Abstract
More than three quarters of all animal species on Earth are insects, successfully inhabiting most ecosystems on the planet. Due to their opulence, insects provide the backbone of many biological processes, but also inflict adverse impacts on agricultural and stored products, buildings and human health. To countermeasure insect pests, the interactions of these animals with their surroundings have to be fully understood. This review focuses on the various forms of insect attachment, natural surfaces that have evolved to counter insect adhesion, and particularly features recently developed synthetic bio-inspired solutions. These bio-inspired solutions often enhance the variety of applicable mechanisms observed in nature and open paths for improved technological solutions that are needed in a changing global society.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Insects form the most multitudinous class of animals on the planet, affecting nature and humans in miscellaneous ways. More than three quarters of all animal species on Earth are estimated to be insects, successfully inhabiting most ecosystems on the planet [1]. Undoubtedly, insects are essential for the large biological networks that distinguish our planet. Insects have upheld human cultures by modulating pest population, producing natural products in conjunction with disposing waste and recycling organic nutrients [2].
These examples accentuate the significance of insects for human life and indicate how different our planet would be in their absence. Nonetheless, insects can also have adverse effects on our agroeconomical system. Each year, insect pests cause considerable damage to plants, buildings and human health worldwide [3–5]. Insect pests spoil plants pre- and post-harvesting [3, 6]. Pre-harvest insect damage is caused by feeding, sap-sucking or infesting different parts of the plants [3]. Post-harvest damage, e.g. during storage, is often caused by insects feeding on the stock and consequently spoiling the crops [6]. While crop losses due to insect infestation vary across the globe and largely depend on crop type, recent studies estimate an annual global crop loss of about 18%–20% with a value exceeding 470 billion USD [7–9].
Apart from crops, insect infestation impacts human life in further ways. Damages to buildings, furniture, books and other objects made of wood or cellulose-based natural products are frequently reported all over the globe [10–13]. Wood-feeding termites, for example, have been reported to consume wooden constructions to the point of their collapse [14].
As disease carriers, insects also inflict significant harm on human health. A simple physical contact can be sufficient to transmit a disease when the insects, such as synanthropic house flies or cockroaches, carry infectious agents on the surface of their bodies [15, 16]. More common are infectious transmissions of a disease vector, such as malaria or dengue fever through mosquito or fly bites, affecting millions of humans every year [17–20].
Due to the severe impact of insect infestation on human lives, a tremendous increase in human-induced countermeasures against insects has been observed. Effective countermeasures include intoxication of the pest, often causing a range of negative effects on the ecosystem as a whole. Hence, alternative methods for efficient pest control have been of great recent interest, including the control of adhesion of insects to surfaces [21–25]. A fundamental understanding of the adhesion mechanism of insects and how to repel them is often missing and insight into this is anticipated to open up new paths to potentially useful and technologically relevant materials that can limit exposure to insects.
Current strategies to tackle insect infestation are often based on toxic chemicals and include insecticides and other insect-repellent chemicals. Since these pose toxic dangers to the environment, alternative eco-friendly strategies are needed [26]. Nature itself provides established biological concepts that do not merely rely on chemical defenses to deter insects but also on physical defence mechanisms, often through surface structuring. Imitating these structures to aid new material designs enables the manufacture of materials that deter insects in a non-toxic way. Indeed, many recent technological examples derive their innovative function from simple observation and replication of examples found in nature [27]. The surfaces of plant leaves encompass a bio-inspirational source of such mechanisms that, among others, inspire eco-friendly alternatives to current insecticides [28–31].
This topical review presents the recent developments on bio-inspired countermeasures against insect infestations, which rely on varying functional principles. Therefore, the following sections first provide an insight into the adhesion mechanisms of insects and the biological anti-adhesive systems of plants before current progress in bio-inspired artificial anti-adhesive systems is presented.
2. Insect adhesion mechanisms
The world is full of surfaces that are structured on a range of length scales and that are continuously in (passive or active) motion. Insects frequently have to adhere to these surfaces to guarantee their survival for foraging or reproduction, which mainly occurs on the surfaces of plants. To do so, evolution brought forward a remarkable diversity of attachment mechanisms that produce outstanding attachment forces to an enormous variety of surface structures, morphologies and chemistries [22, 32]. Surfaces differ in their physicochemical characteristics including hydrophilicity, roughness and material strength [33, 34].
The subsequent sections outline the functional basis of the different adhesion mechanisms encountered in insects. Other reviews [26, 35, 36] have already laid the groundwork for this wide topic and we therefore provide a general overview of the adhesion methods, their biomechanical functions and existing countermeasures to reduce adhesion.
