Abstract
Three-dimensional (3D) printing enhances the production of on-demand fabrication of patient-specific devices, as well as anatomically fitting implants with high complexity in a cost-effective manner. Additive systems that employ vat photopolymerisation such as stereolithography (SLA) and digital light projection are used widely in the field of biomedical science and engineering. However, additive manufacturing methods can be limited by the types of materials that can be used. In this study, we present an isosorbide-based formulation for a polymer resin yielding a range of elastic moduli between 1.7 and 3 GN mm−2 dependent on the photoinitiator system used as well as the amount of calcium phosphate filler added. The monomer was prepared and enhanced for 3D-printing using an SLA technique that delivered stable and optimized 3D-printed models. The resin discussed could potentially be used following major surgery for the correction of congenital defects, the removal of oral tumours and the reconstruction of the head and neck region. The surgeon is usually limited with devices available to restore both function and appearance and with the ever-increasing demand for low-priced and efficient facial implants, there is an urgent need to advance new manufacturing approaches and implants with a higher osseointegration performance.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The craniofacial region comprises several different tissue types that include bone, cartilage, muscles, ligaments and skin, as well as essential supporting structures such as blood vessels and nerves [1]. There are approximately 60 000 craniofacial reconstruction surgeries carried out each year in the UK alone [2]. These operations are needed as a result of trauma, such as road traffic accidents, surgery to remove tumours or to correct congenital anomalies in babies and children born with conditions such as cleft lip and palate. In some cases, the reconstructive surgery is needed to correct functional issues, such as creating more space inside the skull to enable a person's brain to grow or even to provide better protection for their eyes. Oral and maxillofacial surgery specialises in treating many conditions and diseases in the head, neck, face and jaw region [3]. With the ever-growing demand for a suitable material to restore both function and appearance for patients there have been developments taking place in the field of dental materials to best suit the ideal selection criteria to satisfy the functionality, biocompatibility, aesthetics, durability and ease of manipulation and contourability as a maxillofacial material. Due to excellent osseointegration and osteogenesis properties, autologous bone grafts remain a gold standard technique for surgery in this area [4]. However, the use of autologous bone grafts has disadvantages such as the risk of infection, risk of rejection and multiple operations, which lead to postoperative pain and discomfort for patients.
Several other grafting methods include; alloplastic bone substitutes, collagen grafts, tricalcium phosphate grafts and bioglass grafts, which all have varying advantages and disadvantages that are to be examined before the relevant method is chosen for surgery [5]. Biomaterials are also used in maxillofacial surgery and can be defined as natural or synthetic materials that are used to replace parts of a living system [6]. The basic requirements for a successful material are that it should be biocompatible, degradable, and corrosion resistant and must be able to withstand stress as well as interfere minimally in normal growth, remodelling and development of bone [7]. Natural materials including collagen, gelatine and hyaluronic acid have been approved by the FDA but come with unpredictable degradation kinetics and poor mechanical properties, which leads to the emergence of synthetic polymers.
Synthetic polymers are widely used in the biomedical field as their mechanical properties can be tailored for individual applications [8]. Synthetic polymers represent the largest group of biodegradable polymers and exhibit predictable and reproducible mechanical properties including tensile strength, elastic modulus and degradation rates [9]. Poly(glycolic acid), polylactic acid and poly(l-lactide-co-glycolide) copolymers are the most commonly used synthetic polymers in the clinical environment [10]. Poly( -caprolactone) (PCL) is a synthetic biodegradable polymer that has been extensively explored over the last few years for its use in medical applications [11]. Photo curable polymers such as PCL diacrylate and polyethylene glycol diacrylate can be suitable for biomedical applications in tissue engineering as they have been found to have good mechanical stiffness properties and low degradation rates [12].
-caprolactone) (PCL) is a synthetic biodegradable polymer that has been extensively explored over the last few years for its use in medical applications [11]. Photo curable polymers such as PCL diacrylate and polyethylene glycol diacrylate can be suitable for biomedical applications in tissue engineering as they have been found to have good mechanical stiffness properties and low degradation rates [12].
While the materials discussed have exhibited some excellent properties, they have also exhibited deficiencies such as the formation of acidic species which can provoke problems for long-term stability [13] as well as their unsuitability to withstanding high temperatures. These polymers therefore lack the ability to best restore a maxillofacial defect. There is a great need to explore a new material which demonstrates biocompatibility, chemical stability, degradability and is aesthetically pleasing for patients.
Bioceramics, such as calcium phosphates (CaPs) are some of the most widely studied materials that interface with bone. CaPs have been found to be osteogenic [14], have a similar structure to the mineral phase of bone [15], and reported to be naturally osteoinductive [16]. In some cases CaPs have a high affinity for proteins such as BMP-2 which plays an important role in proliferation and differentiation of osteoprogenitor cells in bone and cartilage [17].
Three-dimensional (3D) printing, also referred to as additive manufacturing, is currently attracting increasing attention across the biomedical and tissue engineering sectors [18]. Stereolithography (SLA) is a technique that utilizes direct 3D printing enabling rapid and direct fabrication of intricate 3D models with micron sized resolution [19]. SLA printing has distinctive advantages such as the low cost, high-resolution prints, a wide set of materials available and support-structure-free printing [20]. To eliminate the damaging effects of UV exposure on cells, visible light-sensitive photoinitiators can be utilized [21].
Photoinitiators are compounds that produce radicals when exposed to UV light which react with monomers to initiate polymerisation. There are several types of photoinitiators that can be used depending on the type of material in question. The most commonly used type of photoinitiation system used in dental resins is camphorquinone (CQ) [22]. However, CQ has been proven to have a few disadvantages when activated for dental resins mainly due to its yellow colour which compromises aesthetic restorations in the field of dentistry [23]. Alternative initiators are constantly under investigation to overcome such issues.
