Abstract
Ecological studies on the impacts of timber harvesting contributed to inform sustainable management strategies of tropical forests. However, these studies rely strongly on two major assumptions: (i) strong seedlings recruitment predispose for positive population dynamics, and (ii) more adult trees is a guarantee for a strong reproductive capability of the population. These assumptions are applied without accounting for the life history of the harvested species. Here, we revisit these assumptions in light of the life history theory, which predicts different responses of short- and long-lived species to perturbation. We predict that harvesting adults, rather than reducing seedling recruitment of long-lived species, would have greater impact on population dynamics. We tested our prediction on three mangrove species in South Africa. First, we reconstructed the projection matrices for three mangrove species in the Mngazana Estuary of Eastern Cape province of South Africa. Next, we simulated a range of harvest intensities for different life stages and explored how harvesting influences population dynamics. Finally, we examined the age-specific mortality trajectories for all three species. We found that populations of all three species were closer to their stable stage distribution. Contrary to popular belief, we found that changes in seedling recruitment will have minimal effects on mangrove long-term population dynamics, a finding consistent with the life history theory. However, contrary to expectation, simulating harvest of adults had minimal effect on population dynamics. This is due to low reproductive values for these late stages. Our analysis of age-specific mortality trajectories further provided evidence for positive senescence particularly for Avicennia which was the least resilient to harvest. Our findings cast doubt on the traditional forest management strategies that rely strongly on seedling density as a metric of sustainability and forbid unquestionably harvesting large individuals. We call for caution while generalizing forest management strategies irrespective of the life history of the species at hand.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Most of tropical forests are bound to be logged, and it is critically important to understand the most sustainable ways to harvest these forests before they are cleared for agricultural purpose (Putz et al 2012, Mohren 2019). Classical forest management strategies often call for the maintenance of a specific seedling density as a way of ensuring sustainable forest exploitation (Veríssimo et al 1992, Dauber et al 2005, Zimmerman and Kormos 2012). This infers that strong seedling recruitment would be sufficient to prevent harvested mangrove forest from declining. This assumption is grounded in the traditional view that more seedlings recruitment can ensure better forest ecosystems dynamics (FAO 1994, Ashton and Macintosh 2002, Bosire et al 2008). This common indiscriminate use of seedling density as a silver bullet for all forest management strategy, irrespective of species life history (FAO 1994, Ashton and Macintosh 2002, Bosire et al 2008, Rajkaran and Adams 2012), is questionable in light of the predictions of the life history theory and empirical evidence in plant demography (Silvertown et al 1993). Life history theory suggests that long-term population growth rates of long-lived species are most sensitive to the survival of reproductive adults (Silvertown et al 1993, Schmidt et al 2011, Adler et al 2014) as these species invest preferentially more in long-term survival strategy (Morris et al 2008). In such scenario, low seedling survival rate would have limited effect on their population dynamics as opposed to adult survival (but see Gaoue 2016). Consequently, informing management decisions of long-lived species based on seedling density could be misleading. However, this management strategy is commonly recommended for mangrove species (FAO 1994, Bosire et al 2008, Rajkaran and Adams 2012).
Mangroves are ecologically important tropical and subtropical ecosystems that have gone through drastic reduction in their natural distribution range because of persistent anthropogenic disturbance (Alongi 2002). Mangrove forests cover 0.12% of the world's total land area (Dodd and Ong 2008) and 0.7% of the total global area of tropical forests (Giri et al 2011). Mangroves buffer coastal regions from storm surges and erosion (Das and Vincent 2009), play an important role in carbon sequestration (Donato et al 2011, Siikamäki et al 2012, Fatoyinbo et al 2018) and support the livelihood of dwelling populations (Walters et al 2008). This ecosystem provides a variety of important provisional ecosystem services to local communities, including wood, firewood or building supplies for houses, huts, fences, matting and scaffolds (Millenium Ecosystem Assessment 2005, Duke et al 2007, Ellison 2008, Walters et al 2008, Rajkaran and Adams 2012, Adite et al 2013). They are also key sources of ecological services, e.g. protection against coastal erosion and storms (Fosberg and Chapman 1971, Dahdouh-Guebas et al 2005, Barbier et al 2008, Das and Vincent 2009), regulation of fluxes of energy and nutrients, hydrology and food webs (Ellison et al 2005).