2.1. Insect adhesive organs
Many insects possess adhesive organs which enable them to climb a variety of surfaces. They can be classified into two categories: (i) mechanical hook systems, i.e. 'simple' attachment devices, such as claws; and (ii) microstructured adhesive systems, i.e. more structurally defined organs, such as hairs (so-called setae) or smooth pads. Figure 1 highlights a variety of these organs. The attachment and detachment movements can be performed by virtue of either interlocking or by adhesion forces exerted by the adhesive organs. Locomotion based on the latter requires the rapid attachment and detachment of these adhesive organs via a peeling mechanism, comparable to the function of pressure adhesive tapes [37]. The two types of attachment organs are typically situated along the length of the leg or only at the end [38].
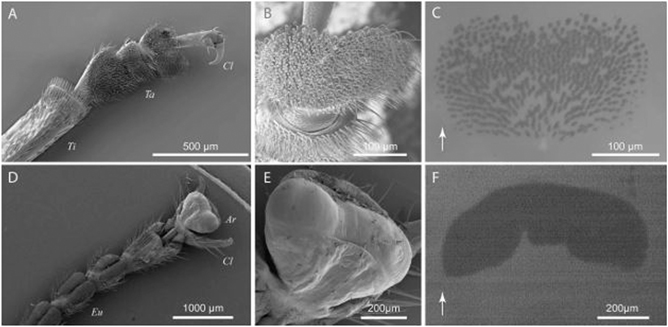
Figure 1. Attachment devices of a male dock beetle (G. viridula, (A)–(C)) and an Indian stick insect (C. morosus, (D)–(F)), showing both the simple and structurally more defined organs of the hind (A) and front tarsal segments (D). Simple attachment devices such as claws are situated behind (A) or around (D) the structurally finer distal adhesive pads ((B) and (E)). With the aid of epi-illumination, the contact area of the adhesive pads in contact with glass ((C) and (F)) was visualised. Both systems employ an adhesive fluid, which is indicated by the dark shadow on the glass surface, displaying the contact between the mediating fluid, secreted by the setae (C) or cuticular sac (F). The arrows in (C) and (F) indicate the distal direction. The tibia (Ti), claws (Cl), tarsal segments (Ta), euplantulae (Eu) and arolium (Ar) are also indicated. Reproduced with permission from [42]. © The Company of Biologists Limited 2008.
Download figure:
Standard image High-resolution imageClaws, employed for attachment or grabbing and holding onto prey, are usually found on the tarsi of insect legs (figures 1(A) and (D)). The tarsus (foot, pl. tarsi) is commonly described as the final segment (furthest from the main body) in the anatomy of insect legs. The tarsus can consist of one to five smaller sections, called tarsomeres, and conventionally features one or two claws at the end [39]. These well-developed organs allow the insect to attach to rough surfaces when the surface asperities (i.e. surface irregularities) are larger than the corresponding claw tip diameter [40, 41], but fail to provide attachment to smoother surfaces. The effectiveness of the claws is highly dependent on several factors, such as the stiffness, the morphology and especially the sharpness of the claw tip [1]. For instance, stiff claws are able to penetrate soft surfaces and attach to them, while they can interlock only with sufficiently large surface asperities of rigid surfaces [40, 41]. To attach to surfaces which are too 'smooth' for claws, such as many plant leaves or glass surfaces, different adhesion strategies are needed.
Attachment to smooth surfaces is achieved through the use of more structured adhesive organs, compared to the stiff claw organs. These refined attachment systems, found in the exoskeletons of most insects, are almost exclusively made of cuticle, a stiff layered composite structure consisting of two main materials: chitin and proteins [43]. To minimise the effective stiffness of the adhesive organs, two different systems have evolved: (i) fibrillar adhesion systems based on a hierarchically structured hairy system (figure 1(B)); and (ii) flexible sac-like systems supported by smooth adhesion pads (figure 1(E)) [44]. Figure 1 shows a morphological comparison of the two mechanisms.
While the two adhesion systems are intrinsically different in their contact morphologies, both rely on the increase of the contact area of the adhesive organ with a contacting surface [45, 46]. These highly specialised systems are often located on different parts of the leg, not only the final tarsal segment. It is worth mentioning that research has shown a division of labour between different attachment devices on the same leg of an insect [47], particularly as the attachment frequently functions in a direction-dependent manner. Insect adhesive organs support either downward or upwards climbing, dependent on their directional dependence and whether they can engage in complete contact with the surface or not [48, 49]. The shear-sensitivity of the adhesive organs forms an astute principle allowing for a rapid change between attachment and detachment. Strong attachment is generated by pulling the adhesive pads towards the body, while separation is initiated through a pushing motion [50]. This principle applies to both fibrillar and smooth, sac-like adhesive systems [51–53] that are presented in detail below.