In this study, the synthesis of a light-curable degradable polymer is investigated alongside its 3D-printable properties for potential use in custom-fit facial implants. The monomer, (((((((((((3R,3aR,6S,6aR) - hexahydrofuro[3,2-b] furan-3,6-diyl)bis(oxy)) bis(ethane-2,1-diyl))bis(oxy))bis(carbonyl))bis(azanediyl))bis(3,3,5 - trimethylcyclohexane - 5,1-diyl))bis(azanediyl))bis(carbonyl))bis(oxy))bis(ethane - 2, 1 - diyl) bis(2-methylacrylate) referred to as CSMA-2 was synthesised as previously explored in a study by Owji et al [24] and optimised for enhanced mechanical properties. Two different photoinitiators, as seen in table 1, CQ and phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide) (BAPO) were incorporated to allow polymerisation to take place. In previous studies by Owji et al [24] 1 wt% CQ was the only photoinitiator explored, which gave the starting point for the advancement of this study. The comparison of CQ with a different photoinitiator; BAPO allowed us to understand the differences in photoinitiation systems and their effects on the synthesised monomer. 2 wt% of photoiniator was chosen for this study to give a better chance of 3D printing results. Throughout the study both CQ and BAPO have been compared and contrasted through polymerisation kinetics, mechanical testing and 3D printing results leading to a conclusive optimal formulation to be used in further in vitro studies.
Table 1. Description of photoinitiators used in the study.
| Abbreviation | Molecular structure | Nomenclature | Molecular mass (g/mol) |
|---|---|---|---|
| CQ | Camphorquinone | 166.22 | |
| BAPO | Phenylbis (2,4,6-trimethylbenzoyl)-phosphine oxide | 418.50 |
To further advance this study, a CaP (hydroxyapatite (HA)) was incorporated into both polymer systems to adjust the mechanical properties and printing characterisation. HA was chosen as it is a naturally occurring mineral form of calcium apatite and 70% by weight of natural human bone is made up of HA. In comparison, the previous study on CSMA by Owji et al [24] used a reactive filler combination of mono calcium phosphate monohydrate and beta tri-calcium phosphate (TCP) to form the polymer composites.
Here we present the synthesis of CSMA-2 optimised for 3D Printing via an SLA method comparing both types of photoinitiators and varying range of CaP filler from 0% to 10%.
2. Experimental methods
2.1. Materials
1,3-dioxolan-2-one (Ethylene carbonate, 99%) was purchased from Alfa Aesar (A15735-36), potassium carbonate (99%) was supplied by Scientific laboratory supplies (P5833-500) and 1,4:3,6-dianhydro-D-sorbitol (isosorbide, 98%) (329 207) isophorone diisocyanate (IPDI, 98%) (317 624), 2-hydroxyethyl methacrylate (HEMA, 97%) (128 635), triethylene glycol dimethacrylate (TEGDMA, 95%) (261 548), dibutyltin dilaurate (DBTDL) (95%) (291 234), ethyl acetate (99.8%) (270 989) and methanol (99.8%) (322 415) were obtained from Sigma-Aldrich and distilled to remove inhibitor and increase purity. Silica gel beads (0.015–0.040 mm) were purchased from Merck (11 511) and white quartz sand (274 739) was purchased from Sigma-Aldrich to assist in the column chromatography. To assist in photo polymerisation, the photoinitators, CQ and BAPO were purchased from Sigma-Aldrich. For the addition of CaP fillers, CAPTAL R HA was purchased from Plasma Biotal Limited with a Ca:P ratio of 1.67 and a high surface area of around 6–20 m2 g−1.
2.2. CSMA-2 synthesis
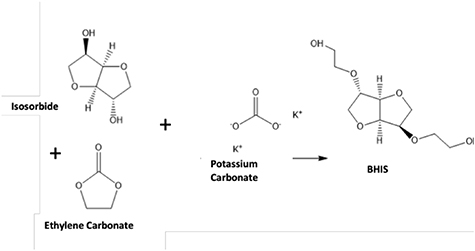
Figure 1 outlines the methodology used in this study starting with the synthesis of the intermediate chemical compound, BHIS, (figure 2) using isosorbide and ethylene carbonate. A mixture of isosorbide (100 g, 684.3 mmol) and ethylene carbonate (132.57 g, 1505.5 mmol) in a 1000 ml, three-necked round-bottom flask was degassed under dry nitrogen for 60 min. The reaction mixture was then heated on a hot plate at 70 °C for 60 min. After the solid contents were melted completely, the reaction mixture was heated to 170 °C and then potassium carbonate (3.0 g, 21.71 mmol) was added to the reaction mixture and left to react for 48 h. The synthesized BHIS was purified by silica column chromatography using methanol/ethyl acetate (1/9) mobile phase and dried at 60 °C for 24 h under vacuum to obtain high-purity BHIS (figure 2).
Figure 1. Schematic describing the methodology involved in this study.
Download figure:
Standard image High-resolution imageFigure 2. Preparation of bis(2-hydroxyethyl) isosorbide—(BHIS).
