Abstract
Cartilage defects are among the most difficult diseases to cure in clinic. Due to the limited regeneration capacity of chondrocytes, cartilage regeneration is very difficult. Tissue engineering is a potential strategy for cartilage regeneration. The choice of scaffold is a key factor for the successful construction of tissue engineering cartilage. In this research, we successfully constructed the silk/silk fibroin/gelatin/polylactic acid porous microspheres (S/SF/G/PLLA-PMs) scaffold, then further evaluated the physical and chemical properties and biocompatibility of the composite cartilage tissue in vitro and in vivo, also the long-term survival of the composite cartilage in large animals was carried out. The research results showed that S/SF/G/PLLA-PMs composite scaffold had good biocompatibility. The addition of L-polylactic acid porous microspheres (PLLA-PMs) could significantly enhance the mechanical strength of the scaffold and achieve a multi-level pore structure. After 4 weeks of culture in vitro, composite cartilage could be constructed. Further immunohistochemical results showed that S/SF/G/PLLA-PMs scaffold could increase the long-term stability of the composite cartilage transplantation in vivo.
Export citation and abstract BibTeX RIS
1. Introduction
Cartilage defects are one of the common clinical diseases. Due to the lack of blood vessels in the cartilage tissue, the ability of chondrocytes to regenerate is limited [1, 2]. As a result, large defects often fail to heal completely, eventually causing pain or even disability. Thus, tissue engineering cartilage came into being. Tissue engineering cartilage has developed rapidly in recent years, and the scaffold material as one of the three key elements in tissue engineering plays an important role in the development of tissue engineering cartilage. Due to the special requirements of cartilage on scaffold materials the composite construction of cartilage scaffolds with multiple materials is still a hot research topic in tissue engineering scaffolds.
Degummed silk (S) is a natural polymer material with good mechanical strength, and can be used as the skeleton structure of the scaffold. The RGD ligand (Arg-Gly-Asp) which is defined as signal that can be recognized by the integrin receptor in the cell membrane further promoting cell differentiation, proliferation, and adhesion naturally present on the soluble silk fibroin (SF) of wild silkworm [3], a further purification product of degummed silk, which is more conducive to cell adhesion and can be used as a scaffold filler. On the other hand, gelatin (G) is a derivative of collagen which has lower immunogenicity than collagen. And G also contains integrin binding site for cell adhesion, migration and differentiation. More importantly, G is derived from natural proteins and has similar components to the extracellular matrix, which can provide cells with a highly similar living environment to the body [4–6]. However, due to its brittleness, high water solubility, low strength, and uncontrollable degradation rate, G is rarely used alone [7]. Thus, a combine of these two natural polymers has been developed as a possible compromise. Some previous studies have shown that SF/G blends might be used as films, scaffolds and hydrogels for tissue engineering and drug-controlled release [8–13]. However, this composite material still has the problems of insufficient mechanical strength and difficulty of cell migration into the scaffold [8, 14].
Poly-L-lactic acid (PLLA) is a synthetic polymer material that has been approved by the US Food and Drug Administration for clinical use [15]. It has been widely used in tissue engineering scaffold according to its characteristics of non-toxic, non-irritating, biodegradable absorption, high mechanical strength, good plasticity, etc [16]. However, the hydrophobic nature of PLLA surface affects it as an ideal scaffold material [17]. Porous microspheres (PMs), as a microcarrier form of tissue engineering scaffold, they have been widely used in the construction of cartilage tissue engineering, which is mainly used for loading drugs and cells [18, 19]. Compared with other forms of scaffolds, PMs have many advantages. Not only can they increase the mechanical strength of the scaffold, the porous structure provides a larger surface area for cell adhesion and a larger void space for cartilage tissue regeneration [20]. Also, degradation products are less comparing with the non-PMs under the same volume conditions, and it is more suitable as a cartilage tissue engineering scaffold.
Therefore, PLLA-PMs were prepared. We hope that the mechanical strength of the composite scaffold can be increased by adding PLLA-PMs to the S/SF/G composite scaffold. At the same time, the multilevel porous structure of the composite scaffold is realized through the addition of PLLA-PMs. Further to achieve the purpose of chondrocyte growth and long-term retention. It is also hoped to reduce the hydrophobicity of PLLA-PMs by mixing with G, which has been described in many studies [17, 21]. Thus, we have successfully prepared S/SF/G/PLLA-PMs composite scaffold, patent number: CN201910119602. In this study, we compared S/SF/G and S/SF/G/PLLA-PMs scaffolds in vitro and in vivo to analyze the preliminary effect of the incorporation of PLLA-PMs on the scaffold formation of cartilage and stability in vivo.