Unfortunately, the future of the mangrove forests is increasingly questioned owing to a number of threats (natural and anthropogenic) that generally affect negatively the ecological and biophysical integrity of this ecosystem (Smith et al 1994, Alongi 2002, Polidoro et al 2010, Daru et al 2013). Natural threats to mangroves are climate related, including tsunami, cyclones, floods, sea level rise (Jiang et al 2016, Simard et al 2019), but also diseases (Spalding et al 1997, Osorio et al 2016). These diseases may affect the dynamic of mangrove populations through their inhibitive effects on mangrove fruit germination (Robertson et al 1992, Smith 1992). These negative impacts are exacerbated by changes in salinity, tidal inundation, sedimentation and soil physico-chemistry, and more critically by anthropogenic perturbation such as harvest (Rajkaran and Adams 2012), mining and aquaculture (Thomas et al 2017, Hamilton and Friess 2018). As a result of anthropogenic disturbances, almost 26%–50% of the world's mangrove forests have already been lost (Valiela et al 2001, Ellison 2008, Spalding 2010) at an annual rate of 1%–8% (FAO 2003, 2007, Miththapala 2008), even though recent studies show a poleward and upward expansion of mangrove in some subtropical regions (Cavanaugh et al 2014, Lenoir and Svenning 2015). If the current trends continue, the entire mangrove biome may be lost within the next 100 years (Duke et al 2007). Such alarming projection poses the challenging question of identifying optimal sustainable harvest limits of mangroves in the context of socioeconomic constraints (Armsworth et al 2010, Gaoue et al 2016, Acuña and Garcia 2019).
In South Africa, 11% of mangrove forest cover is lost every decade (Hoppe-Speer 2013). There has been an increasing interest in the sustainable management of South Africa's mangrove. In one of the rare such studies in tropical Africa, Rajkaran and Adams (2012) set a threshold of harvest intensity at 5% per year for Rhizophora mucronata Lam. (Rhizophoraceae) and Avicennia marina (Forssk.) Vierh. (Acanthaceae) and 0% for Bruguiera gymnorrhiza (L.) Lam. (Rhizophoraceae). They further suggested that an annual harvest of 238 mangrove adult trees ha−1 would ensure the sustainability of the mangroves over time (Rajkaran and Adams 2012). These recommendations were based on the assumption that a minimum of 5000 seedlings ha−1 is enough for a positive population dynamics for these species.
In this study, we seek to understand how the population dynamic of long-lived species such as mangroves species can be affected by which life stages is perturbed by anthropogenic activities. Specifically, we investigated the long-term population dynamics and sensitivity of mangrove species to harvest, and assessed how harvesting different proportions of given plant life stages influences the dynamic of the population. We expected that the studied mangrove species will respond differently to harvest of different life stages. We also hypothesized that, consistent with life history theory, that harvesting late life stages rather than reducing seedling survival will have the greatest effect on the population dynamics of all species. To gain a mechanistic understanding of the sensitivity of mangrove species to harvest of different life stages, we discussed the influence of the (remaining) reproductive values (Keyfitz and Caswell 2005). Our findings provide management strategies that are contrary to previously established protocols for sustainable mangrove harvest.
2. Material and methods
2.1. Study system
We studied the sustainability of harvesting three mangrove tree species, (Bruguiera gymnorrhiza, Rhizophora mucronata, Avicennia marina) in the Eastern Cape province of South Africa using data from Rajkaran and Adams (2012). The authors monitored nine sites in the Mngazana Estuary (31◦42 S, 29◦25 E) from 2005 to 2009. The Mngazana Estuary is 5.3 km long and covers 118 ha with an average water table depth of 33.3 ± 3 cm, a mean monthly rainfall of 87.8 ± 2 mm, and temperatures ranging from 10.5 °C to 28.2 °C (Rajkaran and Adams 2010). During the summer, the estuary received an average monthly rainfall of 115.6 ± 3.4 mm and 46.6 ± 3.1 mm during the winter. The estuary is covered for up to 43% by A. marina stands, 39% by R. mucronata and 18% by stands of B. gymnorrhiza (Rajkaran 2011).
