Abstract
The elasticity, topography, and chemical composition of cell culture substrates influence cell behavior. However, the cellular responses to in vivo extracellular matrix (ECM), a hydrogel of proteins (mainly collagen) and polysaccharides, remain unknown as there is no substrate that preserves the key features of native ECM. This study introduces novel collagen hydrogels that can combine elasticity, topography, and composition and reproduce the correlation between collagen concentration (C) and elastic modulus (E) in native ECM. A simple reagent-free method based on radiation-cross-linking altered ECM-derived collagen I and hydrolyzed collagen (gelatin or collagen peptide) solutions into hydrogels with tunable elastic moduli covering a broad range of soft tissues (E = 1–236 kPa) originating from the final collagen density in the hydrogels (C = 0.3%–14%) and precise microtopographies (⩾1 μm). The amino acid composition ratio was almost unchanged by this method, and the obtained collagen hydrogels maintained enzyme-mediated degradability. These collagen hydrogels enabled investigation of the responses of cell lines (fibroblasts, epithelial cells, and myoblasts) and primary cells (rat cardiomyocytes) to soft topographic cues such as those in vivo under the positive correlation between C and E. These cells adhered directly to the collagen hydrogels and chose to stay atop or spontaneously migrate into them depending on E, that is, the density of the collagen network, C. We revealed that the cell morphology and actin cytoskeleton organization conformed to the topographic cues, even when they are as soft as in vivo ECM. The stiffer microgrooves on collagen hydrogels aligned cells more effectively, except HeLa cells that underwent drastic changes in cell morphology. These collagen hydrogels may not only reduce in vivo and in vitro cell behavioral disparity but also facilitate artificial ECM design to control cell function and fate for applications in tissue engineering and regenerative medicine.
Export citation and abstract BibTeX RIS
1. Introduction
Accumulating evidence demonstrates that the elasticity, topography, and chemical composition of cell culture substrates have significant effects on cell morphogenesis, migration, proliferation, apoptosis, and differentiation [1–5]. Thus, the mechanical and chemical properties of the substrate, in addition to biochemical factors, such as growth factors and hormones [6], cannot be neglected when designing in vitro experimental systems for basic biomedical research, drug development, and precision and regenerative medicine. As a typical case, cells cultured on standard substrates such as plastic and glass exhibit behaviors that differ from those observed in vivo, including decreased differentiation activity in stem cells, induction of tumorigenesis in healthy cells, and aberrant drug responses [7–9]. To reduce this in vivo and in vitro behavioral disparity, it is essential to use a culture substrate that preserves the key features of the native extracellular matrix (ECM), a hydrogel of proteins (mainly collagen) and polysaccharides, to regulate physiological processes.
A key distinguishing feature of native ECM is that the concentration (C) of collagen, which is the primary structural material in ECM, generates the elastic modulus (E) of the ECM. A power-law dependence was reported between C and E of bulk tissue [10], where E ranges from ∼1 kPa to a few 100 kPa in soft tissue [11]. However, this correlation is lost in artificial hydrogels. Although it is possible to obtain a hydrogel from ECM-derived collagen solutions (typically <1%) via fibril self-assembly, which involves neutralization and heating, E is limited to <1 kPa due to its low final collagen concentration. Achieving E values of collagen hydrogels comparable to that of a native ECM (E > 1 kPa) requires chemical cross-linkers. The most common cross-linkers such as aldehydes and carbodiimides alter cytocompatibility and biodegradability [8], as well as damage arginine (R), glutamic acid (E), and aspartic acid (D) residues in cell-binding motifs, such as GxxGER and RGD, by using primary amines and carboxylic acids for cross-linking reactions [12–16]. Although cytocompatible transglutaminases and plant-derived genipin can be employed as alternative cross-linkers as they preserve cell-binding motifs [15–18], they also present some drawbacks: transglutaminases have a limited effect on the mechanical properties of collagen gels [15, 16, 18, 19], and genipin is not a zero-length cross-linker and gives a dark blue color to gels [15, 19]. As a result, protein-coated polyacrylamide (PA) hydrogels are the most commonly used for investigating the effects of E on cell responses owing to well-established protocols for easily tuning E [8, 20], despite uncorrelated C and E.
Another key feature of native ECM is the nanometer- to micrometer-scale topography. In addition to obtaining collagen hydrogels with controlled elasticity, controlling the topography of soft hydrogels is technically challenging. Thus, cell responses to topographic cues have mainly been explored on rigid materials (E of ∼GPa) [1, 21–24], such as silicon and plastics, that can be micro-/nano-patterned with techniques such as electron beam lithography and hot embossing. Several studies have addressed micropatterning on PA hydrogels to combine elastic and topographic cues [25, 26] using the replica molding (imprinting) technique [27]. Comelles et al recently improved the patterning technique and reported defined micropatterning (5–10 μm) on PA hydrogels with tunable stiffness (3–145 kPa) [28]. To the best of our knowledge, this is the smallest pattern on PA hydrogels with the widest range of elasticities. Independent control of the elasticity and topography of PA hydrogels will be used to assess the role of each cue and potential synergistic effects in regulating cell functions.
Artificial materials can mimic the E and/or topography of native ECM; however, they cannot reproduce the correlation between C and E in ECM. Artificial materials are used after coating with protein solutions, and this process can cause problems: different binding states of proteins depend on the coating method and/or substrate properties such as chemical composition and surface morphology, resulting in a different distribution of cell adhesion sites, that is, an incoherent effect of the mechanical inputs. It has been reported that different effects of substrate stiffness on stem cell spreading and differentiation are obtained between polydimethylsiloxane (PDMS) and PA hydrogels, even when they are similarly coated with collagen [29]. Moreover, the density of biochemical ligands coated on PA hydrogels significantly influences localization of the stiffness-dependent yes-associated protein (YAP) [30]. That is, cells can show apparently inconsistent behavior in response to substrates having the same mechanical properties, due to the cell adhesion sites independently distributed from the mechanical cues. In vivo, on the contrary, the distribution of ECM macromolecules generates the elasticity and topographies of the ECM. Due to a lack of ECM-mimicking hydrogels with controlled elasticity and topography originating from the distribution of ECM-derived macromolecules, the respective and/or synergetic effects of the chemical and mechanical properties of the ECM on cell function and fate in vivo remain unclear.
