS and its components, we fitted the relationships of RS, RH and RA with soil temperature across the growing season using the following exponential equation (Hui and Luo 2004):
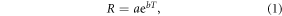
where R is soil respiration rate (mol CO2 m−2 s−1), T is soil temperature at 5 cm (in °C), a and b are constants. Here, b was used to calculate the respiration quotient (Q10), which characterized the respiration changes under an increase by 10 °C of soil temperature, described as the following equation (Zhou et al 2007):
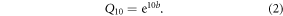
To determine the treatment effects on RS and its components, linear regressions were performed to determine the relationships of response ratios (RR) of RS, RH and RA with RRs of soil temperature and moisture, where the RRs were calculated as the ratios of variables between warming and control groups. Given that data obtained in different years were not independent in statistic, linear mixed-effects models were performed with fixed effects being explanatory variables and the random effect being experimental year (Li et al 2017, Peng et al 2017b). Three data points for RS and RH and two data points for RA were outliers based on Boxplot Procedures (figure S1 is available online at stacks.iop.org/ERL/14/094015/mmedia; Hoaglin and Iglewicz 1987, Sim et al 2005); therefore, these data points were excluded in the final model. All statistical analyses were conducted using R statistical software v3.4.0 (R Core Team 2018).
2.2. Meta-analysis of field experiments in Tibetan alpine meadow and arctic ecosystems
To explore how RS in cold regions responded to warming over a large scale, we synthesized published studies reporting warming effects on RS from the Tibetan alpine meadow and arctic ecosystems using Web of science (http://apps.webofknowledge.com/). To avoid publication bias, selected papers had to meet the following criteria: (i) in situ warming experiments were conducted using one of the three frequently used methods (e.g. OTCs, infrared heater and soil heating cable); (ii) RS was measured for at least one complete growing season; and (iii) the mean value, standard deviation (or standard error) and number of replicate were reported. Based on the above criteria, 11 observations were collected in the form of 'control-warming' pairs from seven studies in the Tibetan alpine meadow, and 23 observations were identified from 10 studies in the arctic and subarctic zones (table S1). The background information, including latitude, longitude, mean annual temperature, mean annual precipitation, experiment duration, and changes in soil temperature as well as moisture in the studies, was also recorded (table S1).
To evaluate warming effects on RS, the RR was calculated as described by Hedges et al (1999):
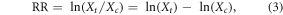
where Xt and Xc are the mean values of control and treatment groups, respectively. Moreover, variance (v1) of individual studies was calculated based on sample size and standard deviation:
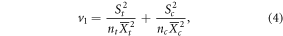
where nt and nc, and st and sc are the sample size and standard deviation for treatment and control groups, respectively. Due to the limited number of data points, the overall mean RRs and the corresponding 95% confidence intervals (CI) were calculated using resampling methods (e.g. bootstrapping; Adams et al 1997). If the 95% CI did not overlap with 1, treatment effects on RS were considered significant. To further explore whether the mean effect size was different between the Tibetan alpine meadow and arctic ecosystems, total heterogeneity (Qt) was partitioned into within-group heterogeneity (Qw) and between-group heterogeneity (Qb). The significant Qb implies that the RRs differ between the two ecosystems (Hedges et al 1999). All above analyses were conducted using MetaWin 2.1 software (Rosenberg et al 1997).
3. Results
3.1. Warming effects on RS and the regulating factors in our field study
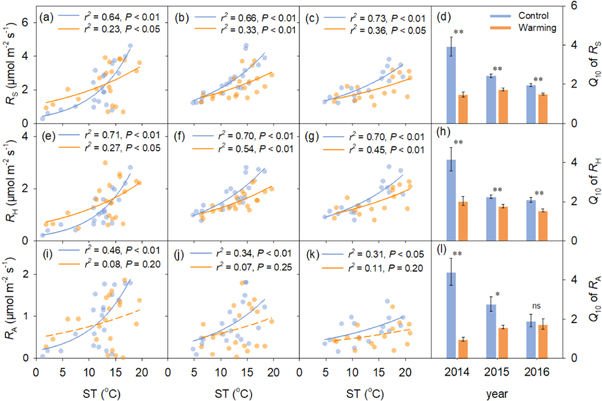
During the growing seasons of 2014–2016, warming treatments significantly increased soil temperature in this alpine steppe by 1.4, 1.6, and 2.0 °C, respectively, while the corresponding soil moisture significantly decreased by 2.5, 3.4, and 2.7%, respectively (figure S2). Experimental warming had no significant effects on RS, RH, and RA at the seasonal scale (table 1), although RS and its components exhibited seasonal pattern (figure 1). Similarly, there were no significant changes in seasonal means of the three parameters (RS, RH, and RA) between warmed and control plots (figure 1 inserts). However, warming significantly reduced the Q10 of the three parameters during the three growing seasons, except for that of RA in 2016 (figures 2(d), (h), (l)).