Download figure:
Standard image High-resolution imageCSMA-2 (figure 3) was prepared by a two-step reaction from BHIS, and a three-fold excess of IPDI in the presence of a diluent such as TEGDMA, followed by a reaction between the NCO-terminated monomer and 2- HEMA. A mixture of the BHIS (32.15 g, 79.37 mmol), IPDI (57.15 g, 257.07 mmol) TEGDMA (125 g, 436.56 mmol) and 5 drops (approximately 0.5 ml) of DBTDL were slowly added into a brown glass beaker and wrapped in tin foil to exclude all light. The reaction mixture was continuously stirred at 25 °C for 4 h. To the resulting NCO-terminated monomer, HEMA (71.42 g, 548.82 mmol) was added into the reaction mixture alongside another 5 drops (approximately 0.5 ml) of DBTDL. The reaction mixture was continuously stirred for at 25 °C for 12 h and then evaporated in a rotary evaporator to give the crude product. Finally, the synthesized monomer was purified by column chromatography using a methanol/ethyl acetate (1/2) mobile phase and dried at 60 °C for 24 h under vacuum.
Figure 3. Preparation of (((((((((((3 R,3aR,6 S,6aR)-hexahydrofuro[3,2-b]furan-3,6-diyl)bis(oxy))bis(ethane-2,1-diyl))bis(oxy))bis(carbonyl))bis(azanediyl))bis(3,3,5-trimethylcyclohexane-5,1-diyl))bis(azanediyl))bis(carbonyl))bis(oxy))bis(ethane-2,1-diyl) bis(2-methylacrylate)—(CSMA-2).
Download figure:
Standard image High-resolution image2.3. Nuclear magnetic resonance (NMR)
The 1 H NMR and 13C spectra were recorded with the JEOL NMR-ECA 600 spectrometer (600 MHz, Tokyo, Japan) and deuterated chloroform (CDCL3) as the solvent for both BHIS and CSMA-2. All data were analysed via 'delta NMR software; Version 4.3.6' and were given as chemical shifts relative to tetramethylsilane (δ, ppm).
2.4. Fourier transform infrared spectroscopy (FTIR)
The degree of monomer conversion was measured and calculated using the FTIR (Perkin Elmer Spectrum one, Llantrisant, United Kingdom). A drop of the polymer premixed with 2 wt% CQ or 2 wt% BAPO was placed on the diamond of an attenuated total reflectance (ATR) (Golden Gate ATR, Specac Ltd., United Kingdom), and upon exposure to blue light spectra were then recorded for 1000 s. At a resolution of 0.5 cm−1. This process was repeated for the polymer mixture with 2 wt% BAPO as well. To calculate the optimum curing time of CSMA-2, a small aliquot of the monomer mixed with 2 wt% of CQ/BAPO was loaded onto the diamond ATR and absorbance profiles were measured at around 1320 cm−1 (C–O stretch bond) and at 1335 cm−2 for the baseline. These absorbance profiles were then used to calculate the conversion.
2.5. Composite preparation
To prepare the composite polymer paste the amounts of HA filler shown in table 2 were mixed with CSMA-2 and 2 wt% CQ or BAPO accordingly using a centrifugal planetary mixer (SpeedMixer, Hauschild Engineering, Hamm, Germany, DAC150.1 FVZ) at 1700RPM for 4 min. The mixtures of (0 wt%, 5 wt%, 10 wt% inorganic phase: organic phase) with either CQ or BAPO were loaded into the 3D printer (Nobel Superfine, XYZ Printing, The Netherlands) where discs with a 10 mm diameter and 1 mm thickness were printed and photopolymerised at 450 nm.
Table 2. Polymer to CaP ratios for the composite polymer paste.
| CSMA-2 (%) | CSMA-2 (g) | Volume CSMA-2 (cm3) | HA (%) | HA (g) | Volume HA (cm3) | Volume ratio HA: CSMA-2 (%) |
|---|---|---|---|---|---|---|
| 100 | 10 | 8.47 | 0 | 0 | 0 | 0 |
| 95 | 9.5 | 8.10 | 5 | 0.5 | 0.16 | 1.97 |
| 90 | 9 | 7.63 | 10 | 1 | 0.32 | 4.19 |
Composite polymer bars were also created to allow for mechanical testing via the dynamic mechanical analysis (DMA) technique. The same mixtures of 0 wt%, 5 wt% 10 wt% inorganic phase: organic phase) with either CQ or BAPO were created and loaded into the 3D printer where bars with dimensions of 20 mm × 5 mm × 1 mm were printed and photopolymerised at 450 nm.
2.6. Mechanical testing
To characterise the mechanical properties of the monomer, the technique of a 3-point biaxial flexural test flexural test which involved applying a load via a 2 kN load cell at a displacement rate of 1 mm min−1 was carried out via the Shimadzu Autograph AGS-X machinery (Shimadzu, Milton Keynes, UK) and data analysed via the Origin Pro 2019 software. The biaxial flexural test was carried out until specimen failure and repeated for eight specimens in each group. The result of these tests allowed evaluation of the flexural strength and the Young's modulus of the materials to be evaluated. The flexural strength, also known as bend strength, represents the highest stress experienced by the material at its moment of yield.
The Young's modulus was also measured using the three-point bend test to determine reproducibility of methodologies. The composite polymer bars of nominal dimensions 5 mm × 1 mm × 20 mm were placed on a 3-point bending rig on the DMA 850 (TA Instruments, New Castle, USA) and a loaded at a displacement rate of 1 mm min−1, up to the instrument load limit of 18 N. The collected data was analysed via the TA Instruments TRIOS software and the elastic modulus was calculated. It was not possible to measure the bend strength as the specimens did not reach a failure point.
The hardness of the specimens was also determined using a Wallace Micro-hardness tester (Wallace, UK). Vickers hardness testing methods are used to measure the hardness of a material. The unit of hardness given by the test is the Vickers pyramid number (HV) and the HV number is determined by the ratio F/A, where F is the force applied to the diamond in kilograms-force and A is the surface area of the resulting indentation in square millimetres.