In order to solve this problem, here we report a novel collagen hydrogel developed with a technique called elastic and topographical control by radiation molding (ET-RaM) that can combine elastic, topographic, and compositional cues similar to those of native ECM. These hydrogels are composed of only ECM-derived purified collagens, collagen I or hydrolyzed collagens (gelatin or collagen peptide), and water, and the networks of collagen molecules not only provide cell adhesion sites but also produce elasticity and microtopography. The ET-RaM required no chemical cross-linkers and ensured the maintenance of the intrinsic functionality of collagen, controlled E values encompassing the broad range of in vivo soft tissue, and formed precise microtopographies. By using collagen hydrogels obtained by ET-RaM, we investigated the responses of cell lines (fibroblasts, epithelial cells, and myoblasts) and primary cells (rat cardiomyocytes) to the complex cues of chemical composition, elasticity, and topography, especially specific topographic cues as soft as the ECM of in vivo soft tissue.
2. Materials and methods
2.1. ET-RaM of collagen
A schematic of the ET-RaM process is shown in figure 1. PDMS molds were prepared in advance as follows. The precursor of PDMS (SIM-260) was mixed with a curing agent (CAT-260) (both from Shin-Etsu Chemical) at a 10:1 ratio, and then degassed and spin-coated on a silicon master mold (DTM1-1; Kyodo International) at 1000 rpm. After curing at 150 °C for 30 min, the molds were peeled from the master mold. A 5 mg ml−1 collagen I solution (porcine skin, Collagen BM, #639-30861; Nitta Gelatin) was concentrated to 50 mg ml−1 by evaporating the solvent at room temperature (∼25 °C). The gelatin (porcine skin, Type A, G1890; Sigma-Aldrich) and collagen peptide (porcine skin, Type A; Nitta Gelatin) solutions were prepared by dissolving in deionized water (supplied by a Millipore Milli-Q system) at 10 wt% by heating at 50 °C for 30 min. After pouring the solution into cell culture dishes (Iwaki 1000-035, non-treated, 35 mm; AGC Techno Glass) (figure 1, Step 1), micro-patterned flexible PDMS molds loaded onto a plastic cover such as polyethylene-terephthalate film were placed in the solution (figure 1, Step 2). The samples were stored overnight at 4 °C for collagen I and peptide and at 20 °C for gelatin. During storage, gelatin and peptide underwent physical gelation. The samples in sealed bags were irradiated with 60Co γ-rays (60Co No.2 Irradiation Facility of Takasaki Advanced Radiation Research Institute, QST) in air at 15 °C–20 °C at a dose rate of 10 J g−1 h−1 (J g−1 = kGy) for ⩾10 J g−1 and at 8 J g−1 h−1 for 8 J g−1 irradiation (figure 1, Step 3). The samples were detached from the PDMS molds and immersed in PBS (pH 7.4, 10010023; Thermo Fisher Scientific) (figure 1, Step 4) at 37 °C for ⩾3 days for collagen I, or at 50 °C for 2–3 h for gelatin and peptide to remove non-cross-linked components and to allow them to equilibrate. Collagen hydrogels prepared by ET-RaM were used for cell culture after immersion in culture medium at 37 °C for 1 h to replace the absorbed PBS (figure 1, Step 5).
Figure 1. Schematic of ET-RaM process. Collagen hydrogels with combined elastic, topographic, and compositional cues that recapitulate native ECM can be obtained without chemical agents.
Download figure:
Standard image High-resolution image2.2. Characterization of collagen hydrogels
Photographs of hydrogels and bright-field images of hydrogel microtopography were obtained using a digital camera (DMC-TZ85; Panasonic) and an inverted microscope (IX83; Olympus) with a digital camera (AdvanVision), respectively.
To determine the final collagen concentration in the hydrogels, the swollen samples after Step 4 were filtered through a stainless steel 200-mesh filter (mesh size: 75 μm) and weighed (Ws). After vacuum-drying overnight at 30 °C and weighing the dried sample (W0), the final collagen concentration, C (%), was calculated as (W0/Ws) × 100.
The elasticity of collagen hydrogels was measured using an indentation tester (RE2-3305B; Yamaden) at 37 °C following Step 5. The samples were compressed to 30% deformation with a 2 N load cell using a plunger (ϕ3 mm or ϕ8 mm) at 50 μm s−1. The compressive modulus was then determined from the slope of the stress–strain curves over the strain range from 0% (surface) to the % value equivalent to a depth of 100 μm in each sample.
The chemical structures of gelatin before and after the ET-RaM process were compared using Fourier transform infrared (FT-IR) spectroscopy. As the pristine sample, 10 wt% physical gelatin gel was vacuum-dried overnight at 30 °C. The hydrogels produced by the ET-RaM process were washed in Milli-Q water at 50 °C for 2–3 h and vacuum-dried overnight at 30 °C. FT-IR spectra were collected using an IRAffinity-1 S instrument (Shimadzu) with a DuraSampl IR-II single-reflection diamond attenuated total reflection attachment (Smiths Detection) at a resolution of 1 cm−1 and averaged over 32 scans.