The two adhesion systems (figure 3) are commonly referred to as 'wet' adhesion mechanisms since the adhesion is mediated by the secretion of adhesive fluids [1]. The excreted liquids allow insects to adapt to a range of surfaces. Furthermore, we note that other adhesion systems, different to the two main attachment devices introduced above, have developed among insects, including structures resembling suction-cup-like structures [54]. Recent studies showed that these devices can range from straightforward passively working mechanisms to highly complex, multi-component, active suction cups [54–56]. However, since these mechanisms are uncommon among typical pest insects, they are not covered in this review.
2.1.1. Smooth adhesion pads
The pad structures (also called arolia) appear smooth on a macroscopic level but display various microscopic patterned surfaces across insect species (figures 2(A)–(F)) [57]. These patterns may consist of hexagonal structures (figure 2(A)), complex patterns of microfolds (figures 2(C) and (F)) or perpendicular lines (figures 2(D) and (E)). Furthermore, the cuticle of the smooth pads carries a fine ultrastructure consisting of cuticular rods [57]. These rod structures are perpendicularly aligned to the surface, branching out into finer rods, thus enabling the increase of the pad's adaptability to adhere to rough surfaces (figure 2) [58]. In stick insects, for example, these rods are 44–74 μm long with an average diameter of 1.65 μm [57].
Figure 2. Different micro- and nanostructures observed in the smooth pads of a range of arthropod tarsi. (A) Hexagonal structures in Tettigonia viridissima (Ensifera). (B) Complex pattern in Apis mellifera (Hymenoptera). (C) Microfolds in Urocerus gigas (Hymenoptera). (D) Perpendicular lines in Panorpa communis (Mecoptera). (E) Perpendicular lines in Paravespula germanica (Hymenoptera). (F) Complex pattern of microfolds in Tipula sp. (Diptera). [57] John Wiley & Sons. Wiley VCH.
Download figure:
Standard image High-resolution imageThe working principle of these smooth adhesive organs, frequently described as soft cuticular structures, is based on their ability to deform in response to pressure. This deformation increases the contact area on rough surfaces (figure 1) [46]. Secretion of a fluid further facilitates intermolecular forces, increasing the contact area and, thus, the strength of the contact. Furthermore, the fluid promotes capillary adhesion by filling gaps and facilitates self-cleaning properties [46]. Such secretion-assisted smooth adhesive organs have been observed for many insect groups including ants, bees, stick insects, grasshoppers, true bugs and cockroaches [38, 57, 59–62]. As smooth pad structures have been reported to be more frequent than the hairy adhesion-based systems described below, it is hypothesised that smooth pads developed earlier in insect evolution [63, 64].
Furthermore, different smooth pads with several subforms of pad structures have developed. These are normally distinguished between arolia and other forms of smooth pads. Smooth attachment pads can include euplantulae (e.g. in Tettigonia viridissima L) or pulvilli (e.g. in Coreus marginatus L.) [65, 66] and various forms of adhesive microstructures on the tarsal attachment pad have been identified [67]. Particularly interesting are attachment pads that developed nubby adhesive microstructures. These conical outgrows of different aspect ratios can vary in length by a few micrometers and, therefore, may partially resemble hairy attachment pads [67, 68]. When comparing the functional abilities of nubby and smooth euplantalue, nubby pads were found to generate stronger forces on a range of different surfaces [49, 67].
2.1.2. Hairy adhesion pads
Hairy adhesion organs feature flexible hairy structures (setae) in a size range of 0.2–8 μm (figures 1(A)–(C)) [69]. Depending on the species, the setae of some beetles branch towards the tip, varying in shape and size, but most insects feature a single terminal element [51]. Tip-shape variations include mushroom-shaped, spatula-like or pointed/conical tips [70–72]. Tarsal adhesive pads that are densely covered with setae are widely prevalent among both arthropods and can also be found in some vertebrates [37]. Their prevalence among animals that vastly differ in size, such as lizards, spiders and several insect orders, demonstrates their advantage regarding substrate adhesion.
Several aspects of hairy pads, including force scaling and fracture mechanics, make them a highly discussed research field. Early research focussed on adhesion on rough surfaces, arguing the high performance of hairy pads stems from the ability to make more intimate contact due to the flexibility of the setae, despite consisting of relatively rigid materials [73, 74]. More recent research outlined a more comprehensive range of functional qualities attributed to hairy adhesion pads, such as self-cleaning properties, controllable detachment and increased adhesion [75, 76]. In this regard, the interplay between designated adhesive and traction pads appears to be crucial for efficient locomotion [53].