2.7. Wettability measurement
The surface wettability i.e. hydrophilicity of the 3D-printed samples was examined using deionized water. The water contact angle (WCA) measurements were carried out for hydrophilicity testing using a KSV instruments Cam 200 optical contact angle meter (Biolin Scientific, UK).
2.8. 3D printing
3D Printing, specifically SLA was carried out using the Nobel Superfine SLA printer from XYZ Printing, The Netherlands. The synthesised CSMA-2 was combined with 2 wt% CQ or 2 wt% BAPO photoinitiator. The 3D models were designed via 'Autodesk AutoCAD 2019' and prepared and sliced via the XYZ printing software 'XYZmaker suite'. The model setup for curing was set to 8300 ms with a power intensity of 100 W m−2. The power level had 3 setting options of 33%, 56% and 80% and it was set at 33% after several trial and error tests to find the best level required to achieve a clear and precise print. The peeling parameter is to choose how fast the printed object peels from the base, and this was set to 0.25 mm s−1 with a distance of 5 mm. Once printed, the final models were washed in 99.9% methanol (Sigma-Aldrich, UK) for a total of 5 min to remove any remaining residues. This was followed by a post curing technique in a UV curing chamber (XYZ Printing, The Netherlands) for 10 min.
2.9. Scanning electron microscopy (SEM)
SEM images (Philips XL30 field emission SEM, Amsterdam, Netherlands) were taken of 3D-printed specimens. Specimens were coated with 95% gold and 5% palladium (Polaron E5000 Sputter Coater, Quorum Technologies, United Kingdom) and SEM was used to visualize the surface of the specimen and to take measurements to compare to the digital STL files.
2.10. Computed tomography (CT)
CT scans of the printed constructs were obtained using a SkyScan 1172 (Bruker), with the following scan settings: 0.5 mm aluminium filter, current 100 mA, voltage 80 kV, exposure time 666 ms, pixel size 23.8 μm, rotation step 0.3°, frame averaging 6. Scans were reconstructed using NRecon (Bruker), 3D renderings were produced in CTVox (Bruker). For numerical analysis, the dataset was reoriented in DataViewer (Bruker), such that the faces of the cylinders were orthogonal to the view direction. The reoriented dataset was then loaded into CTAn (Bruker), where it was thresholded to produce binary data. Random slices through the dataset were then taken in both orthogonal directions, such that only the faces of the cylinders were in view. Individual 2D object analysis was then applied, in order to determine the area and circle-equivalent diameter of each cylindrical cross-section. Four random slices, two in each orthogonal direction, were used for numerical analysis.
2.11. Cell culture
For biocompatibility studies, MG-63 osteosarcoma cell line passage number 10 (European Collection of Authenticated Cell Cultures, UK) were cultured in T-45 polystyrene flasks in Dulbecco's modified Eagle's medium (Gibco, Life Technologies Ltd. UK) with 10% fetal bovine serum (Sigma-Aldrich), 1% of L-Glutamine (Sigma-Aldrich) and 0.1% of penicillin/streptomycin (P/S) (Sigma-Aldrich). The cells in media were incubated at 37 °C, 5% CO2 and used once they reached approximately 80% confluency. The cell culture medium was changed every 2 d.
2.12. Metabolic activity
Triplicates of each composite polymer disc group (0, 5,10 wt% HA) with either CQ or BAPO were placed in the wells of a 24 well tissue culture plates (Thermo Fisher Scientific, Loughborough, UK) and soaked in 70% Ethanol (Sigma-Aldrich) for 15 min and repeated with 99% Ethanol and left under UV light (250 nm) for 30 min for sterilization. Post sterilization, they were transferred to 48 well tissue culture plates (Thermo Fisher Scientific, Loughborough, UK) and 1 × 104 MG-63 cells were seeded in each well. This was repeated for time points of days 1, 4 and 7 and all were left to incubate at 37 °C, 5% CO2.
For the determination of cell proliferation, at each time point, 100 µl of alamarBlue dye (Thermo Fisher Scientific, Loughborough, UK) was added to each well and incubated at 37 °C for 3 h. Prior to the addition of alamarBlue, the samples were transferred into new 48 well plates to avoid any contribution from the cells that had attached to the original well plate. Fluorescence measurements (excitation wavelength 530 nm and emission of 590 nm) were measured using a Biotek FLx800 microplate reader (BioTek, USA).
2.13. Cell adhesion and spreading
MG-63 cells were cultured on 3D-printed samples and then cell morphology was assessed for 1, 4, and 7 d using fluorescence microscopy. Briefly, after the designated time points, the samples were removed from the culture media and transferred to new well plates and then gently washed with phosphate-buffered saline (PBS) at least three times and fixed with 4% paraformaldehyde for 10 min at room temperature. After fixation, samples were washed with PBS three times again and permeated with 0.1% Triton-X100 in PBS for 10 min at room temperature. The samples were then incubated with phalloidin (Thermo Fisher Scientific, Loughborough, UK) for 30 min at room temperature. After removal of phalloidin, the samples were washed with PBS three times to remove residue. Finally, the cell nuclei was stained with 4´,6-diamino-2- phenylindole (DAPI) (Thermo Fisher Scientific, Loughborough, UK) by covering the samples for 10 min at room temperature.
The samples were then imaged via a Leica DMIRB fluorescence microscope (Leica Microsystems UK, Milton Keynes, UK) with a QImaging QICam Mono (Media Cybernetics UK, Marlow, UK) camera. The images were captured via the QCapture software (Media Cybernetics UK, Marlow, UK).
2.14. Statistical analysis
The results were statistically analysed using one-way analysis of variance with Tukey's post hoc test; p < 0.05 was considered to be statistically significant.