Table 1. Results of repeated ANOVA on the effects by warming (W), date (D) and their interactions on RS, RH and RA during the growing seasons between 2014 and 2016.
| 2014 | 2015 | 2016 | |||||
|---|---|---|---|---|---|---|---|
| Effect | F | P | F | P | F | P | |
| RS | W | 2.08 | 0.17 | 3.7 | 0.07 | 3.16 | 0.09 |
| D | 82.59 | <0.001 | 70.09 | <0.001 | 78.09 | <0.001 | |
| W × D | 5.62 | <0.001 | 9.24 | <0.001 | 3.99 | <0.01 | |
| RH | W | 4.25 | 0.05 | 2.2 | 0.16 | 0.45 | 0.51 |
| D | 42.42 | <0.001 | 34.23 | <0.001 | 54.76 | <0.001 | |
| W × D | 3.31 | 0.01 | 3.66 | <0.01 | 5.01 | <0.01 | |
| RA | W | <0.001 | 0.99 | 1.01 | 0.32 | 1.93 | 0.18 |
| D | 25.43 | <0.001 | 22.84 | <0.001 | 3.58 | 0.02 | |
| W × D | 4.37 | <0.01 | 3.81 | <0.01 | 0.7 | 0.55 | |
Note: Values in bold denote statistically significance.
Figure 1. Seasonal dynamics of RS (a)–(c), RH (d)–(f), RA (g)–(i) in 2014 (left panels), 2015 (middle panels) and 2016 (right panels). The inserts show means of RS, RH and RA in 2014–2016. Error bars denote standard errors, and ns denotes no significance.
Download figure:
Standard image High-resolution imageFigure 2. Relationships of RS, RH, and RA with soil temperature across the growing seasons in 2014 (a), (e), (i), 2015 (b), (f), (j) and 2016 (c), (g), (k); temperature sensitivity (Q10) of RS (d), RH (h), RA (l) under control and warming treatments between 2014 and 2016. Insignificant relationships are shown with dashed lines in subfigures (i), (j) and (k). Level of significance: *, P < 0.05; **, P < 0.01; ns, no significance.
Download figure:
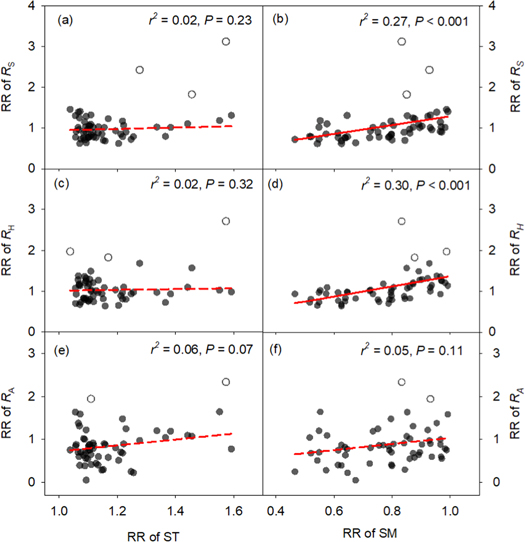
Standard image High-resolution imageThe insignificant responses of RS, RH, and RA in this alpine steppe were likely associated with the lowered soil moisture (figure S2), particularly for RS and RH. Consistent with the above results of insignificant warming-induced changes in RS, RH and RA, no obvious relationships were observed between RRs of RS and its components and RR of soil temperature across the three years (RS: r2 = 0.02, P = 0.23; RH: r2 = 0.02, P = 0.32; RA: r2 = 0.06, P = 0.07; figures 3(a), (c), (e)). However, results from the linear mixed-effects models showed that RRs of RS and RH were positively correlated with the RR of soil moisture (RS: r2 = 0.27, P < 0.001; RH: r2 = 0.30, P < 0.001; figures 3(b), (d)), although it had little effects on RA (r2 = 0.05, P = 0.11; figure 3(f)).