These study species all have anatomical and physiological strategies to adapt to such a particular environment. For example, R. mucronata is equipped with several aerial stilt roots which allow the plant to maintain itself upright in the mangrove ecosystems. B. gymnorrhiza and A. marina (Acanthaceae) are also equipped with upright pneumatophores (up to 45 cm above ground) which are used by these species for gas exchanges (Van Wyk and van Wyk 1997, Rippey and Rowland 2004, Bagust and Tout-Smith 2005). Seeds from both R. mucronata and A. marina often germinate on the tree and fall in the mud as a seedlings. As a result, seed germination rates are high but seedling survival is limited by herbivory from crabs (Rajkaran 2011). The wood of these species are often durable and used in building construction, as firewood and to make fish traps (Little 1983, Van Wyk and van Wyk 1997, Rajkaran 2011).
2.2. Matrix projection model
We reconstructed projection matrices for the three mangrove species (Bruguiera gymnorrhiza, Rhizophora mucronata, Avicennia marina) from size class specific data on mortality (M), transition (T) and persistence rates (P) published in a recent study on South Africa's mangrove (table 1 in Rajkaran and Adams 2012). Rajkaran and Adams (2012) defined five life stages using individual height and knowledge of the ecology of these species: seedlings (S1: <0.50 m height), saplings (S2: 0.51−1.50 m height), small adults (S3: 1.51−2.50 m height), medium adults (S4: 2.51−3.50 m height), and large adults (S5: ≥3.51 m height). For each mangrove species, we built a 5 × 5 stage-based projection matrix A (Caswell 2001). To estimate the long-term population growth rate for each species, we developed the species specific matrix projection models following Caswell (2001):
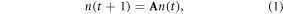
where n(t) and n(t + 1) represent population structures (size class distribution) at time t and t + 1, and A is a 5 × 5 primitive and irreducible Lefkovitch matrix which elements aij summarize various stage transitions within the population, including growth, fertility and survival. We estimated the long-term population growth rate, λ, of each species as the dominant eigenvalue of corresponding matrix A. We also estimated the damping ratio, ρ, as the ratio of the subdominant (λ1) and dominant (λ) eigenvalues:  (Haridas and Tuljapurkar 2007). This damping ratio varies between 0 and 1 and is a measure of how fast a population structure will converge to the stable stage distribution. For each mangrove species we estimated the elasticities eij of λ to perturbation of matrix elements aij (de Kroon et al 1986, Caswell 2000):
(Haridas and Tuljapurkar 2007). This damping ratio varies between 0 and 1 and is a measure of how fast a population structure will converge to the stable stage distribution. For each mangrove species we estimated the elasticities eij of λ to perturbation of matrix elements aij (de Kroon et al 1986, Caswell 2000):
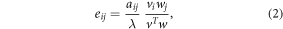
where w and v are the right and left eigenvectors associated with the dominant eigenvalue, λ, and where T denote a transpose. Biologically, w represents the stable stage distribution or the proportional distribution of individuals across life stages at equilibrium. The vector v represents the reproductive value. As it can be concluded from equations (2), the elasticity of λ to changes in a given matrix element aij will depend upon the proportion of individuals in the corresponding life stage (j) at equilibrium (w) and its contribution to future population (v). We used that understanding of the decomposition of elasticity equation to interpret how various life stages will contribute to the dynamics of the three mangrove species. From this, we made assumptions on, and investigated how various types of harvesting and life stages that are targeted can affect the population dynamics of the mangrove species. λ, ρ and eij were all estimated using the package popbio (Stubben and Milligan et al 2007) in R version 3.4.0 (R Core Team 2017).
2.3. Mangrove harvesting simulations
In the communities living around our study system, poles from mangrove species are used to build houses, and individuals which can produce poles that are ≥3 m are preferred by harvesters (Rajkaran and Adams 2010). However, harvesters can target tree as small as 2 m in height (Traynor and Hill 2008). Rhizophora mucronata and Bruguiera gymnorrhiza were the most preferred sources of building poles and Avicennia marina was harvested more for firewood (Traynor and Hill 2008). To simulate harvesting, we increased life stage mortality rate, mainly by decreasing stasis (which comprises mortality rate) of each life stage independently. Because our goal was to identify the life stages that can be harvested sustainably, in addition to determining the intensity of harvest, we simulated harvesting for each life stage independently. We first reduced survival of large adults (>3.5 m height) by percentages varying from 0 (no harvest) to 100% (all individuals in this life stage are harvested) in increments of 10%. We conducted similar simulations for other life stages. For seedlings, we also simulated the effect of increasing mortality rate to test if classic assumption that sparing 2500–10000 seedlings ha−1 is enough to ensure the sustainability of harvesting poles (see FAO 1994, Ashton and Macintosh 2002, Bosire et al 2008, Rajkaran and Adams 2012). We measured the effect of these changes in survival rates on the long-term population growth rates for each study species and compared it with the threshold of λ = 1.