2. Materials and methods
2.1. Materials
All the experiments in the present study were performed according to the Declaration of Helsinki. Ethical approval was obtained from the Ethical Committee of the Jilin University First Hospital. Carboxyl-terminated PLLA (MW 5000) (Sigma, USA), Type VI Collagenase (Nordmark, Germany); SF (Beijing Shengnuodede Medical Technology Co., Ltd, China); dichloromethane (Tianjin University Kewei Company, China); anhydrous ethanol (No. 6 Tianjin Chemical Reagent Factory, China); sodium hydroxide (Tianjin Fengchuan Chemical Reagent Technology Co., Ltd, China) Dulbecco's modified Eagle medium (DMEM)/high glucose culture medium (Hyclone, USA), fetal bovine serum (Clack, Australia), penicillin-streptomyces–amphotericin B mixed solution (Solaribo, China), Collagen type II Rabbit Polyclonal antibody (Proteintech, USA), cell counting kit-8 (CCK-8) kit (Invigentech, USA), and calcein-AM/PI double-staining kit (Solaribo, China). All other chemical reagents were obtained from commercial sources.
2.2. PLLA porous microspheres preparation
Carboxyl-terminated PLLA (Mw: 50 000) powder was dissolved in dichloromethane to prepare an oil phase solution with a concentration of 2.5%; NH4HCO3 was dissolved in deionized water to prepare an internal aqueous phase with a concentration of 1% NH4HCO3. Under high speed disperser stirring, the internal aqueous phase solution was added drop-wise to the prepared oil phase solution with a syringe pump. After the addition was complete, stirring was continued for 2 min to form W1/O emulsion. Quickly transfer W1/O emulsion into a stirred 0.1% PVA aqueous solution to form a double emulsion (W1/O/W2). Stir for 3 h to volatilize the organic solvent and solidify the PMs. The PMs were collected and washed with deionized water three times. Resuspend the PLLA PMs with 0.1 mol l−1 NaOH solution at room temperature for 20 min, and then washed three times, pre-frozen in separate packaging, and freeze-dried to obtain porous microsphere solid powder.
2.3. Scaffolds preparation
Physical blending was applied for the preparation of S/SF/G/PLLA-PMs scaffold. The detailed steps refer to the patent. Here, a brief description was introduced. Degummed silk was evenly spread into a net-like structure in the mold. The G was dissolved in deionized water so that the concentration of the G solution was 30%. The PLLA PMs were blended with SF solution and G solution. The weight of PMs in 15 ml blend was 0 or 200 mg. SF concentration was 3 mg ml−1, and G concentration was 7%. Fifteen milliliters of the solution was injected into a mold containing degummed silk, and freeze-dried to obtain a composite scaffold material. The composite material was immersed in 1% carbodiimide (EDC) for 8 h, washed with water, and lyophilized to finally obtain a composite tissue engineering scaffold material with or without PLLA PMs.
2.4. Surface morphology
Gold was sprayed on the S/SF/G and S/SF/G/PLLA-PMs scaffolds, and the surface of the scaffolds was observed under an acceleration voltage of 5 kV through a scanning electron microscope (SEM; Hitachi S3400, Japan). The diameter and pore size of PLLA-PMs were obtained by analyzing SEM images of microspheres.
2.5. Determination of porosity
The porosity of S/SF/G and S/SF/G/PLLA-PMs scaffolds was measured using an ethanol infiltration method [22]. The results were calculated as following:
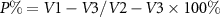
where V1 is the known volume of ethanol before immersing lyophilized scaffold, V2 is the total volume of ethanol and immersed scaffold after the pores are completely immersed in ethanol, and V3 is the volume of the ethanol remaining in the tube after removing the scaffold.
2.6. Determination of swelling
Dry samples of scaffolds weighed W1 were incubated in 10 ml PBS (pH 7.4) at 37 °C for 24 h. After removing the unabsorbed solution on the surface of the scaffolds with filter paper, the weight of the wet samples was recorded as W2. The swelling fold of the scaffolds was calculated as following:
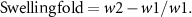
2.7. Fourier transform infrared spectroscopy (FTIR)
Structural analysis of the scaffolds was performed using FTIR spectroscopy (6800–50/NEXUS, Nicolet, America) in the attenuated total reflection mode. Samples of S/SF/G and S/SF/G/PLLA-PMs scaffolds were prepared by freeze-drying. Transmittance readings were measured by scanning at a resolution of 0.09 cm−1 in the 4000–500 cm spectral region.
2.8. Young's modulus
A universal material testing machine (Measuring range: 0–5KN, accuracy: within ±0.5% of the indicated value, speed range: 0.001–500 mm min−1) was used to evaluated the Young's Modulus of the S/SF/G and S/SF/G/PLLA-P-MPs scaffolds, in triplicate for each scaffold. The scaffolds were fabricated into samples with a cuboid (10 × 10 × 5 mm) and attached to the material testing machine. A compression load was applied at a compression speed of 1 mm min−1 until failure was observed. Simultaneously, Young's Modulus were obtained.