3. Results and discussion
3.1. Synthesis and characterisation of CSMA-2
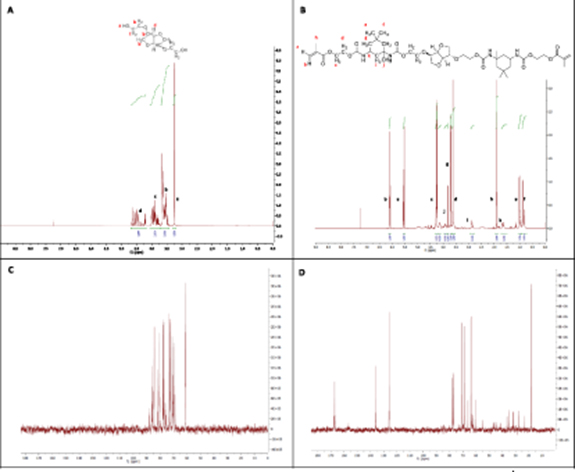
1 H NMR and 13 C NMR (figure 4) analysis confirmed the formation of the starting monomer precursor, BHIS and the final monomer CSMA-2 which were fabricated from Isosorbide. The 1H NMR spectrum confirms the synthesis of BHIS (figure 4(A) is confirmed by the proton signal at 3.58–3.73 ppm, which represents the H2–C–C–H2 protons. Figure 4(B) of CSMA-2 shows that the three singlets present at 6.14, 5.59, and 1.96 ppm correspond to the protons of the methacryl group (CH2═C–CH3). The signals between 0.8 and 1.2 ppm correspond to the protons of the isophorone cycle. However, the protons of the urethane groups which link the methacrylate units to Isosorbide, were not seen, which could be due to them being superimposed by other signals. These findings match the 1H NMR spectra previously recorded by Owji et al [24]
Figure 4. 1H NMR spectra showing the presence of chemical bond formation of BHIS (A) and the final CSMA-2 (B) monomers. Also, 13C NMR spectra showing the carbon environments of BHIS (C) and CSMA-2 (D).
Download figure:
Standard image High-resolution imageTo further confirm the formation of the monomers, 13C NMR was performed to allow the identification of carbon atoms in BHIS CSMA-2 (figures 4(C) and (D), respectively). 13C NMR only detects the 13C isotope of carbon, whose natural abundance is only 1.1%, because the main carbon isotope, 12C, is not detectable by NMR since its nucleus has zero spin and hence 13C NMR is in general much less sensitive compared to 1H NMR. 13C NMR spectra of BHIS (figure 4(C)) shows ten visible carbon environments are present which represent the ten carbon environments in the structure of BHIS. Image (figure 4(D)) confirms the 19 visible carbon environments which are present in CSMA-2 and represent the 19 carbon environments in the structure of the monomer. As the structure is mirrored only one side of the chain is counted.
Time dependent FTIR spectra of CSMA-2 with the different photoinitiators was recorded to determine the monomer conversion rate upon photopolymerisation of the liquid phase. The physical and mechanical properties of photo-cured composites are directly influenced by the level of conversion attained during polymerization. The degree of conversion (DC) is determined by measuring the intensity decrease of the methacrylate as the methacrylate monomer is converted to polymer [25]. A high DC can affect the durability and the performance of the polymer [22] and an incomplete (or low degree) polymerisation of any methacrylate monomer could lead to biological properties being affected, due to the presence of unreacted monomers, which could ultimately lead to cytotoxic effects [26]. As seen in figure 5, the absorbance profiles were used to calculate the conversion and found that within the first 40 s of the spectra, 65% conversion of CSMA-2 was observed in the sample with CQ and 72% conversion of CSMA-2 was observed in the sample with BAPO. These results suggested that a curing time of 40 s is enough for further composite preparation. It can be seen that the degree of monomer conversion is significantly higher with the BAPO photoinitiator. A study by Galvão et al [27] discussed the DC of dental composites where the dental composites tested in the study had DC values ranging from 55% to 68% which is the highest achievable range for these materials. The optimal results in this study are comparable to the DC values we obtained for CSMA-2. This could be due to its chemical structure having more photocrosslinking groups [22] to allow a faster polymerisation time leading to more of the monomer polymerising in the same amount of time as in the system with CQ.
Figure 5. FTIR spectra of CSMA-2 showing the rate of monomer conversion post exposure to UV blue light in 1000 s for the CQ system (grey) and BAPO system (red).
Download figure:
Standard image High-resolution image3.2. Mechanical testing
In order to determine the potential clinical use of a material for bone tissue engineering, mechanical tests need to be carried out to ensure the material will perform appropriately in a mechanically loaded environment, as well as determining the materials biological properties, both in vitro and in vivo. The CaP ceramic of choice for this study was HA, with the chemical composition Ca5(PO4)3OH and a calcium to phosphorous ratio of 1.67, and it is the most commonly used CaP in clinical applications in bone tissue engineering area particularly for craniofacial defects [28].
The main mechanical properties to measure for bone scaffolds are elastic modulus and strength. The elastic modulus of cancellous bone in non-load bearing regions is within the range of 0.1–0.5 GN mm−2. Due to the viscous nature of CSMA-2 only a relatively low amount of HA was added to allow optimum formulations for printing via the SLA method. The flexural strength and elastic modulus, also known as Young's modulus, was investigated for CSMA-2 with CQ and CSMA-2 with BAPO, with 0%, 5% and 10% HA addition. As there is some debate about the values determined via biaxial flexure testing compared to tensile/three/four point bend methods we carried out testing using a biaxial flexure test and also a three point bend test to compare the values obtained, and it should be noted that the composite polymer discs suitable for biaxial flexure testing and composite polymer bars suitable for three point bend testing were 3D-printed to ensure accuracy and identical specimens and also to allow values of printed polymer specimens to be determined with significantly different geometries to allow assessment of whether the geometry/size affected the properties due to the printing.