2.4. Age-specific mortality and demographic trajectories
We split each matrix A into a fertility matrix F and a transition matrix Q, the latter representing only transitions among stages of already existing individuals: A = Q + F (Cochran and Ellner 1992, Caswell 2001). We estimated mean survivorship to age x for each mangrove species following Caswell (2001):
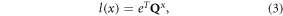
where eT represent a 5 × 1 column vector of ones and it is used here to sum up the columns of matrix Qx. We estimated the age-specific mortality rate μ(x), which is the rate of decrease in survivorship, as the ratio of the age-specific survivorship to age x, l(x), and to age x + 1, l(x + 1) on a log-scale (Horvitz and Tuljapurkar 2008):

To further understand changes in the demography of each of the mangrove species which affect these age-specific mortality trajectories, we established the age-specific stage structure by varying the age x in Qx and deriving the proportion of individuals in each stage class over time without summing the matrix we obtain at each time step. This captures the variation in the proportion of each life stages over time. All the simulations and estimations were developed using R version 3.4.0 (R Core Team 2017).
3. Results
3.1. The long-term dynamics and sensitivity of mangrove species
The populations of all three mangrove species were closer to their stable stage distribution than not (damping ratio for Avicennia: ρavic = 0.833, Bruguiera: ρbrug = 0.857, and Rhizophora: ρrhiz = 0.835), suggesting that our long- (rather than transients) term analysis of population growth rate is appropriate. The populations of all three studied species were projected to increase over the long term. Avicennia and Bruguiera were projected to grow at a similar rate of 3.23% at equilibrium (λavic = λbrug = 1.032) which is three times slower than the 8.90% growth rate projected for Rhizophora (λrhiz = 1.088).
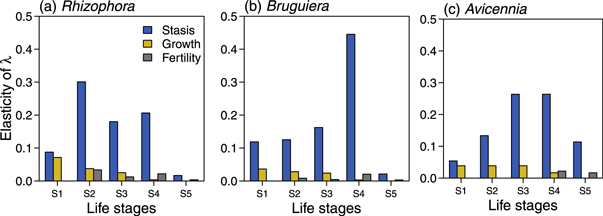
At equilibrium, the population structure of Rhizophora was dominated by seedlings (S1, 29%) and juveniles (S2, 41%) with the last adults stage, S5, represented only by 2% of the individuals (figure 1(a)). However the reproductive values of S1 and S2 which dominated the stable stage distribution were the lowest (figure 1(b)). As a result, the population growth rate of Rhizophora, λrhiz, was more sensitive to changes in juvenile (S2), small (S3) and medium (S4) adults stasis (figure 2(a)). The elasticities of fertility and seedling stasis were relatively small suggesting that changes in these demographic processes are less likely to affect population dynamics of Rhizophora than changes in the stasis of juvenile and medium adults.
Figure 1. (a) Stable stage distribution and (b) reproductive values for three mangrove species (Rhizophora mucronata, Bruguiera gymnorrhiza and Avicennia marina) at the Mzangwa Estuary of Eastern Cape. Life stages represent seedlings (S1), juveniles (S2), small (S3), medium (S4) and large adults (S5).
Download figure:
Standard image High-resolution imageFigure 2. Elasticities of population growth rate, λ to perturbation of matrix elements for three mangrove species: (a) Rhizophora mucronata, (b) Bruguiera gymnorrhiza and (c) Avicennia marina at the Mzangwa Estuary in Eastern Cape.
Download figure:
Standard image High-resolution imageThe stable stage distribution for Bruguiera indicated that half of the population at equilibrium was represented by seedlings (S1, 50%), followed by medium adults (S4, 21%) and juvenile (S2, 17%). The large adults stage, S5, represented only 3% of the population at equilibrium (figure 1(a)), and also had the second lowest reproductive value (v5 = 2.50, figure 1(b)) after the seedling stage. As a result, the population growth rate of Bruguiera, λbrug, was the least sensitive to changes in the survival of large adults (e5,5 = 0.021, figure 2(b)). λbrug was most sensitive to changes in the survival of medium adults (S4) which had the highest elasticity value (e4,4 = 0.445). The elasticity of λbrug to perturbation of seedling was weak (e1,1 = 0.119, figure 2(b)), suggesting that changes in seedling recruitment may not strongly affect the long-term population dynamics of Bruguiera.