2.9. Cell isolation and culture
Fresh ear cartilage was harvested from the ears of 4 week old pigs under sterile conditions, digested with 0.25% trypsin plus 0.02% EDTA at 37 °C for 60 min, and then cut into pieces of about 1 mm3. The cartilage pieces were washed with PBS (supplemented with 100 U ml−1 penicillin and 100 mg ml−1 streptomycin) three times and digested using 0.2% collagenase VI in serum-free DMEM at 37 °C for about 8–10 h. The digested tissue was filtered through a 200 mm nylon mesh and centrifuged at 1000 rpm for 10 min to obtain chondrocytes after removal of the supernatant. The chondrocytes were re suspended in DMEM supplemented with 15% FBS, 100 U ml−1 penicillin, and 100 mg ml−1 streptomycin and seeded in a culture flask at a density of 5 × 105 cells ml−1. The culture flask was incubated in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. The culture medium was refreshed every 2–3 d, and chondrocytes were subcultured when they reached 80%–90% confluence. Chondrocytes at passage 2 were used for the following experiments.
2.10. Cell seeding on scaffolds
Prior to use, the scaffolds were sterilized by Co60 irradiation. Before inoculating chondrocytes, the S/SF/G and S/SF/G/PLLA-PMs scaffolds were sterilized and moistened. In short, the scaffolds were made into cube samples with a size of 0.7 × 0.7 × 1.5 mm. Then, the scaffolds were immersed in ethanol for 30 min, and finally washed three times with PBS for 5 min each time. The scaffolds were immersed in DMEM/High glucose supplemented with 10% FBS, 100 units ml−1 penicillin, and 100 mg ml−1 streptomycin overnight. Then absorbed culture medium. Subsequently, cell suspension with the concentration of 1 × 108 ml−1 was added to the scaffolds, about 100 μl/scaffold. Then the scaffolds were further incubated in an atmosphere of 5% CO2/95% humidified air at 37 °C for 5 h to attach the cells. After the cells adhered to the scaffold for 5 h, 1–2 ml of culture medium was added to immerse the scaffold–cell construct. The culture medium was refreshed every 2–3 d.
2.11. Scaffold cytotoxicity
The cytotoxicity tests of S/SF/G and S/SF/G/PLLA-PMs scaffolds were performed using the CCK-8 method. Initially, the scaffolds extract was prepared by soaking a sterile scaffold in DMEM supplemented with 10% FBS at 37 °C for 72 h. After the extraction was completed, the filter was used for sterilization, and the scaffold extract was used as the culture medium for the experimental group. Additionally, DMEM containing 10% FBS was utilized as the culture medium of control groups. The chondrocytes were seeded into the wells of a 96-well culture plate at a density of 3 × 103 cells/well, and then 100 μl culture media of the control or experimental group was added (n = 6 per group). The culture plate was transferred to the incubator, and chondrocytes were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. After culturing for 1, 3, 5, and 7 d, 10 μl CCK-8 solution was added to each well and incubated for 2.5 h. An enzyme labeling instrument (Model 680, Bio-Rad, USA) was used to measure absorbance at an excitation wavelength of 450 nm. The experiments were independently repeated in quintuple [23].
2.12. Chondrocyte proliferation
CCK-8 was used to measure the proliferation of the chondrocytes seeded on the scaffolds (1 × 105 cells/scaffold). After incubation for 1 and 5 d, the cell-seeded scaffolds were relocated to a new 96-well plate (1 scaffold in 1 well) with 10 μl CCK-8 solution added. After incubating with CCK-8 at 37 °C for 2.5 h, the absorbance value of the scaffolds was detected at 450 nm by using an enzyme labeling instrument. The experiments were independently repeated in triplicate.
2.13. Cell adhesion and spreading
The viability of chondrocytes in the scaffolds was evaluated by using live/dead assay. Cells were fluorescently stained by using calcein-AM for live cells and propidium iodide (PI) for dead cells. Presoaked the scaffolds (7 × 7 × 1.5 mm3) in the culture medium in a confocal special Petri dishes for 30 min, and then seeded the chondrocytes into scaffolds (2 × 105 cells/scaffold). After 1, 7 d, scaffolds were washed with PBS and stained with calcein-AM and PI for 30 min at room temperature. Scaffolds were washed again with PBS, and fluorescent images were acquired using the confocal microscope.
The cell morphology and extra cellular matrix distribution on the scaffolds were further studies by SEM observation. Briefly, at day 7 and 30 of cultures, the cell/scaffold constructs were washed twice with PBS, and then fixed in 2.5% glutaraldehyde for 4 h at room temperature. After rinsing with PBS three times, the samples were sequentially dehydrated with gradient ethanol and freeze-dried, and then sprayed with gold for SEM observation.
2.14. In vitro cartilage construction
Cell-scaffold complexes (n = 12 per group) were constructed with pig auricular chondrocytes density of 6 × 107 ml−1 cells, nearly 200 μl onto each scaffold followed by 5 h incubation to promote cell adhesion [24, 25]. All the samples were statically cultured in DMEM/High glucose supplemented with 10% FBS, 100 units ml−1 penicillin, and 100 mg ml−1 streptomycin for 4 weeks in vitro. Medium was refreshed every 2–3 d.