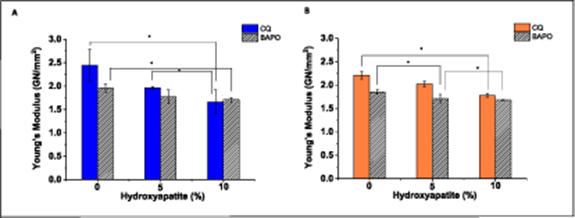
The Young's modulus of CSMA-2 was significantly lower than the literature values for the modulus of similar bone cements such as PMMA. According to a study carried out by Reham M. Abdallah [29] the Young's modulus of PMMA with no added substitutes was 4.45 GN mm−2 which compares significantly to CSMA-2 with a modulus value of 2.0–2.4 GN mm−2. This could be due to its increased degree of crosslinking which leads to the structure becoming more rigid and less elastic. Also, being of a highly viscous nature, the monomer exhibits a higher resistance to flow which can significantly affect powder dispersion and therefore forming a stiffer composite with a higher Young's modulus. However, with the addition of 5% HA and 10% HA, respectively, the Young's modulus decreased slightly, highlighting the difficulty in attaining uniform ceramic phase dispersion in the monomer phase. Both testing methods displayed similar results for the Young's modulus.
Via the biaxial flexure test, (figure 6(A)), CSMA-2 + CQ with no added HA gave a modulus value of 2.44 GN mm−2 whilst the same group tested via three-point bend testing (figure 6(B)) gave a modulus value of 2.21 GN mm−2. These results showed no statistically significant difference at *p < 0.05. For the remaining groups with HA, both determination methods also gave similar results with no statistically significant difference for the modulus therefore concluding that both methods are applicable for the testing of the modulus. When comparing between the BAPO and CQ results for modulus there was no statistically significant difference between each set and therefore there is no proven difference between their results as expected considering the photoinitiator is used to help the photopolymerisation process rather than cross-linking with the polymer.
Figure 6. (A) Young's modulus comparison between CSMA-2 with CQ with the addition of HA incorporated (blue) and CSMA-2 with BAPO photoinitiator with the addition of HA incorporated (grey) determined via a biaxial flexure test (*p < 0.05) and (B) Young's modulus comparison between CSMA-2 with CQ with the addition of HA incorporated (orange) and CSMA-2 with BAPO photoinitiator with the addition of HA incorporated (grey) determined via a three point bend test (*p < 0.05).
Download figure:
Standard image High-resolution imageWhen comparing between the BAPO and CQ results for modulus there was no statistically significant difference between each set and therefore there is no proven difference between their results as expected considering the photoinitiator is used to help the photopolymerisation process rather than cross-linking with the polymer.
The flexural bend strength (figure 7(A)) values for CSMA-2 with BAPO were found to be significantly higher than when CSMA-2 was cured using CQ. With the addition of HA, the flexural bend strength decreases in both CQ and BAPO systems as well as the Young's modulus decreases with increasing HA addition. The hardness determination for CSMA-2 and the composite discs containing 0%–10% CaP (figure 7(B)) show that with increasing amounts of HA, both the CQ and BAPO incorporated formulations increase, suggesting the material becomes harder with higher amounts of HA. This compares to a study by Musib et al [30] where the hardness of PMMA bone cements were measured against similar bone cements and the results showed that PMMA has similar hardness properties as CSMA-2 with 10% CaP incorporated.
Figure 7. (A) Flexural strength comparison between CSMA-2 with CQ with the addition of HA incorporated (red) and CSMA-2 with BAPO with the addition of HA incorporated (grey) (*p < 0.05). (B) Vickers hardness test comparison between CSMA-2 with CQ with the addition of HA incorporated (green) and CSMA-2 with BAPO with the addition of HA incorporated (grey) (*p < 0.05). (C) Contact angle measurement comparison between CSMA-2 with CQ with the addition of HA incorporated (purple) and CSMA-2 with BAPO with the addition of HA incorporated (grey) (*p < 0.05).
Download figure:
Standard image High-resolution imageThe WCA is a common way of measuring the hydrophilicity/hydrophobicity of a material. The contact angle is an angle that a liquid creates with a solid surface or the capillary walls of a porous material when both materials are in contact with each other. This angle is determined by both the properties of the solid, the liquid and the gas phase, as well as the interaction and repulsion forces between the liquid and the solid. The higher the contact angle, the stronger the cohesive forces and the molecules of the liquid tend to interact more with each other than with the solid molecules-making it hydrophobic. On the other hand, the smaller the contact angle, the weaker the cohesive forces are therefore the liquid particles tend to interact more with the solid molecules than the liquid-making it hydrophilic [31]. Figure 7(C) shows the results from the contact angle readings for the polymer and the composite discs for 0, 5 and 10 wt% HA. These results reveal that the polymer by itself has hydrophilic behaviour (CQ-59.3°) and (BAPO-54.3°). The results also show that the more CaP added to the material, the more hydrophilic the material became as the angle decreased slightly. A study carried out by Shah et al [32] investigated the surface modifications of PMMA and the effect of UV irradiated PMMA substrates. The results from the contact angle measurements showed that PMMA displays hydrophilic behaviour as the contact angle measured as 52° but this decreased with significant exposure to UV light. This study allows us to understand the effect of UV exposure and this can be applied to our printing applications to ensure the correct hydrophilic properties are met.