Figure 3. Relationships of the response ratios (RRs) of RS, RH and RA with the RR of soil temperature (ST) (a), (c), (e) and moisture (SM) (b), (d), (f). Hollow points are the outliers determined by Boxplot Procedures and excluded from the fittings. Insignificant relationships are shown with dashed lines in subfigures (a), (c), (e) and (f).
Download figure:
Standard image High-resolution image3.2. Responses of RS in Tibetan alpine meadow and arctic ecosystems
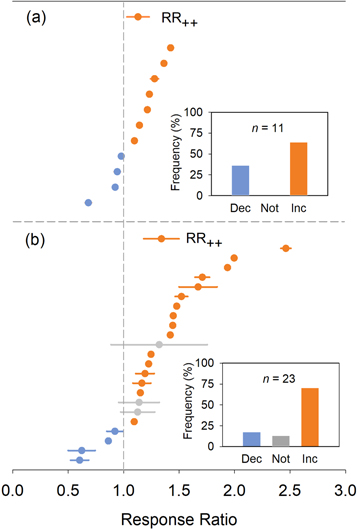
Meta-analysis revealed that the mean RS exhibited a significantly positive response under experimental warming in both Tibetan alpine meadow and arctic ecosystems. Specifically, in the Tibetan alpine meadow, a significant RS response was observed in seven out of 11 individual observations, accounting for 64% of the total. The mean RR of RS was 1.13 and the corresponding 95% bootstrap CI ranged from 1.04 to 1.23 (figure 4(a)). In arctic ecosystems, a significant RS response to warming was observed in 16 out of 23 study sites, accounting for 70% of the total. The mean effect size of RS was 1.23 and the corresponding 95% CI ranged from 1.14 to 1.34 (figure 4(b)). Qb test indicated that no significant warming effects on RS occurred between the two ecosystems (P = 0.09).
Figure 4. Mean effect sizes (responses ratios, RRs) and 95% confidence interval (CI) for individual sites in Tibetan alpine meadow (a) and arctic ecosystems (b). Grand mean effect sizes (RR++) for the two regions are shown at the top of each panel. Circles and columns in orange, grey, and blue indicate significant increase, no change, and significant decrease of RS in response to experimental warming, respectively.
Download figure:
Standard image High-resolution image4. Discussion
Our results showed that RS and its components exhibited insignificant responses to experimental warming in the Tibetan alpine steppe (figure 1). Generally, climate warming should increase RS in cold regions due to the alleviated temperature limitation (Schuur et al 2015). Yet reductions in soil moisture which accompany warming can also affect RS responses (Carey et al 2016). In our field study, the warming-induced changes in RS were significantly related to changes in soil moisture (figure 3(b)). Although slight decline in soil moisture can still increase growth and activity of soil organisms, and thus stimulate RH, severe soil drying would depress RH (figure 3(d)), eventually leading to unchanged RH. However, the non-significant RA response cannot be explained by the changes in soil temperature or moisture (figures 3(e), (f)), indicating that other factors may regulate this process, and the potential mechanisms require further investigation. Altogether, the unchanged RH and RA resulted in a non-significant RS response to experimental warming in this alpine steppe.
Although investigating RH and RA can provide mechanical understanding of the RS response to warming (Bond-Lamberty et al 2018), the root exclusion method, which has been widely used for distinguishing RH and RA (Dorrepaal et al 2009, Hasselquist et al 2012, Suseela and Dukes 2013, Peng et al 2017a), may create some artifacts. Deep collar installation could temporarily stimulate RH due to the decomposition of dead roots. Considering that this transient increase was reported to last for approximately five months (Zhou et al 2007), we started the respiration measurements one year after collars were installed to minimize this limitation. Live root exclusion in deep collars also affected the presences of root exudates, which would in turn affect RH. Despite these inevitable uncertainties, we found that the ratio of RH to RS was between 57% and 72% during the experimental period, within the previously reported range (25%–90%) for global grasslands (Subke et al 2006), indicating that the method is feasible to partition RH and RA in our experiment.