2.15. Real-time fluorescent quantitative polymerase chain reaction (RT-qPCR)
Cartilage specific genes were further analyzed by RT-qPCR to evaluate the in vitro cartilage formation. The total ribonucleic acid (RNA) was extracted with Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions and reverse transcribed into complementary DNA (cDNA) using a real-time PCR kit (TransGen Biotech, Beijing, China). RT-qPCR was performed using the SYBR Green I PCR master mix kit (TAKARA, Beijing, China). The amplification protocol was carried out as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 1 min, and 72 °C for 10 min. The PCR primers for specific mouse genes are as follows: GAPDH(F:5'-CTGCCCCTTCTGCTGAT GC-3',R:5'-TCCACGATGCCGAAGTTGTC-3'), SRY (sex determining region Y)-BOX 9 (SOX9) (5'-ACTACACAGACCAGAACTCCG-3',R:5'-TGAAGG TGGAGTAGAGCACAGAGC-3'), collagen type II (COL2A1) (F:5'-TGGGCAGAGGTATAATGATAAGGA-3', R:5'-CTTCACAGATTATGTCGTCGCAG-3'), aggrecan (ACAN)(F:5'-TGGGAAAGAAGACTTGGC TGG-3', R:5'-CTCCACCAATGTAGTATCCACGA-3').
2.16. In vivo implantation
After 4 weeks, constructs (n = 12 per group) were autologously transplanted into individual subcutaneous pockets of each pig and harvested for analysis 2, and 8 weeks after implantation.
2.17. Histological and immunohistochemical examinations
Samples from each group were harvested after 4 weeks in vitro and 2 or 8 weeks in vivo. The specimens were fixed with 10% buffered formalin in PBS for 24 h, embedded in paraffin, sectioned into 5 mm sections, the sections were stained with Hematoxylin-Eosin (HE) staining for histological analyses, and Safranin O-Fast Green Staining to visualize the glycosaminoglycans (GAG) deposits and type II collagen expression was detected by using a rabbit anti pig type II collagen monoclonal antibody followed by horseradish peroxidase-conjugated anti-rabbit antibody. Images of the type II collagen positive area were analyzed by using ImageJ (NIH), as reported in the published study [9].
3. Statistical analysis
Data was expressed as the mean ± standard deviation. Statistical significance was evaluated by using two tailed T-Test analysis of variance in SPSS Statistics 23.0 software. A value of p < 0.05 was considered statistically significant.
4. Results
4.1. Characterization of the structure
4.1.1. Macroscopic appearance
The macroscopic appearance of S/SF/G and S/SF/G/PLLA-PMs scaffolds was shown without using microscopy, we found S/SF/G/PLLA-PMs scaffolds to be pure white, while S/SF/G scaffolds were yellowish-white, and white PLLA-PMs could be observed in S/SF/G/PLLA-PMs scaffolds (figure 1).
Figure 1. The macroscopic appearance of S/SF/G and S/SF/G/PLLA-PMs scaffolds. (a) S/SF/GEL scaffold. (b) S/SF/G/PLLA-PMs scaffold.
Download figure:
Standard image High-resolution image4.1.2. Scanning electron microscopy
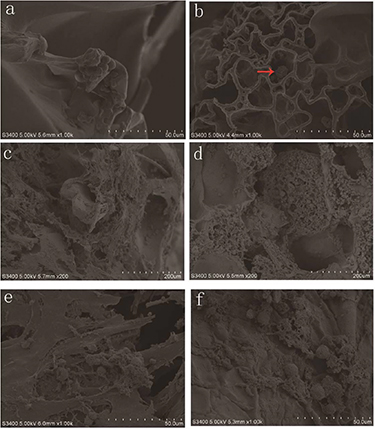
Herewith, the PLLA-PMs were prepared using the reported procedure, which contained both micro- and macro-interconnected pores (figure 2(a)). PLLA-PMs exhibited dense pore distribution. The mean particle size was from 100 to 300 μm; average 245 ± 35 μm, pore size was 20–40 μm, mean 27 ± 4.8 μm. We used SEM to study the internal structure of the S/SF/G and S/SF/G/PLLA-PMs scaffolds. The SEM images were presented in figures 2(b) and (c). Both the scaffolds had porous network structures and good connectivity between the pores. The pore sizes ranging from 80 to 600 μm, average 307 ± 142 μm. However, in S/SF/G/PLLA-PMs scaffolds, part of the pore structure was occupied by PLLA-PMs.
Figure 2. SEM observation of Surface morphology of PLLA PMs, S/SF/G and S/SF/G/PLLA-PMs scaffold. (a) PLLA PM with large number of interconnected pores. (b) S/SF/G scaffold. (c) S/SF/G/PLLA-PMs scaffold.
Download figure:
Standard image High-resolution image4.2. Determination of porosity
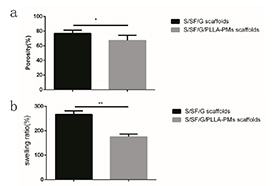
The porosity was measured using the reported procedure. The porosity of the S/SF/G scaffolds was 77.00 ± 1.956%, while the porosity of S/SF/G/PLLA-PMs scaffolds was decreased to 67.25 ± 3.174% due to the corporation of PLLA-PMs (figure 3(a)).