Avicennia and Bruguiera had similar population growth rates but their dynamics were sensitive to different processes. The population structure of Avicennia at equilibrium was balanced with similar proportions of individuals for the first 3 life stages (S1: 30%, S2: 26%, S3: 19%) and decreasing proportion of individuals from the first (S1) to the last (S5) life stage (figure 1(a)). However, the reproductive values were highest for small (v3 = 5.20) and medium (v4 = 6.64) size adults (figure 1(b)). Large adults had the third lowest reproductive value (v5 = 3.80, figure 1(b)). Consequently, the population growth rate of Avicennia, λavic, was most sensitive to changes in the survival of small (e3,3 = 0.264) and medium (e4,4 = 0.265) size adults and less to perturbation of large adults (e5,5 = 0.114, figure 2(c)). The elasticity of λavic to perturbation of seedlings stasis was one of the lowest (e1,1 = 0.054, figure 2(c)). Altogether, this suggests that changes in seedling density are less likely to have significant effect on the long-term dynamics of Avicennia, and harvest of the large adults will also have limited effects on λavic.
3.2. Sustainable harvest of mangrove species
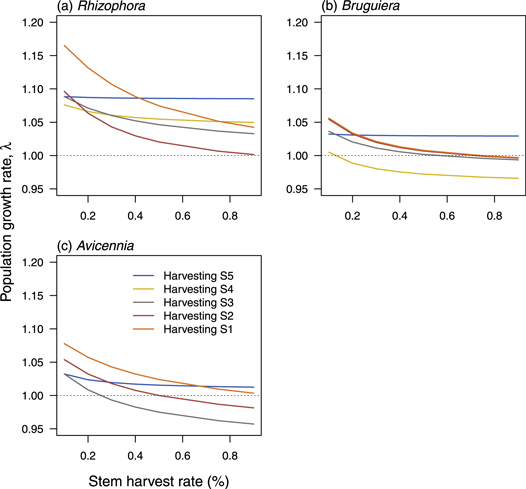
We simulated harvest intensity as the removal of a proportion of given individual life stage from the population because harvest in this case leads to death. The three mangrove species, consistent with differences in their elasticity patterns, show differing resilience to harvesting (figure 3). Regardless of the life stage that is harvested, Rhizophora was the most resilient to harvest (figure 3(a)). Harvesting the large adults did not have any significant effect on λrhiz. However, juvenile (S2) removal had the highest negative effect (10% reduction in λrhiz) on the population dynamics of Rhizophora (figure 3(a)). None of the harvesting scenarios led to negative population growth rate, suggesting a more sustainable harvesting potential for Rhizophora. Similarly, reducing seedling survival to mimic loss in seedling reduced λrhiz by 13% but the population growth rate was still above 1 (figure 3(a)), suggesting that maintaining high seedling recruitment alone is not enough to ensure sustainable harvest.
Figure 3. Simulations of the effect of lethal harvesting of mangrove species on the population growth rate λ of (a) Rhizophora mucronata, (b) Bruguiera gymnorrhiza and (c) Avicennia marina at the Mzangwa Estuary of Eastern Cape. We simulated harvesting as a reduction in the stasis of each life stages: seedlings (S1), juveniles (S2), small (S3), medium (S4) and large adults (S5). For seedling and juvenile, instead of harvesting we simulated reduction in density to test common mangrove conservation and management recommendations.
Download figure:
Standard image High-resolution imageHarvesting large adults from populations of Bruguiera did not affect λbrug, indicating that harvesting this stage is a potential sustainable harvesting strategy (figure 3(b)). However, harvesting more than 10% of medium size adults (S4) or 50% of juvenile (S3) will reduce λbrug below 1, suggesting unsustainable harvest strategies. Similarly to the other two mangrove species, harvesting large adults from Avicennia populations did not affect their dynamics (figure 3(c)). Also, decreasing seedling survival still maintained λavic > 1, indicating that seedling density is a poor metric of harvest sustainability. However, unlike the other mangrove species, Avicennia was the most vulnerable to harvesting. In particular, harvesting 50% of juvenile (S2) or 25% of small- (S3) or medium- (S4) sized adults decrease λavic below 1, suggesting unsustainable harvesting scenarios (figure 3(c)).