The biodegradation properties of gelatin hydrogels were analyzed using collagenase (034-22363; Fujifilm Wako Pure Chemical). The hydrogels produced by the ET-RaM process were used after Step 4. The pristine samples and hydrogels in 35 mm dishes (initial volume of 1.5 ml) were incubated in collagenase solution (0.04% in PBS) at 37 °C. At different time points, the samples were filtered through a stainless steel 200-mesh filter and washed with Milli-Q water. After vacuum-drying overnight at 30 °C, the samples were weighed (Wt), and the percentage of remaining weight was calculated as (Wt /W0) × 100.
Amino acid analysis of the gelatin hydrogels was carried out by acidic hydrolysis using a PICO-TAG Work Station (Waters) followed by high-performance liquid chromatography (HPLC). After irradiation in Step 3, the samples were washed in Milli-Q water at 50 °C for 48 h and vacuum-dried at 30 °C for 24 h. The samples and pristine gelatin powder (1 mg each) in separate glass test tubes were transferred to a reaction vial containing 0.2 ml of 1% phenol (161-01025; Fujifilm Wako Pure Chemical) in 6 M HCl (080-01066; Fujifilm Wako Pure Chemical). The reaction vial was tightly closed and heated at 110 °C for 21 h under vacuum. The test tubes were then vacuum-dried to remove any remaining HCl, and the obtained hydrolysates were filtered through a 0.45 μm membrane (SFCA033045S; AS ONE) after adding 20 ml of Milli-Q water to each tube. Amino acid standard samples were prepared from an amino acid mixture standard solution (013-08391; Fujifilm Wako Pure Chemical) containing 2.5 × 10−6 M of 17 amino acids diluted 1:20 with Milli-Q water and filtered through a 45 μm membrane. Borate buffer (pH 9.50) was prepared by mixing 0.05 M boric acid (021-02195) and 0.05 M NaOH (198-13765) (both from Fujifilm Wako Pure Chemical) at a 2:1 volume ratio. Each 10 μl sample solution was mixed with 20 μl of borate buffer and 20 μl of 0.05 M 4-fluoro-7-nitrobenzofurazan (342-04751; Dojindo Laboratories)/acetonitrile (015-08633; Fujifilm Wako Pure Chemical). The solutions were heated at 60 °C for 1 min and then cooled at −18 °C for 2 min; 50 μl of 0.3 M HCl was then added to terminate the labeling reaction. A 10 μl volume of each labeled sample was analyzed by HPLC (Model 1100; Agilent Technologies) with an InertSustainSwift C18 column (GL Sciences) and a fluorescence detector (2475; Waters) at 40 °C. The HPLC column was eluted with 0.1% trifluoroacetic acid (208-02741; Fujifilm Wako Pure Chemical) and acetonitrile with the gradient mixture ratio as recommended by GL Sciences (data no. LB316-0848) at a flow rate of 1.0 ml min−1.
2.3. Cell culture
Madin-Darby canine kidney (MDCK) epithelial cells (RCB0995), HeLa human cervical carcinoma epithelial cells (RCB0007), and 3T3-Swiss albino mouse embryonic fibroblasts (RCB1642) were obtained from the RIKEN BRC Cell Bank. C2C12 cells (EC91031101) were obtained from DS Pharma Biomedical. Cardiomyocytes were obtained from neonatal Wistar rats (Japan SLC) according to our published protocol [31]. Animal experiments were approved by the Institutional Animal Care and Use Committee of QST (approval no. 18-T001-1) and were performed in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
MDCK and HeLa cells were cultured in minimum essential Eagle's medium (MEM) (M5650; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (SH30910.03; GE Healthcare Japan and 10437-028; Thermo Fisher Scientific, respectively), 100 U ml−1 penicillin, 100 μg ml−1 streptomycin (15140-122; Thermo Fisher Scientific), and 0.002 M L-glutamine (10378016; Thermo Fisher Scientific and G7513; Sigma-Aldrich, respectively). 3T3-Swiss cells, C2C12 cells, and cardiomyocytes were cultured in Dulbecco's modified Eagle's medium (DMEM, 08488-55; Nacalai Tesque) supplemented with 10% FBS, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, and 0.002 M L-glutamine (Sigma-Aldrich). A 2 ml volume of each cell suspension (1 × 104 MDCK, HeLa, or 3T3-Swiss cells ml−1), 5 × 104 C2C12 cells ml−1, or 1.5 × 105 cardiomyocytes ml−1 prepared using the Countess II FL automated cell counter (AMQAF1000; Thermo Fisher Scientific) was introduced onto collagen hydrogels in 35 mm cell culture dishes without any reagent coating, or polystyrene (PS) cell culture dishes (Iwaki 3000-035, treated, 35 mm; AGC Techno Glass) without protein coating. To induce the differentiation of C2C12 cells, the culture medium was replaced with differentiation medium composed of DMEM supplemented with 2% horse serum (26050-070; Thermo Fisher Scientific), 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 0.002 M L-glutamine (Sigma-Aldrich), and 1 μg ml−1 insulin (I0516; Sigma-Aldrich) 2 d after seeding. The differentiation medium was replenished every 1–2 days. The cells were cultured at 37 °C in 5% CO2.