Figure 3. Porosity and swelling ratio. (a) The porosity of the S/SF/G scaffolds was 77.00 ± 1.956%, while the porosity of S/SF/G/PLLA-PMs scaffolds was decreased to 67.25 ± 3.174% (*p ⩽ 0.05). (b) The swelling ratio of S/SF/GEL scaffold was 267.47 ± 11.93%, while the swelling ratio of S/SF/G/PLLA-PMs scaffold was decreased to 175.57 ± 9.61%. Swelling ratio of S/SF/G/PLLA-PMs scaffold was significantly lower than that of S/SF/G scaffold (**p ⩽ 0.01).
Download figure:
Standard image High-resolution image4.3. Determination of swelling
After incubating for 24 h, the swelling ratio of S/SF/G scaffold was 267.47 ± 11.93%, while the swelling ratio of S/SF/G/PLLA-PMs scaffold was decreased to 175.57 ± 9.61% due to the corporation of PLLA-PMs(figure 3(b)).
4.4. Fourier transform infrared spectroscopy (FTIR)
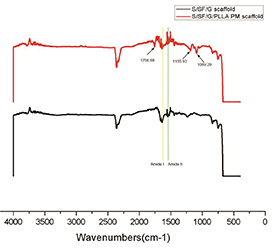
FITR spectra showed that FITR spectra showed that both scaffolds had peaks around 1620 ∼ 1640 cm−1, 1520 ∼ 1540 cm−1 respectively corresponding to amide I and amide II. These regions are the major characteristic peaks of the protein-based substances that define the scaffold composition. The characteristic peaks of PLLA at 1758.68 cm−1 (stretching C = O), 1185.93 cm−1(stretching –C–O–), 1089.20 cm−1 (tension –C–O–) were observed in S/SF/G/PLLA-PMs scaffold, indicating that the characteristic functional group has not changed during the fabricating process of the scaffold (figure 4).
Figure 4. FITR of S/SF/G and S/SF/G/PLLA-PMs scaffold.
Download figure:
Standard image High-resolution image4.5. Young's modulus
The Mechanical tests showed Young's Modulus of S/SF/G and the S/SF/G/PLLA-PMs scaffold in dry condition was 2.304 ± 0.629 and 4.409 ± 0.325 respectively (figure 5(a)). In wet condition, the Young's modulus was 0.6471 ± 0.08358 and 1.452 ± 0.1343, respectively (figure 5(b)). Young's modulus was significantly greater in the S/SF/G/PLLA-PMs scaffold than in the S/SF/G scaffold, which indicated that PLLA-PMs could remarkably improve the mechanical properties of the S/SF/G scaffolds.
Figure 5. Compressive mechanical tests. (a) Young's modulus of S/SF/G scaffolds and S/SF/G/PLLA-PMs scaffolds in dry condition. (b) Young's modulus of S/SF/G and S/SF/G/PLLA-PMs scaffold in wet condition.
Download figure:
Standard image High-resolution image4.6. Scaffold cytotoxicity
Toxicity of the S/SF/G scaffolds and S/SF/G/PLLA-PMs scaffolds were tested by the CCK-8 assay. Chondrocytes were cultured at the leach liquors of scaffolds in the medium for 1, 3, and 5 d. It was observed that most of the cells (>90%) at each leach liquor grew well. The viability of the S/SF/G and the S/SF/G/PLLA-PMs composite scaffolds was not significant compared to the control group during the process of culture, indicating no significant cytotoxicity of both composite scaffolds. Thus, the S/SF/G and the S/SF/G/PLLA-PMs composite scaffolds resulted in excellent biocompatibility (figure 6(a)).
Figure 6. Cell viability and proliferation. (a) Cell viability of pig auricular chondrocytes after 1, 3, and 5 d in contact with conditioned media that has been exposed to the different samples for 72 h. (b) The proliferation of auricular chondrocytes on scaffolds for 1, 3, 5, and 7 d.
Download figure:
Standard image High-resolution image4.7. Cell proliferation
The cell proliferation assay has shown that after 5 d of culture, the cell proliferation rate increased significantly. In the 1, 3, and 7 d of culture, cells seeded on the S/SF/G scaffolds generally seemed to have a higher proliferation rate than that of S/SF/G/PLLA-PMs scaffolds. But at day 5 the proliferation of S/SF/G/PLLA-PMs scaffolds were higher than that of the S/SF/G scaffolds though no statistically significant difference was found (figure 6(b)). Thus, addition of PLLA PMs did not significantly reduce cell proliferation.
4.8. Cell adhesion and spreading
Fluorescent images of live/dead cells showed that chondrocytes had good viability on both the scaffolds and only a few dead cells were observed in these scaffolds (figures 7(a) and (b)). It could appear that at day 1 chondrocytes were well attached on the surface of the two scaffolds (figures 7(e) and (f)). After 7 d culture, the number of cells has increased dramatically and cells on both scaffolds were still in a dispersed state (figures 7(c) and (d)). A few cells entering the PLLA-PMs could be seen after the scaffolds were developed, but most cells were attached to the surface of both scaffolds (figures 7(g) and (h)). The magnified image and 3D image show that the cells were attached to the surface of the microspheres at different depth (figure 7(i)).