3.3. Demographic senescence in mangrove species
To understand if the low reproductive values of large adults and low elasticity of population growth rates to perturbation of this particular life stage is explained by demographic senescence, we examined the age-specific mortality trajectories for all three mangrove species. While Bruguiera and Rhizophora showed constant rates of mortality (figures 4(a), (b)) after reaching the reproductive stage (negligible senescence), mortality rate increased at old age for Avicennia, a trajectory indicating positive senescence (figure 4(c)). The lack of increasing mortality rate after 20 years of projection in Bruguiera and Rhizophora is due to the dominance of the age-specific demographic structures of these species by medium size adults (figures 4(d), (e)). Medium size adults (S4) which had one of the highest reproductive (figure 1(b)) and elasticity values (figures 2(a), (b)) represented 70% of the population structure for Rhizophora (figure 4(d)) and 80% for Bruguiera (figure 4(e)). Contrary to these trends, the age-specific demographic structure of Avicennia was dominated by large adults (S5) which represented 80% of the population after 100 years of simulation (figure 4(f)). This suggests a fast transition of smaller life stages to large adults of Avicennia which, in this case, had some of the lowest reproductive values with limited influence on λavic (figures 1(b), 2(c)).
Figure 4. Age-specific mortality trajectories (a, b, c) and dynamic population structure (d)–(f) for Rhizophora mucronata, Bruguiera gymnorrhiza and Avicennia marina. For the age-specific population structure, seedlings (S1) are in red, juveniles (S2) in yellow, small (S3) adults in green, medium size adults (S4) in blue and large adults (S5) in purple.
Download figure:
Standard image High-resolution image4. Discussion
In the face of the ongoing extinction crisis (Barnosky et al 2011) and the urgent need to reconcile human needs of ecosystem goods and services with the sustainable growth of natural resources (Armsworth et al 2010, Gaoue et al 2016), a poorly informed management decision is ill afforded. In this regard, the present study provides two key take home messages that contribute to centering the debate about mangrove dynamics, sustainable forest harvest and conservation. Our results indicate (1) that ensuring a given seedling recruitment or (2) sparing old or large individuals from harvesting does not guarantee the sustainability of mangrove harvest.
First, common management and sustainable harvest recommendation of ensuring a given seedling recruitment (FAO 1994, Ashton and Macintosh 2002, Bosire et al 2008, Rajkaran and Adams 2012) is obsolete. This should be anticipated from life history theory given that mangroves are long-lived species. We found from elasticity analysis and simulation studies that reducing or increasing density of seedling will have minimal effects on the dynamics of the three mangrove species in the Mngazana Estuary of Eastern Cape (figures 2 and 3). Similar results were also reported for Avicennia marina populations in New Zealand (Burns and Ogden 1985) and for Rhizophora mangle in Venezuela (López-Hoffman et al 2006). These results are not surprising and altogether suggest that, although most studies on the dynamics of mangrove species focus on propagule and seedling predation, the dynamics of such system are driven by other processes that have received far less attention (Clarke 1995).
For long-lived species, the survival of adults is often the most elastic demographic process (Silvertown et al 1993, Salguero-Gómez 2017). Even though seedling survival is an important process, its contribution is often weak (but see Gaoue 2016). The disproportionate importance of large adults for future population dynamics can be explained by the life history theory. Long-lived species invest more in defense to protect the biomass they accumulated over several decades and through a slow growth process. Biomass in seedling is relatively limited and just a few years old. For long-lived species, loss of such recently accumulated biomass can be compensated over time by producing more seedling at appropriate time when the probability of seedling survival is higher. However over the short term, recent studies showed that the transient dynamics of even long-lived species such as African mahogany is disproportionately sensitive to survival of early life stages including seedlings and saplings (Gaoue 2016). This suggests that, for a management tailored toward short term goals, maintaining appropriate seedling density is important. For our studied species, the damping ratios were closer to unity, suggesting that these populations were closer to equilibrium than not. As a result, one would expect the short-term dynamics will be similar to the long-term dynamics we studied.