2.4. Fluorescence staining and imaging
Nuclei, the actin cytoskeleton, and sarcomeres were fluorescently labeled to analyze cell responses to collagen hydrogels. On day 3 (MDCK, HeLa, and 3T3-Swiss), 6 (C2C12), or 4 (cardiomyocytes) of cell culture, samples were washed with PBS, and cells were fixed with 4% paraformaldehyde (163-20145; Fujifilm Wako Pure Chemical) for 10–15 min. After washing with PBS, the cells were incubated in PBS containing 0.1% Triton X-100 (35501-02; Nacalai Tesque) for 5 min and washed with PBS. MDCK, HeLa, and 3T3-Swiss cells were incubated in PBS containing 0.2 μg ml−1 tetramethylrhodamine B isothiocyanate (TRITC)-conjugated phalloidin (P1951; Sigma-Aldrich), 2 μg ml−1 4',6-diamidino-2-phenylindole (DAPI) (D523; Dojindo Laboratories), and 1% bovine serum albumin (BSA) (019-27051; Fujifilm Wako Pure Chemical) for 20 min. C2C12 cells were incubated in PBS containing 0.1 μg ml−1 TRITC-conjugated phalloidin, 1 μg ml−1 DAPI, and 1% BSA (P-6154; Biowest) for 20 min. To visualize sarcomeres, cardiomyocytes were incubated in blocking solution composed of PBS with 1% BSA (Fujifilm Wako Pure Chemical) for 60 min, followed by blocking solution containing mouse anti-α-actinin antibody (1:300, A7811; Sigma-Aldrich) for 60 min. After washing with blocking solution, cardiomyocytes were incubated in blocking solution containing Alexa Fluor® Plus 488-conjugated goat anti-mouse IgG (1:300, A32723; Thermo Fisher Scientific) for 60 min. To visualize the surface of collagen hydrogels, samples were washed with PBS and incubated for 10 min in PBS containing 1 mg ml−1 of 0.2 μm green fluorescent microspheres (F8811) or 0.2 mg ml−1 of 0.2 μm red fluorescent microspheres (F8810) (both from Thermo Fisher Scientific). The samples were then washed with PBS and examined with an upright microscope (BX61WI) with a disk scanning unit (BX-DSU), objective lens (LUMPLFLN 60XW) (all from Olympus), and a complementary metal oxide semiconductor camera (ORCA-Flash4.0 V3; Hamamatsu Photonics K.K.). Staining and observation were performed at room temperature (∼25 °C). Three-dimensional (3D) image stacks were deconvoluted using cellSens software (Olympus). Image processing and 3D rendering were performed using ImageJ software (National Institutes of Health).
2.5. Analysis of cell morphology and actin cytoskeleton orientation
Cell morphology was analyzed in terms of spread area, aspect ratio (length of major axis/length of minor axis), and orientation (angle from 0° to 90°) from bright-field images randomly acquired on an inverted microscope (IX70; Olympus) with a CellPad E digital camera system (Allied Scientific Pro) using ImageJ software (n = 3). For the proliferation assay, HeLa cells cultured for 2 h or 1–3 days were stained with 1 μg ml−1 Hoechst 33342 solution (H342; Dojindo Laboratories). Nuclei were visualized using a light source (130 W mercury lamp, U-HGLGPS) and mirror units (U-MWU2) (both from Olympus), and the number of HeLa cells was counted from randomly acquired fluorescence images (n = 3). The viability of HeLa cells on day 3 was analyzed after staining with 1 μg ml−1 calcein-AM (C396; Dojindo Laboratories) and 1 μg ml−1 DAPI. Viable and dead cells were visualized using mirror units (U-MWU2 and NIBA; Olympus), and the percent viability was calculated (n = 3).
The orientation of the actin cytoskeleton was analyzed using a Fourier transformation-based method. An optical section of the actin cytoskeleton beneath the nucleus was first extracted from z-stack images and cropped to 128 × 128 pixels (13.9 μm2). The 2D Fourier spectrum for the cropped image was then calculated. After removing the direct current offset, a histogram in polar coordinates was generated from the Fourier spectrum data, which was approximated by an ellipse. The orthogonal direction of the major axis of this ellipse was defined as the orientation direction of the actin filaments. The angular difference between this and the major axis of the microgrooves on collagen hydrogels was defined as the orientation angle of the actin cytoskeleton relative to the microgrooves. The 2D Fourier spectrum was calculated with MATLAB (MathWorks) software, and other image analyses were performed using a custom-made macro in ImageJ software.
2.6. Statistical analysis
Data were analyzed using Welch's t-test and one-way analysis of variance followed by a post-hoc Tukey's honestly significant difference test using OriginPro2018J software (OriginLab). The analyzed datasets of cell responses are summarized in (supplementary table S1 (available online at stacks.iop.org/BMM/16/045037/mmedia)). All experiments were performed at least three times.
3. Results
3.1. ET-RaM technique for creating collagen hydrogels
To produce collagen hydrogels without using chemical cross-linkers, we applied radiation-induced chemical cross-linking reactions. Although collagens are dominantly decomposed by ionizing radiation in a dried state [32–34], cross-linking can also be induced in the presence of water [32–35]. At low concentrations (<1%), the probability of decomposition is still higher than that of cross-linking [36], owing to the long intermolecular distances, and cross-linking only produces nano-/micro-particles in low yield. We found that at a certain concentration (a few to several 10 s%) in water, both in the solution state and physically formed hydrogel state, both ECM-derived native collagen and hydrolyzed collagens are predominantly cross-linked through a reaction with hydroxyl radicals generated in water. Water-containing 3D polymeric networks, that is, chemically cross-linked collagen hydrogels, are thus formed without requiring a cross-linking agent.
We developed an ET-RaM technique (figure 1, see section 2) after optimizing the production of collagen hydrogels by radiation-induced chemical cross-linking with high yield and high reproducibility. As an ionizing radiation source, we chose 60Co γ-rays, which are commonly used to sterilize medical products. Owing to their high penetrability, γ-rays provide a uniform dose in samples, even in batch processing. Flexible PDMS molds placed before radiation cross-linking were easily demolded from the soft collagen hydrogels. ET-RaM successfully altered ECM-derived collagen I and hydrolyzed collagen (gelatin or collagen peptide) solutions into micropatterned hydrogels without using chemical cross-linkers. The irradiation dose required for gelation was roughly the same range as that usually adopted for radiation sterilization (4–25 J g−1) [37], as described later.