Figure 7. Calcein-AM/PI-stained images of chondrocytes cultured on scaffolds after 1 and 7 d. (a) Image of chondrocytes cultured on S/SF/G scaffold after 1 d (green: live cells; red: dead cells). (b) Image of chondrocytes cultured on S/SF/G/PLLA-PMs scaffold after 1 d. (c) Image of chondrocytes cultured on S/SF/G scaffold after 7 d. (d) Image of chondrocytes cultured on S/SF/G/PLLA-PMs scaffold after 7 d. (e) Image of chondrocytes cultured on S/SF/G scaffold after 1 d with scaffold development. (f) Image of chondrocytes cultured on S/SF/GPLLA-PMs scaffold after 1 d with scaffold development. (g) Image of chondrocytes cultured on S/SF/G scaffold after 7 d with scaffold development. (h) Image of chondrocytes cultured on S/SF/G/PLLA-PMs scaffold after 7 d with scaffold development(scale bar: 50 μm). (i) Image of cells attached to the surface of microspheres under high magnification at different depth (scale bar: 20 μm), the last image was the three-dimensional of the microsphere with cells attached to the surface.
Download figure:
Standard image High-resolution image4.9. Scanning electron microscope
The SEM image of the cell morphology on the scaffold was shown in figure 8. After 7 d of culture, on the S/SF/G scaffolds the cells adhered well to the surface of the scaffold and they displayed round (figure 8(a)). On the S/SF/G/PLLA-PMs scaffold, cells did not adhere to the scaffold to the full extent, they gathered and clung to each other and maintained their original shape just like they were in vivo (figure 8(b)).
Figure 8. SEM observation of chondrocyte growth on the scaffolds. (a) Chondrocytes on S/SF/G scaffold after 7 d culture. (b) Chondrocytes on S/SF/G/PLLA-PMs scaffold after 7 d culture (red row indicates chondrocytes). (c) Chondrocytes on S/SF/G scaffold after 4 weeks culture. (d) Chondrocytes on S/SF/G/PLLA-PMs scaffold after 4 weeks culture. (e) Extracellular matrix secretion of chondrocytes on S/SF/G scaffold after 4 weeks culture. (f) Extracellular matrix secretion of chondrocytes on S/SF/G/PLLA-PMs scaffold after 4 weeks culture.
Download figure:
Standard image High-resolution imageWith time, the cells grew more and spread all over the internal surface of S/SF/G scaffolds (figure 8(c)); instead, on the S/SF/G/PLLA-PMs scaffolds, cells were located both inside the PLLA-PMs and along the surface of the scaffolds (figure 8(d)). Cells located on both scaffolds have strong matrix secretion capacity (figures 8(e) and (f)).
4.10. RT-qPCR analysis of composite cartilage in vitro
Cartilage specific genes were analyzed by RT-qPCR to evaluate the cartilage formation after 4 weeks culture in vitro. According to the current results, there was no significant difference on expression levels of cartilage specific genes ACAN, COL2A1, SOX9 between S/SF/G scaffolds and S/SF/G/PLLA-PMs scaffolds (figure 9).
Figure 9. Cartilage-related gene analyses of in vitro engineered tissues at 4 weeks. All expressions of cartilage-related genes showed no significant difference between the two kinds of scaffolds after 4 weeks culture.
Download figure:
Standard image High-resolution image4.11. Histological and Immunohistochemical examinations of composite cartilage in vitro
At 4 weeks, HE staining revealed that a large number of cells were distributed on the scaffolds, cells gather together, and the extracellular matrix deposits initially formed. On S/SF/G/PLLA-PMs scaffolds, the cells were mainly distributed on the surface of the PMs, and few cells enter the interior of the PMs. (figures 10(a)–(d)) Immunohistochemical results showed that both the two scaffolds showed positive aggregation of type II collagen (figures 10(e)–(h)).
Figure 10. Histological and immunohistochemical examinations of composite cartilage in vitro. After cell seeding, all samples at 4 weeks retain their original shape. On S/SF/G/PLLA-PMs scaffolds, the cells were mainly distributed on the surface of the PMs, and few cells enter the interior of the PMs (a)–(d). Immunohistochemical results showed that both the two scaffolds showed positive aggregation of type II collagen (e)–(h).