3.3. 3D printing and SEM
As already discussed, the addition of CaP fillers can play a significant role in the biological and mechanical properties of a polymer. 3D printing is an emerging technology which has been widely used in bone tissue engineering to help guide the potential gain of personalised treatments and patient-specific devices [33]. A study carried out by Yi et al [34] looks at 3D printing patient-specific devices for nasal cartilage and gives the scope of the advantages that 3D printing technology holds in the field of bone tissue engineering. In this study, patient-specific nasal cartilage implants were 3D-printed and tested in vitro to observe chondrogenic markers before implanting the engineered cartilage in vivo. This process could potentially be beneficial in generating implants for other types of tissue such as that of the maxillofacial region after facial reconstruction surgery.
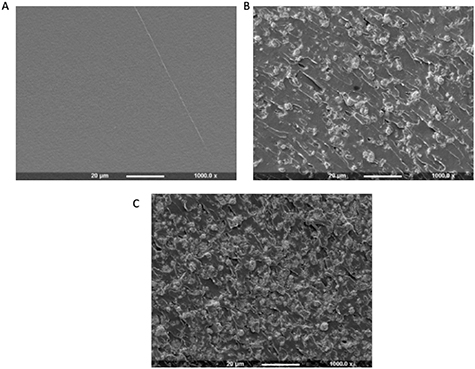
Although CaP powders are known to show improved osteoconductivity and biocompatibility the optimum dispersion of the filler into the liquid is critical. Figure 8 displays an array of SEM images highlighting the fracture surface of the composite-polymer discs with varying amounts of HA incorporated. Image (A) shows a smooth fracture surface of the disc as it has no CaP incorporated. As the amount of HA is increased from 0 to 10 wt % the SEM images show clusters of the HA forming and dispersing on the surface of the polymer discs.
Figure 8. SEM imaging to show the fracture surface of the composite polymer discs with CaP incorporated. (A) CSMA-2 with 0 wt% HA, (B) CSMA-2 with 5 wt% HA and (C) CSMA-2 with 10 wt% HA.
Download figure:
Standard image High-resolution imageThe dimensions highlighted in figure 9 display the cross-sectional measurements between the matrixes. The original Stereolithography (STL) file, designed and loaded into the XYZprint software, had cross-sectional dimensions of 0.50 mm × 1.00 mm (A) After printing, the SEM shows the dimensions as an average of 0.56 mm ± 0.10 mm × 0.91 mm ± 0.14 mm (B). In this study 2 wt% of BAPO photoinitiator was incorporated for printing. The addition of photoinitiator can impact the quality of the final 3D-printed model. However, an increase in the amount of photo-initiator can have an adverse effect on the biocompatibility of the polymeric matrix. Therefore, it is crucial to establish an ideal balance between the curing time, power intensity, and the amount of photo-initiator to optimize the printability of a scaffold.
Figure 9. Shows the final 3D-printed matrix using 2 wt % BAPO photoinitiator (A) followed by the matrix viewed via SEM at 19 × magnification with highlighted dimensions (B).
Download figure:
Standard image High-resolution imageThe method of 3D printing of bio ceramic scaffolds has been found to be advantageous in several ways. The fabrication process can be seen to be quick and also decrease the chances of experimental errors. Using 3D printing, the characteristics of a scaffold such as the pore shapes, porosity and interconnectivity can be controlled to be suitable for patient-specific demands [35]. Having an ideal 3D-printed scaffold could provide structure for cell migration and proliferation to promote the regeneration of bone tissues [36]. Finally, in the area of craniofacial reconstruction surgery, in particular maxillofacial surgery, the reduction in the number of operations for the patient is a major advantage as the degradable printed material will not need additional surgery after implantation. This also links to the aesthetic concerns that current implants in the region have as the 3D-printed models will fit uniquely with the contours of the craniofacial defects [37].
Figure 10 displays macro images taken via an SLR camera of a 3D-printed scaffold. The model was imported from STL files into the XYZ printing software and printed using CSMA-2 with 2 wt% of BAPO initiator. The log structure took 3 h 11 min to print at power intensity level 33. After printing was complete, the model was washed in 99.9% methanol to remove all remaining residues and then placed in a UV curing chamber for 10 min to post cure and solidify all parts of the print.
Figure 10. 3D-printed scaffold of a log structure showing how cortical bone is built, using CSMA-2 with 2% wt BAPO photoinitiator.
Download figure:
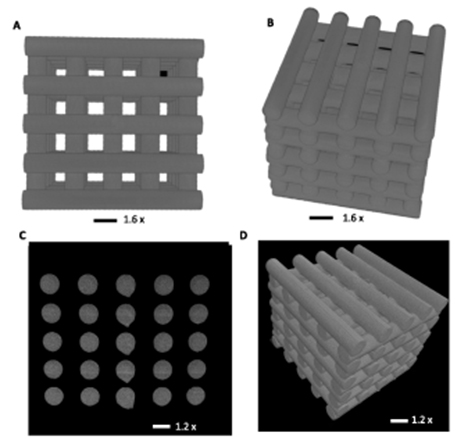
Standard image High-resolution imageMicroCT (Bruker) was used to compare the accuracy of the print of the final product to the original CAD design. As shown in figure 11, scans were reconstructed and the dataset reorientated such that the faces of the cylinders were orthogonal to the view. Random orthogonal slices, displaying the cylinderical cross sections, were taken from both directions, and the cross-sectional area and circle equivalent diameter were calculated for each cylinder face (table 3). It can be seen that the logs during printing were kept quite uniform as they displayed similar measurements to the other layers. These measurements also compare to the original STL file with the set diameter for the logs as 3.46 mm.
Figure 11. (A), (B) Log structure design displayed via AutoCAD and (C), (D) the scaffold displayed and sliced via the 'Bruker micro- CT' software.