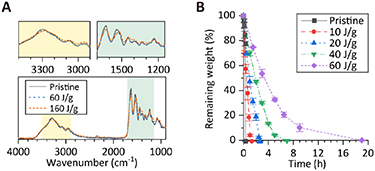
First, the chemical properties of the obtained collagen hydrogels were analyzed to determine whether the irradiation process influences the intrinsic functionality of collagen. Figure 2(A) shows the FT-IR spectra of gelatin before and after ET-RaM processing with irradiation doses of 60 and 160 J g−1. The amide bands of polypeptides (A: 3297 cm−1, B: 3075 cm−1, I: 1630 cm−1, II: 1537 cm−1, and III: 1237 cm−1) related to peptide bonds and helical structure remained essentially unaltered by ET-RaM. A slight shift to a lower wavenumber for amide A and amide II after irradiation at 160 J g−1 suggests a small change in the secondary structure of gelatin. In vitro enzymatic biodegradation of the gelatin hydrogel was evaluated using collagenase (figure 2(B)). Biodegradability was maintained after the ET-RaM process, although it slowed down with increasing irradiation dose, that is, with increasing matrix density. These results were supported by the observation that the amino acid composition ratio of 15 amino acids, including R, G, D, and E, which comprise cell-binding motifs, were almost unchanged by ET-RaM at doses of 30 and 60 J g−1 (supplementary table S2). These chemical analyses showed that ET-RaM ensured the maintenance of the intrinsic functionality of collagen.
Figure 2. (A) Fourier-transform infrared spectra of gelatin before and after ET-RaM processing with irradiation doses of 60 and 160 J g−1. (B) In vitro enzymatic biodegradation of the gelatin hydrogel evaluated using collagenase. Error bars, SEM (n = 3).
Download figure:
Standard image High-resolution image3.2. Elastic and topographical control of collagen hydrogels by ET-RaM
Collagen hydrogels with a regulated elastic modulus, E, were obtained by tuning the irradiation dose of ET-RaM from 8 to 120 J g−1. E values determined by the indentation test (supplementary figure S1) ranged from 1 to 236 kPa (figure 3(A)) without the addition of any reagent. Collagen I and gelatin showed similar E values at the same irradiation dose, while collagen peptide, which has the lowest molecular weight among the three collagen types, required the highest irradiation dose to form hydrogels. Higher irradiation doses resulted in higher cross-linking densities, thus resulting in higher E values. E showed power law-dependence on the final concentration of collagen in the hydrogels, C, which ranged from 0.3% to 14%, except for the softest hydrogel obtained with collagen I (figure 3(B)).
Figure 3. (A) Controlling elastic modulus, E, of collagen hydrogels by adjusting γ-ray irradiation dose (Step 3). (B) Dependence of E values on collagen concentration in hydrogels, C. The hydrogels were produced using γ-ray irradiation doses of 10, 20, 40, and 60 J g−1 for collagen I and gelatin, and 40, 60, 100, and 120 J g−1 for peptide, in the ascending order of C. (C) Photograph of a typical collagen hydrogel (iridescent reflections are due to microtopography diffraction) and micrographs of representative microtopographies formed on the hydrogel surface. (D) Micrographs of representative topographies formed on the collagen hydrogel surface with an E of 1.2 kPa. (E) Diameter of collagen I hydrogel and representative photographs taken just after irradiation with 20 J g−1 (left) and after heating at 37 °C in PBS for 3 days (right). Micrographs show representative topographies formed on the surface of compacted collagen I hydrogels. (F) Elasticity and topographic properties of collagen hydrogels prepared by ET-RaM (green colored area) compared to conventional cell culture materials and in vivo soft tissue. Error bars in (A), (B), and (E) represent SEM (n = 3).
Download figure:
Standard image High-resolution imageWe obtained micropatterned collagen hydrogels ranging from 7 to 236 kPa. Microtopographies such as microgrooves with widths and spacings of 1, 5, and 10 μm and a height of 2 μm, were successfully formed on the hydrogel surfaces (figure 3(C)). The softest hydrogel in which micropatterns were observed had an E of 1.2 kPa, which corresponds to the stiffness of the human brain [11]; however, the patterns easily collapsed due to their honey-like fluidity (figure 3(D)). Interestingly, the volume of collagen I hydrogels reduced during soaking in PBS at 37 °C (figure 1, Step 4), and reached an equilibrium after 3 d (figure 3(E)). Since the compaction of collagen I was isotropic, microtopographies were formed with a fixed reduction rate; the width of the transferred microgrooves on hydrogels produced with 20 J g−1 irradiation decreased from 5 and 10 μm to 3.6 and 7.2 μm, respectively. On the other hand, mold patterns were directly transferred to gelatin and collagen peptide hydrogels. As a result, our collagen hydrogels covered a broad range of elasticities and topographies of the native ECM of in vivo soft tissue (figure 3(F)), which were never achieved with conventional dishes, collagen hydrogels, or Matrigel [8, 38].
3.3. Cell responses to elastic and topographical combinational cues of collagen hydrogels
As described above, the developed collagen hydrogels can combine elastic, topographic, and compositional cues. We used collagen hydrogels to investigate cell responses to chemical and physical complex cues, especially soft topographic cues, such as those existing in vivo.