Download figure:
Standard image High-resolution image4.12. Stability of the cartilage construction in vivo
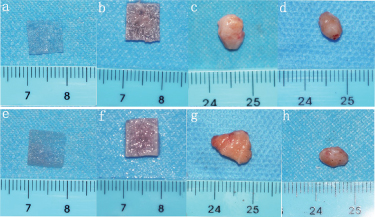
Long-term stability of cartilage construction in vivo was a key factor in determining whether the scaffold material could be used in clinic. After 4 weeks cultures in vitro, all the samples (12/12) formed ivory-white cartilage-like tissue with elasticity (figures 11(a), (b), (e) and (f)). However, it was observed that ivory-white tissue structure was distributed in stripes along the S/SF/G scaffold while not seen in the S/SF/G/PLLA-PMs scaffold. After subcutaneous implantation at 2 and 8 weeks in the mini-pig model. Both samples shrunk to vary degrees over time. They adhered with the surrounding connective tissue. The boundary between harvested samples and surrounding tissues was not very clear and their original shape and size were changed to some degree (figures 11(c), (d), (g) and (h)).
Figure 11. Macroscopic appearance of implanted S/SF/G and S/SF/G/PLLA-PMs scaffold after 2- and 8-weeks durations of implantation. (a) S/SF/G scaffold. (b) Composite cartilage cultured in vitro for 4 weeks of S/SF/G scaffold. (c) 2 weeks after implantation of S/SF/G scaffold composite cartilage. (d) 8 weeks after implantation of S/SF/G scaffold composite cartilage. (e) S/SF/G/PLLA-PMs scaffold. (f) Composite cartilage cultured in vitro for 4 weeks of S/SF/G/PLLA-PMs scaffold. (g) 2 weeks after implantation of S/SF/G/PLLA-PMs scaffold composite cartilage. (d) 8 weeks after implantation of S/SF/G/PLLA-PMs scaffold composite cartilage.
Download figure:
Standard image High-resolution imageHistology showed that compared with S/SF/G scaffolds, S/SF/G/PLLA-PMs scaffolds had a more integrated structure. Both the S/SF/G and S/SF/G/PLLA-PMs scaffolds showed inflammatory reaction, such as lymphocyte aggregation, foreign body giant cell reaction (figures 12(a), (b), (e) and (f)). Safranin O staining showed obvious extracellular matrix deposition for 2 weeks culture in vivo, and a significant decrease in extracellular matrix deposition for 8 weeks. And S/SF/G/PLLA-PMs scaffold has more obvious extracellular matrix deposition than S/SF/G scaffold (figures 12(c), (d), (g), (h), (k), (l), (o) and (p)). Immunohistochemical staining showed intense staining of cartilage-specific collagen II after 2 weeks of transplantation in vivo. The PLLA-PMs were stained darker than the rest part of the scaffold, although statistics showed no significant difference in the ratio of the staining area between the two scaffolds (figures 13(a)–(d) and (i)). At week 8 post transplantation, the type II collagen staining of the S/SF/G and S/SF/G/PLLA-PMs scaffolds reduced significantly. However, compared with S/SF/G scaffold, the S/SF/G/PLLA-PMs scaffold was stained darker, indicating that the PLLA-PMs had a better effect of maintaining long-term stability of cartilage (figures 13(e)–(i)).
Figure 12. Histology examinations of implanted S/SF/G and S/SF/G/PLLA-PMs scaffold after 2- and 8-weeks durations of implantation. (a)–(h) Composite cartilage cultured in vivo for 2 weeks of S/SF/G scaffold. (i)–(p) 8 weeks after implantation of S/SF/G scaffold and S/SF/G/PLLA-PMs scaffold composite cartilage. Green arrows indicated foreign body giant cells, yellow arrows indicated lymphocyte aggregation.
Download figure:
Standard image High-resolution imageFigure 13. Immunohistochemical staining images (scale bar = 500 μm) and higher magnification of Immunohistochemical staining images (scale bar = 200 μm). (a) Type II collagen immunohistochemical staining of S/SF/G scaffold for 2 weeks in vivo. (b) Type II collagen immunohistochemical staining of S/SF/G/PLLA-PMs scaffold for 2 weeks in vivo. (c) Higher magnification of a. (d) Higher magnification of image b. (e) Type II collagen immunohistochemical staining of S/SF/G scaffold for 8 weeks in vivo. (f) Type II collagen immunohistochemical staining of S/SF/G/PLLA-PMs scaffold for 8 weeks in vivo. (g) Higher magnification of e. (h) Higher magnification of image f. (i) Quantitative data of positive type II collagen (*p ⩽ 0.05).
Download figure:
Standard image High-resolution image5. Discussion
In our research, we successfully constructed S/SF/G/PLLA-PMs composite scaffolds. By adding PLLA-PMs to achieve a significant increase in the mechanical strength of the composite scaffold, which reached the mechanical strength of cartilage scaffold 0.2–2 MPa [26]. Further, we successfully constructed composite tissue engineered cartilage in vitro and evaluated its long-term stability in vivo. Our research found that at day 7 in vitro culture, the cells were mainly attached to the surface of G, SF and PLLA-PMs, few chondrocytes migrated into the interior of the PLLA-PMs. After 30 d of in vitro culture, it could be seen that a large number of cells mainly located both inside the PLLA-PMs and along the surface of the scaffolds. The results also showed that there were no significant differences in the effect of the composite cartilage constructed by the two scaffolds on the expression of cartilage-specific collagen type II early after in vivo transplantation. The long-term in vivo transplantation showed that the expression of collagen type II with PLLA-PMs was higher, indicating that the PMs had a certain stabilizing effect on the long-term survival of the composite cartilage after transplantation in vivo. However, more research results are needed in order to prove this finding.