Download figure:
Standard image High-resolution imageTable 3. CT scan analysis results highlighting the accuracy of the print.
| Slice # | Area-equivalent circle diameter (mm) | Cross sectional area (mm2) |
|---|---|---|
| 1 | 3.33 ± 0.02 | 34.90 ± 5.61 |
| 2 | 3.34 ± 0.03 | 35.03 ± 0.66 |
| 3 | 3.36 ± 0.04 | 34.41 ± 8.65 |
| 4 | 3.36 ± 0.04 | 35.50 ± 1.01 |
| Average | 3.35 ± 0.04 | 34.96 ± 3.98 |
| Dimensions from digital file | 3.46 ± 0.20 | 37.68 ± 4.32 |
3.4. Cell viability, adhesion and morphology
MG-63 osteosarcoma cells were seeded in order to evaluate the biocompatibility of the composite polymer discs with the different photoinitiators and were compared to a control of cells grown in the well plate. The alamarBlue assay gave a quantitative measurement of cell metabolism and the results can be seen in figure 12. The results show that the both CQ and BAPO groups continued to proliferate throughout the study but were all significantly lower than the tissue culture plastic (TCP) control group. TCP offers a good platform for cells to proliferate in as it is pre-treated to modify the hydrophobic plastic surface to make it more hydrophilic therefore leading to advanced cell attachment. This, as well as the cell seeding efficiency could explain why the control has continuous proliferation of cells in comparison to the experimental group. The addition of HA also had an impact on the cell metabolic activity, whether or not the effect was positive cannot be decided until further testing has been completed however, a study by Ingole et al [38] looked at the effects of varying composition ratios of HA in combination with nanocomposites for a bone regeneration application. They studied the structural, crystalline and morphological properties of the composites as well as in vitro studies on human bone-derived osteoblast cells. Their results showed that the addition of HA exhibited no observable negative effect on cell growth. This could allow us to believe that with subsequent repeats of the alamarBlue assay as well as other assays such as the alkaline phosphatase assay the results could be clearer as to whether a higher content of HA has a positive impact on the cell metabolic activity rate.
Figure 12. The fluorescence intensity measurements showing metabolic activity of MG-63 osteosarcoma cells.
Download figure:
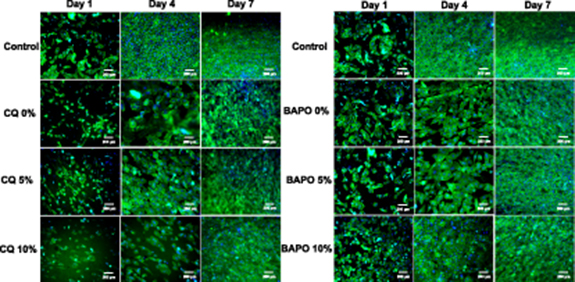
Standard image High-resolution imageFigure 13 displays the results from the staining and cell attachment. DAPI and phalloidin staining highlights the nucleus and the cytoskeleton of the cell which can be seen in blue and green, respectively. The results for the CQ groups are harder to visualise as the discs became slightly concave after curing under UV for a prolonged period of time. Moreover, the photo-initiator used for UV curing (450 nm) and DAPI (abs. 358 nm, emi. = 461 nm) staining wavelengths are approximately in the same range, which caused autofluorescence and made it difficult to visualise the nucleus under the fluorescence microscope.
Figure 13. The results from DAPI/phalloidin staining viewed via a fluorescence microscope for experiments days 1–7.
Download figure:
Standard image High-resolution imageThe variation of cell morphologies at different sample surfaces with time can be explained on the basis of the change in surface topography/roughness due to the incorporation of CaP (HA) microparticles. The varying concentration of HA microparticles (0, 5, and 10 wt.%) in polymers generate varying surface texture as clearly observed in the SEM image (figure 8). The microtexture surfaces are due to the presence of varying concentration of HA which can influence the cell response on the scaffolds and can cause changes in the morphologies and phenotypes as previously reported [39]. Moreover, it has been said that nanoroughness can also affect the cell morphologies [40].
4. Conclusions
In this study, a light-curable mechanically strong polymer was successfully synthesised and integrated with stereolithographic 3D printing technology with potential to prepare high resolution, patient-specific facial implants with desired properties to meet various requirements for applications in the biomedical and tissue engineering field. The monomer synthesised, CSMA-2 was prepared with two different photoinitiators to find the optimum formulation for printing. Both photoinitiator systems were incorporated with various ratios of HA in order to potentially improve osseointegration properties for bone regeneration, the bone integration performance and to assess its printability to enhance the fabrication of the final product and its key characteristics such as its flexibility and stiffness. The results show that the material itself has potential promise in manufacturing and improves implants for maxillofacial reconstruction surgery compared to the current conventional implants that are used. It can be said that BAPO, as a photoinitiator is the more preferable choice for this material as the mechanical properties proved to be more ideal for printing formulations and the in vitro study showed more promising results for BAPO. Furthermore, the photocrosslinking groups in the structure of BAPO allow a faster photopolymerisation to occur, which is a benefit for 3D printing with this material. The work investigated in this study will aid in the future research which will focus on the in vivo properties and toxicity studies of the material. It will further guide us into evaluating improved modification and optimisation properties for 3D printing as well as assessing the degradation mechanics of the material synthesised.
Acknowledgments
The authors acknowledge the financial support provided by the Engineering and Physical Sciences Research Council (EPSRC), UK, Grant code EP/R513143/1, the Restoration of Appearance and Function Trust (RAFT), Registered Charity No. 299811, UK, and that the research was supported in part by the National Research Foundation of Korea (2018K1A4A3A01064257).
Conflicts of interest
There are no conflicts of interest to declare.