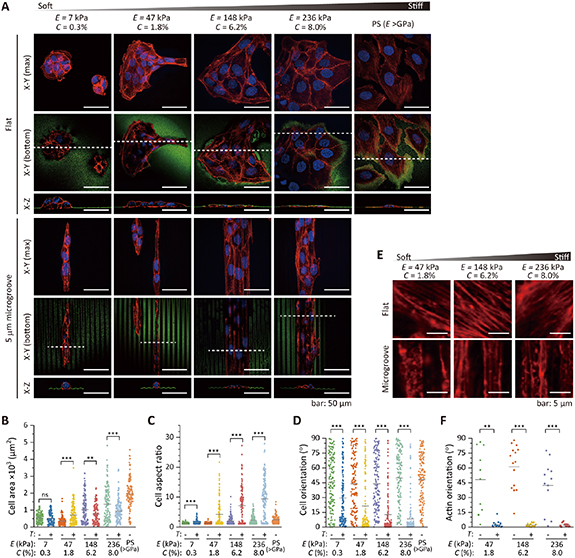
Gelatin was used as the base polymer owing to the broad range of E and precise microtopography without the random fibrous structures of collagen I that could affect the cellular response. We analyzed the morphology and actin cytoskeleton organization of MDCK, HeLa, and 3T3-Swiss cells grown on uncoated 7, 47, 148, and 236 kPa collagen hydrogels produced by ET-RaM at irradiation doses of 10, 20, 40, and 60 J g−1. These E values were selected to cover the range of soft tissues and to ensure precise micropatterning: 7, 47, 148, and 236 kPa roughly correspond to the stiffness of the human kidney, cardiac muscle, skeletal muscle, and skin, respectively [11]. The E values of collagen hydrogels originated from C of 0.3%, 1.8%, 6.2%, and 8.0%, respectively (figure 3(B)). Hydrogels with a flat surface or with microgrooves (width and spacing = 5 μm, height = 2 μm) were prepared for each E. The topographical cues of sub-micrometers to 10 μm were considered to act on the actin cytoskeleton [1]. Within this range, we selected 5 μm microgrooves, owing to its high reproducibility on all the above E values.
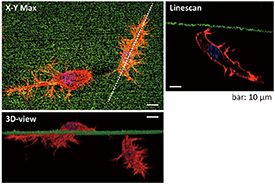
First, we investigated cell responses to elastic cues using flat collagen hydrogels. The morphology of MDCK, HeLa, and 3T3-Swiss cells changed drastically on day 1 after seeding depending on the E (supplementary figures S2–S4). Cells became rounded and formed aggregates over 3 days on the softest 7 kPa (C = 0.3%) hydrogels (figure 4(A) and supplementary figures S3, S4). We found that MDCK and HeLa cell aggregates partially infiltrated the 7 kPa hydrogels (figure 4(A) and supplementary figure S3). 3T3-Swiss cells extended pseudopodia and actively migrated into these hydrogels (figure 5 and supplementary figure S4), which were similar to their movement between micropillars [39, 40]. On the contrary, with increasing E (i.e. with increasing C), these cells chose to stay atop the collagen hydrogels and gradually flattened and spread over a larger area (figures 4(A), (B) and supplementary figures S2–S4). The morphology of HeLa cells became more spindle-shaped with increasing E. The morphology on stiff collagen hydrogels was different from that observed on a PS dish. The flattened cells and aggregates adhered to the hydrogels. Proliferation over 3 days was independent of E and comparable to that of cells grown on a PS dish (supplementary figure S5).
Figure 4. (A) Confocal fluorescence images of nuclei (blue) and actin (red) staining in MDCK cells grown on hydrogels with a flat surface or with 5 μm microgrooves, and on a conventional polystyrene (PS) cell culture dish on Day 3. The hydrogel and PS surfaces were visualized using fluorescent microspheres (green). (B)–(D) Cell area (B), morphological aspect ratio (C), and orientation (D) of MDCK cells on hydrogels with various elasticity (E) values and a flat surface (T:—) or 5 μm microgrooves (T: +) and on a PS dish. (E) Actin cytoskeleton beneath the nucleus (red) and (F) actin orientation of MDCK cells on collagen hydrogels on Day 3. Cells on 7 kPa hydrogels were excluded from analyses due to their multi-layer complexity. Inserted bars in (B)–(D) and (F) indicate the mean values, and statistical significance was assessed with Welch's t-test. ns, not significant (P ⩾ 0.05); **P < 0.01, ***P < 0.001 (supplementary table S1).
Download figure:
Standard image High-resolution imageFigure 5. Confocal fluorescence micrographs of nuclei (blue) and actin (red) staining in 3T3-Swiss albino cells that migrated into collagen hydrogels prepared by ET-RaM on Day 3 (E = 7 kPa, C = 0.3%). The hydrogel surface was visualized using fluorescent microspheres (green).
Download figure:
Standard image High-resolution imageNext, we combined specific topographic cues with elastic cues from collagen hydrogels. Microgrooves (width and spacing = 5 μm, height = 2 μm) were formed on the collagen hydrogels with the same E (7, 47, 148, and 236 kPa) as the flat ones. Interestingly, these soft topographic cues induced all cell types to spread and become aligned parallel to the microgrooves, even on the softest 7 kPa (C = 0.3%) hydrogels, where cells formed aggregates on the flat surface (figure 4(A) and supplementary figures S3, S4), except for some 3T3-Swiss cells that migrated into the hydrogels. The morphological aspect ratio and orientation angle relative to the longitudinal direction of the microgrooves significantly differed from those of cells on flat hydrogels (figures 4(C), (D) and supplementary figures S2–S4). These results indicate that the cell morphology conformed to the microtopographic cues even when they are as soft as in vivo ECM. Stiffer hydrogels structurally constrained the alignment of the cells effectively, which was clearly shown by the higher aspect ratio and smaller variation in the orientation angle of MDCK and 3T3-Swiss cells (figures 4(C), (D) and supplementary figures S2, S4). In contrast, the response of HeLa cells to topography was independent of E due to the drastic morphological change observed on both flat and micropatterned collagen hydrogels (supplementary figure S3). The organization of the actin cytoskeleton was also affected by the soft topographic cues, with actin filaments in MDCK, HeLa, and 3T3-Swiss cells aligned parallel to the microgrooves (figures 4(E), (F) and supplementary figures S3, S4). The effect of the microgrooves on the actin filament orientation was independent of the E values, unlike that on the cell morphology.