Second, the recommendation that harvest of old or large individuals must be limited to ensure reproduction (Rajkaran and Adams 2012) is also not appropriate in this case. Our results show that for most species, harvesting large adults (>3.5 m height) will have negligible effects on the population dynamics of all three studied species because this life stage has one of the lowest elasticity values (figure 3). Similar results were obtained on Garcinia lucida in Cameroon where the young adults of 5–10 cm diameter at breast height had the highest elasticity values (Guedje et al 2007) and also for Rhizophora mangle in Venezuela where the highest elasticity values were reported for juvenile (López-Hoffman et al 2006). These results are surprising and contrary to expectations from life history theory that survival of large adults drives future population dynamics in long-lived species (Silvertown et al 1993, Franco and Silvertown 2004, Adler et al 2014). However, empirical evidence from the demography studies of long-lived species lends support for this expectation (e.g. Olmsted and Alvarez-Buylla 1995, van Mantgem and Stephenson 2005, Gaoue et al 2011, Dalgleish et al 2011). The elasticity of population mean fitness, λ, is positively associated with leaf lifespan and wood density (Adler et al 2014), suggesting that such disproportionate relative importance of adult survival for λ is driven by species need to conserve expensive biomass which was accumulated slowly over the long-term.
The weak elasticity of λ to survival of large adults in the mangrove species that we studied is explained by the low reproductive value for this life stage across all species (figure 1(b)). Mathematically, the reproductive value is an eigenvector that can be decomposed into two main components: current reproduction, which is the direct contribution from fertility, and residual reproductive value, which is an indirect contribution from survival to and reproduction at maturity (Keyfitz and Caswell 2005, p. 212). The current reproduction value for large adults in our study species was the highest. Consequently, the low reproductive value for this life stage is likely due to their low residual reproductive value, a weak potential contribution of this life stage to future population size beyond its direct fertility. To further understand the role of such low residual reproductive values on species resilience to harvest, we examined the age-specific mortality trajectories for all three studied species. Persistence of a large proportion of large adults with low reproductive values led to increased mortality rates at old age in Avicennia (figures 4(c), (f)). Associated with this positive senescence, is the low resilience of Avicennia to harvest (figure 3(c)) as compared to other two mangrove species. Positive senescence captures intrinsic physiological decline including reduction in reproductive performance (Roach and Carey 2014). In the case of Avicennia, the accumulation of elderly individuals in the population at old ages could be due to the limitation in the functions of pneumatophores over time.
What are the implications of our findings for the management of mangroves? Our study provides several practical recommendations for the sustainable harvest of mangrove. As indicated above, there is a global recommendation that a threshold of seedling density be maintained in mangrove forest to ensure that poles harvesting is sustainable (FAO 1994, Ashton and Macintosh 2002, Bosire et al 2008). Our study along with other existing studies on mangrove dynamics demonstrate that such recommendation should not be considered because the dynamics of mangrove species were less sensitive to perturbation of seedlings. Instead, our study showed that for the mangrove species in the Mzangwa Estuary of Eastern Cape, harvesting that ensures sustainability of the systems should target large adults which had low reproductive values, and as a result contribute weakly to the future dynamics of the populations. Particularly, harvesting poles that are >3.5 m is more recommended than harvesting smaller poles. Harvest regulations for Rhizophora which was the most resilient to harvest, should be more flexible. However, for Avicennia, to meet potential demands of poles beyond that provided by individuals that are >3.5 m in height, a maximum of 25% of the medium size adults can be harvested. For Bruguiera, that harvest limits must be < 10%.
Overall we investigated the effects of size specific harvest of mangrove species and additionally tested common assumption that ensuring a threshold seedling density is necessary for sustainable harvest. Our study shows that such recommendation is not supported by the results from our analysis and this is consistent with predictions from life history theory. We also show that harvesting old individuals in all of our study species had negligible effects on their population dynamics because this particular life stages has low residual reproductive value which reduces its contribution to future population dynamics.
Acknowledgments
This work was initiated as a result of a Carnegie African Diaspora Fellowship offered to OGG and supported by a start-up grant from the University of Tennessee, Knoxville to OGG. KY acknowledges funding support from the National Research Foundation, South Africa (Grant # 103944 and 112113).
Data availability
The projection matrices reconstructed from table 1 of (Rajkaran and Adams 2012) and the R script developed to run the simulations in this paper are available on Github https://github.com/ogaoue/SAmangroveR.git.