In contrast to the observations in the dry alpine steppe, our results also revealed that experimental warming significantly increased RS in the Tibetan alpine meadow and arctic ecosystems (figure 4). The different responses of RS to warming among various ecosystems may be caused by the following three aspects. First, regional differences in environmental factors, including temperature and water availability, may have led to different RS responses. It has been reported that mean annual temperature in the Tibetan alpine steppe (0.80 °C) was higher than those in the Tibetan alpine meadow (0.15 °C, Chen et al 2019). Moreover, background information of synthesized studies indicated that the sites in Tibetan alpine meadow had a higher temperature range than those in arctic ecosystems (table S1). This temperature difference may have led to lower RS response levels to experimental warming in our field experiment, since RS in cold regions often show higher temperature sensitivity (Carey et al 2016, Hursh et al 2017). In addition, soil moisture in the Tibetan alpine steppe (10.9%) was lower than the Tibetan alpine meadow (22.3%, Chen et al 2019), and the soil moisture in arctic tundra were likely to remain unchanged under experimental warming (table S1). Consequently, RS in the Tibetan alpine meadow and arctic tundra are not severely impacted by the limited water supply, and may be stimulated by experimental warming.
Second, SOC differences across regions may contribute to different patterns of RS under warming. SOC density (C amount per area) within the 1 m depth was reported as 4.5 kg C m−2 in the Tibetan alpine steppe, lower than 9.0 kg C m−2 in the Tibetan alpine meadow (Yang et al 2008, Ding et al 2016) and 34.8 kg m−2 in arctic tundra (Ping et al 2008). It has been suggested that the responses of RS to climate warming are directly related to SOC stock, with larger SOC triggering stronger RS response (Crowther et al 2016). Consequently, elevated temperature may lead to higher RS levels in the Tibetan alpine meadow and arctic ecosystems than in the Tibetan alpine steppe. In addition, SOC quality is another important factor controlling RS response. Laboratory incubation experiments have shown that SOM is highly decomposable in both Tibetan alpine meadow and arctic tundra (Schadel et al 2014, Treat et al 2014, Chen et al 2016a), which may contribute to more sensitive RS changes under climate warming in these regions (Eberwein et al 2015).
Third, differences in plant and microbial growth may cause the heterogeneity in RS response among various regions. Previous research revealed that the average aboveground biomass in the Tibetan alpine steppe was 50.1 g m−2 (Yang et al 2009), while that in the Tibetan alpine meadow and arctic tundra was 90.8 and 315.4 g m−2, respectively (Yang et al 2009, Howard et al 2012). The higher aboveground biomass may be responsible for larger RA fluxes in the Tibetan alpine meadow and arctic ecosystems. In addition to plants, soil microorganisms are also of great importance in determining the response of RS to warming (Allison et al 2010, Wieder et al 2013). Soil microbial biomass in the Tibetan alpine steppe was reported to be 13.5 mmol C kg−1, lower than that in the Tibetan alpine meadow (33.5 mmol C kg−1; Chen et al 2016b) and arctic tundra (340.5 mmol C kg−1; Xu et al 2013). The lower soil microbial biomass indicates that soil microbes in the Tibetan alpine steppe may respire less CO2 under warming. Overall, the larger plant and microbial biomass may contribute to a significant warming-induced RS increase in the Tibetan alpine meadow and arctic ecosystems.
In summary, our study demonstrated that changes to RS under climate warming in the Tibetan alpine steppe were different from those reported in Tibetan alpine meadow and arctic ecosystems. Although warming significantly increased RS in the Tibetan alpine meadow and arctic tundra, it exerted no significant effect on RS in the Tibetan alpine steppe. This finding does not support the traditional view that climate warming would induce a significant increase in RS in cold regions, suggesting that a more complex C-climate feedback mechanism exists in cold regions. Therefore, future evaluations on terrestrial C-feedback, even in warming-sensitive ecosystems, should call for a comprehensive consideration of the heterogeneous ecosystem responses and complex regulating mechanisms.
Acknowledgments
The data that support the findings of this study are available from the corresponding author upon reasonable request. We appreciate the editor and two anonymous reviewers for their critical comments on an early version of this manuscript. We thank Dr Tianfeng Han for his help in field measurements, and acknowledge the financial support from the National Natural Science Foundation of China (31825006 and 41877046), Key Research Program of Frontier Sciences, Chinese Academy of Sciences (QYZDB-SSWSMC049), and Chinese Academy of Sciences-Peking University Pioneer Cooperation Team.