Collagen hydrogels with tunable elasticity and microtopography can be utilized to induce and control diverse cell behaviors in vitro. Figure 6 shows rat cardiomyocytes and C2C12 mouse myoblasts seeded onto collagen hydrogels. Both cells adhered well to the hydrogels without any reagent coating and were aligned parallel to the microgrooves. Aligned sarcomeres (the actin-based internal structure) that drive muscle contraction were formed in cardiomyocytes (figure 6(A)). C2C12 myoblasts formed aligned myotubes after culture in differentiation medium for 4 days (figure 6(B)). These cells reacting to soft topographic cues recapitulated their morphology and cytoskeleton as observed in vivo.
Figure 6. (A) Confocal fluorescence micrograph of nuclei (blue) and sarcomeric α-actinin (green) staining in cardiomyocytes grown on microgroove-patterned collagen hydrogels (red) prepared by ET-RaM (E = 236 kPa, C = 8.0%, width and spacing = 5 μm, height = 2 μm). (B) Confocal fluorescence micrographs of nuclei (blue) and actin (red) staining in C2C12 myotubes grown on microgroove-patterned collagen hydrogels prepared by ET-RaM (E = 148 kPa, C = 6.2%, width and spacing = 5 μm, height = 2 μm).
Download figure:
Standard image High-resolution image4. Discussion
We developed a simple yet effective ET-RaM technique suitable for the production of collagen hydrogels using only ECM-derived purified collagens, collagen I or hydrolyzed collagens (gelatin or collagen peptide), and water (figure 1). A distinguishing feature of the collagen hydrogels created by ET-RaM is the preservation of the biological functionality of collagen, unlike conventional cross-linking methods using chemical cross-linkers that alter cytocompatibility and biodegradability [8], and damaged cell-binding motifs in collagens [12–14]. The chemical structures and amino acid composition ratios were almost unchanged by ET-RaM (figure 2(A) and supplementary table S2). The obtained collagen hydrogels can be used for cell culture without any sterilization process, which may alter the hydrogel properties [8] because the irradiation step in ET-RaM not only induces cross-linking but also sterilizes the sample. In addition, owing to the enzyme-mediated degradability (figure 2(B)), the cultured cells on/in the collagen hydrogels can be recovered by digesting the hydrogels using collagenase.
The collagen hydrogels have tunable E from 1 kPa to 236 kPa, covering the elasticity of a broad range of soft tissues (figure 3(A)). We found a power-law correlation between E and the final collagen concentration in hydrogels, C (figure 3(B)), similar to the reported correlation between in vivo collagen concentration and the E of bulk tissue [10]. The results indicate that collagen hydrogels have their elasticity originating from the matrix density, that is, providing compositional and elastic combinational cues similar to those of the native ECM. The volume of collagen I hydrogels was isotropically reduced during soaking in PBS at 37 °C (figure 3(E)), similar to the contraction displayed by a fibroblast-containing collagen I hydrogel [41, 42]. Raub et al reported that fibroblasts concentrated physically cross-linked collagen hydrogels to ∼15% by 16 days of cell culture [42]. In our hydrogels, we believe that collagen I molecules are held in place by uniformly induced cross-linking, forming locally dense cores that promote fibril contraction. The final concentration of 8–157 kPa collagen I hydrogels was 6%–14% (figure 3(B)), which cannot be achieved with a conventional physically-cross-linked (by neutralization and heating) collagen I hydrogel (<0.5%) and is comparable to that in vivo [42].
Moreover, artificial micropatterns can be formed on the hydrogel surfaces by ET-RaM. The softest micropatterned hydrogel had an E of 1.2 kPa, but the patterns easily collapsed due to its fluidity (figure 3(D)). Precise micropatterning (⩾1 μm) was achieved with high reproducibility on hydrogels with E ⩾ 7 kPa (figure 3(C)). Finally, collagen hydrogels prepared by ET-RaM covered a wide range of elasticities and topographies, such as those existent in vivo (figure 3(F)). Although physical collagen hydrogels formed via self-assembly can also be patterned by imprinting, it is challenging to retain the transferred patterns due to its fluidic nature, as experienced with our collagen hydrogel with an E of 1.2 kPa (figure 3(D)). Thus, the reported pattern sizes are larger than several dozens of micrometers [43, 44]. Precise nano/micropatterning of collagens requires mechanical stabilization by chemical cross-linkers and drying [45, 46] or coupling with photo-reactive materials for adopting the photolithography technique [47, 48]. To the best of our knowledge, our ET-RaM, for the first time, accomplished precise control of both E and microtopography of collagen hydrogels without employing any chemical reagents.
We investigated the responses of MDCK, HeLa, and 3T3-Swiss cells to specific topographic cues as soft as the ECM of in vivo soft tissue by using micropatterned collagen hydrogels with an E of 7, 47, 148, and 236 kPa (corresponding to C of 0.3%, 1.8%, 6.2%, and 8.0%, respectively). We demonstrated that the cells responded to elastic cues and changed their morphologies from a round to a flattened shape with increasing E (figure 4 and supplementary figures S2–S4). Depending on E, that is, the density of the collagen network, C, cells chose to stay on top of the collagen hydrogels or spontaneously migrate into them (especially 3T3-Swiss cells) as they can deform the biodegradable collagen hydrogels (figures 4 and 5, and supplementary figures S3, S4). These hydrogels can be used to study cell-ECM interactions in both 2D and 3D. Moreover, we found that HeLa cells became spindle-shaped with increasing E. From the obtained aspect ratio and orientation angle of the cells, this morphological change was considered to be induced between 47 kPa (C = 1.8%) and 148 kPa (C = 6.2%). Epithelial-mesenchymal transition (EMT) would be induced on collagen hydrogels, similar to the report using PA hydrogels [49].