Due to the unique role of cartilage in the human body, the mechanical strength of tissue engineering cartilage is often the key to whether the composite cartilage can be further applied. Studies have shown that degummed silk had high mechanical strength. Gelatin and SF from wild silkworms had a large number of RGD sequences [4, 23–25, 27]. RGD sequence has been proved to be recognized by integrin on the cell membrane to promote cell adhesion [27]. In recent years, there have been a lot of reports on the use of SF RGD sequences to increase cell adhesion and proliferation [23, 25]. Therefore, we applied degummed silk as the mechanical support of the scaffold, and G and soluble SF as the filling material of the scaffold to increase the adhesion and proliferation of chondrocytes. Similar reports have been recorded [14]. However, the results of the other study have shown that the elastic modulus was still in sufficient though degummed silk was mixed [8]. Therefore, PLLA with stronger strength still need to be added to the composite scaffold.
In the previous studies, the microspheres usually used as a drug or cell carrier. Thus, PLGA microspheres with relatively lower mechanical strength and faster degradation rate were often used [28, 29]. Compared with PLGA, PLLA was demonstrated to have greater mechanical strength and longer degradation time. This conclusion could also be confirmed by the integrated PLLA-PMs structure observed in 8-week tissue transplantation in vivo. In addition to improve the mechanical strength of the composite scaffold, the purpose of adding PLLA-PMs in this study was also to attain the purpose of enriching the pore size of the scaffold and achieving multi-level pore size distribution. It has been reported that the pore size of the scaffold has a very significant effect on the proliferation and differentiation of chondrocytes [30, 31]. Previous studies have suggested that small pores could allow higher cell contact and signaling [32]. Our research also found that chondrocytes entering the PMs were more likely to adhere to each other to form a mass which was believed by many other researchers that the aggregation was a prerequisite for chondrocytes to express cartilage-specific matrix [33–37]. The development of cell connections and chondrocytes differentiation was induced during the aggregation process [38]. In addition, hypoxia was regarded as a stimulus that increased chondrocyte phenotype expression. Studies suggested that in the small pore scaffold, due to the increase of the diffusion barrier, the smaller pore size of the three-dimensional aggregation of cells was associated with lower oxygen levels. This might be the condition for the synthesis of cartilage matrix protein in the small pore scaffold [39]. On the other hand, there are a large number of studies that believed that larger pore size scaffolds were more beneficial to the growth of chondrocytes [40, 41]. In fact, chondrocytes cultured on three-dimensional micro porous scaffolds have shown that small pore size could damage cell infiltration and might cause uneven cell distribution throughout the scaffold [36]. Actually, larger pore size would allow a more effective nutrient supply and waste removal, which were a key factor in cell activity. Therefore, chondrocytes cultured in a scaffold with a larger pore size might be affected by better nutrient supply and chondrogenic medium [32]. However, these contradictory results might be due to the complexity of various factors in the three-dimensional culture. These factors might affect the penetration, distribution of cells and the diffusion of nutrients [37, 41]. Therefore, in this study, PLLA-PMs with an average pore diameter of 27 ± 4.8 μm and scaffolds with an average pore size of 307 ± 142 μm were used to form a multi-layered pore size, hoping to promote chondrocyte proliferation and specific protein expression.
In this study, a composite scaffold with mechanically enhanced multi-level pore structure was successfully constructed. Furthermore, composite cartilage was constructed in vitro. It was preliminary proved that adding PLLA-PMs to the S/SF/G scaffold could enhance its mechanical properties and promote the long-term stability of the composite cartilage in vivo to a certain extent. However, there are still deficiencies in research. The effect of the addition of PLLA-PMs on the secretion of chondrocyte extracellular matrix and the effect of chondrocytes migrating into the scaffold still needs to be further quantified. Furthermore, the long-term effects of different cultural time in vitro on the composite cartilage implantation in vivo also need to be further verified. Long-term observation of implantation in vivo still needs to refine research indicators.
6. Conclusions
This study confirmed the feasibility of S/SF/G/PLLA-PMs scaffold as a tissue engineering cartilage scaffold. Studies have shown that PLLA-PMs could enhance the mechanical properties of S/SF/G scaffolds, promote early chondrocyte aggregation. The long-term stability of composite cartilage with PLLA-PMs in vivo was better than that without PLLA-PMs. The scaffold could be utilized to the regeneration of cartilage. The study of the inflammatory response of PLLA-PMs in large animals will more fully confirm the therapeutic potential of the scaffold.
Acknowledgments
Thanks to Zhimi Zhou and Yuzhou Yang from Biomedical Engineering Research of Chinese Academy of Medical Sciences for scaffold fabrication. Thanks to the supported by the CAMS Innovation Fund for Medical Sciences [Grant Number CAMS-2017- I2M-1-007].