We also demonstrated that microgrooves (width and spacing = 5 μm, height = 2 μm) patterned on 7, 47, 148, and 236 kPa affected cell morphology (figure 4 and supplementary figures S2–S4). These soft topographic cues induced all cell types to spread and become aligned parallel to the microgrooves, even on 7 kPa hydrogels, where cells formed aggregates on the flat surface. We also found that stiffer microgrooves on collagen hydrogels aligned cells more effectively, except HeLa cells, that underwent EMT-like morphological changes. The obtained results support our view that the elasticity of the micro/nanostructures can serve as a parameter for fine-tuning the effectiveness of topography as a structural constraint of cellular morphology [1]. The actin cytoskeleton organization was also affected by the soft topographic cues of collagen hydrogels. In contrast to the cell morphology, actin filament orientation was independent of the stiffness of the microgrooves. Although we evaluated the actin filament orientation from the projected image of the actin cytoskeleton, the 3D morphology of the actin cytoskeleton might reflect the effect of stiffness. The actin cytoskeleton not only supports changes in cell morphology and migration, but also serves as a determinant of cell fate. Our findings suggest that ECM topographies of in vivo soft tissue, even when they are as soft as several kPa, affect cell migration, proliferation, and differentiation.
We also demonstrated that micropatterned collagen hydrogels can align muscle cells. Rat cardiomyocytes were aligned with aligned sarcomeres, which drive muscle contraction, and C2C12 myoblasts formed aligned myotubes. Collagen hydrogels can therefore be utilized to induce and control diverse cell behaviors in vitro.
Although the influence of elasticity and microtopography of cell culture substrates have been extensively studied using PDMS and PA hydrogels, this study offers new insights that could be obtained by novel collagen hydrogels that can combine elastic, topographic, and compositional cues that recapitulate the native ECM properties. Importantly, our collagen hydrogels have a positive correlation between C (i.e. density of cell adhesion sites) and E, similar to the reported correlation between in vivo collagen concentration and the E of bulk tissue [10]. Thus, the cell responses to elasticity and topographic cues from collagen hydrogels can be different from those obtained using protein-coated artificial materials. Comelles et al enabled the combination of elastic and topographic cues from PA hydrogels and reported 3T3 cells elongated on 5 μm microgrooves with an E of 13, 37, and 145 kPa [28]. The E values and micropatterns were similar to our experiments; however, their results did not show clear dependence of cell alignment on elasticity, as we found in this study. We believe that this difference was caused by the cell adhesion sites independently distributed from the mechanical cues on PA hydrogels, considering that they obtained different results with two different functional densities of fibronectin [28]. This complexity induced by different densities of coated proteins has recently been reported [29, 30]. In addition, the migration of cells into the matrix cannot be observed with PA hydrogels or PDMS. While PA hydrogels used after protein coating are advantageous for understanding the role of mechanical cues and the density of cell adhesion sites separately, our collagen hydrogels recapitulate a critical feature of in vivo ECM, specifically, the elasticity and topography originating from the distribution of the ECM-derived macromolecules. The superiority of our collagen hydrogels will be demonstrated by further research, such as by comparing induced cellular behavior with that observed on protein-coated conventional gels and with that observed in vivo. The combined use of collagen hydrogels and PA hydrogels will elucidate the synergetic effects of the chemical and mechanical properties of the ECM on cell function and fate. Revealing how ECM guidance cues are integrated and transduced into the nucleus to drive the functional response using collagen hydrogels is an important topic for future investigations.
5. Conclusion
We have developed the ET-RaM technique and produced novel collagen hydrogels that can combine elasticity, topography, and composition and reproduce the positive correlation between C (i.e. density of cell adhesion sites) and E in ECM. Without altering the amino acid composition ratio, cytocompatibility, and biodegradability, ECM-derived collagen I and hydrolyzed collagen (gelatin or collagen peptide) solutions changed into hydrogels with tunable elastic moduli covering a broad range of soft tissues (E = 1–236 kPa) originating from the final collagen density in the hydrogels (C = 0.3%–14%) and precise microtopographies (⩾1 μm). Using collagen hydrogels, we demonstrated various cell responses that could not be observed with conventional cell culture materials, especially clear dependence on E (i.e. C) on cell behaviors such as spontaneous migration into hydrogels and cell alignment induced by microtopography. In addition to reducing in vivo and in vitro behavioral disparity of cells, our collagen hydrogels may be useful for strategically inducing and controlling cell function and fate in tissue formation, maintenance, regeneration, and repair [1–5], and contribute to applications in tissue engineering and regenerative medicine.
Acknowledgments
The authors thank Ms Ryoko Mezaki (QST), Ms Noriko Uchida (QST), Ms Noriko Tawara (QST), and Ms Bin Jeremiah Duenas Barba (Philippine Nuclear Research Institute) for technical assistance. We would like to thank Editage for the English language editing. T G O was supported by the Japan Society for the Promotion of Science KAKENHI (Grant Nos. JP26790070 and JP18K18390) and the JST ACT-X (Grant No. JPMJAX2014). K O was supported by a PRESTO (Grant No. JPMJPR17P3) from the JST. H M was supported by an AMED PRIME (Grant No. JP18gm5810012).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
T G O, K O, A K, and M T are co-inventors on a filed patent application related to this work.