Nevertheless, ongoing discussions attempt to explain how adhesive forces are maximised. The 'force scaling' hypothesis claims that dividing the contact zone/area into innumerable microscopic sub-units allows for increased adhesion, if the adhesive forces scale linearly with the dimensions of the contact areas [77]. 'Fracture mechanics', on the other hand, states that adhesive forces are only maximised when the size of the adhesive contacts are smaller than the critical crack length [78]. The third model, 'work of adhesion', addresses energy losses during detachment, suggesting that the bending and stretching of setae leads to increased adhesion forces [79]. The 'work of adhesion' model is popular and is often employed to justify morphological traits of the setae. It explains that setae with convex and oblique tip morphologies enhance adhesion, while at the same time avoiding self-matting [37]. Anisotropic seta tip morphologies maximise adhesion on one side while minimising adhesion on the other side, to prevent the setae from sticking to each other [80]. The 'work of adhesion' model predicts that adhesion of pads with unbranched setae cannot be increased by subdividing the contact zone into ever finer subcontacts, because of the increase of self-matting [79].
Contact splitting can hence efficiently increase adhesion if the setae are branched [81]. The crucial difference between 'wet' and 'dry' adhesive systems illustrates that seta-branching is a valuable concept to maximise adhesion when needed. While insects that utilize adhesive fluids adhere well to surfaces with small-scale roughness with fairly blunt seta tips, adhesion to dry systems requires much finer tips afforded by branched setae [37].
While purely 'dry' adhesive pads have not been reported in insects, other species (e.g. geckos and anoles) demonstrate the importance of the weak interfacial forces involved in their attachment. Indeed, accounting for the millions of setae on their feet, some gecko species should theoretically be able to generate enough adhesion force to carry the weight of two humans, when neglecting adhesive pad scaling effects [82, 83]. Nonetheless, the adhesive fluids in insects are significant.
2.2. Adhesive fluids
Insects that have either hairy or smooth pads employ a mediating fluid between the substrate and their adhesive organs to stick to surfaces [23, 84].
Adhesive fluids contain long chain hydrocarbons (C16–C22), carbohydrates and amino acids, but their detailed chemical composition is not fully known [44]. Vötsch et al concluded that the adhesive fluids are oil in water emulsions based on hydrophobic lipid-like droplets dispersed in a watery continuous phase [86]. The composition of the carbohydrates has a major influence on the fluid viscosity and is hence linked to the adhesive strength [86]. The preparation and discharge mechanism of the adhesive fluids are not understood in detail, but studies have shown that insects have the ability to conserve them by minimising the contact area between the adhesive organ and the substrate [87]. Furthermore, some insects can recover the excreted fluid by reabsorbing it through the pad during detachment [87].
Adhesive fluids have been observed in all insect groups, such as cockroaches and stick insects as well as in flies and beetles [23, 88, 89]. While the composition of the adhesive fluids for smooth pads has been partially investigated and seems to consist mostly of a two-phase microemulsion, the detailed chemical composition of adhesive fluids secreted by hairy pads is still unresolved. Analysis of the latter is more complicated due to their small contact areas and therefore small fluid amounts. Recent studies indicate, however, that the lipophilic phase of the adhesive fluid and the cuticular hydrocarbon layer show similarities [90, 91].
Adhesive liquids are not only employed to increase adhesion [92, 93], but also to facilitate self-cleaning of the adhesive organs [75]. Moreover, insects generate both adhesion (perpendicular to the substrate) as well as friction (parallel to the substrate) forces and optimise the contact release, requiring an intricate interplay of the fluid with the contacted surface. To determine the underlying physical principles of the fluid-mediated attachment of insect feet, simple force models that include the surface tension of the liquid, the Laplace pressure and viscous forces are often used [85, 94].
2.2.1. Adhesive forces
Several studies have focussed on the mechanisms underlying the generation of adhesive forces by insects. Several parameters have been found to play a crucial role. For instance, Drechsler and Federle investigated the influence of the mediating fluid film thickness and found that adhesion, as well as friction forces on smooth surfaces, decreased with increasing fluid volume, but increased on rough surfaces [95]. On rough surfaces, piled up adhesive fluid compensated for the surface roughness by filling gaps between the adhesive organ and the surface, thereby increasing the effective contact area. This mechanism resulted in increased traction and adhesion [95]. In contrast, excess adhesive liquid decreased friction and adhesive forces on smooth surfaces [42]. Nonetheless, when equipped with deformable adhesive organs (both smooth and hairy), insects achieve higher adhesive forces than predicted by simple models on account of larger achieved contact areas which increase viscous dissipation [44, 96].
2.2.2. Friction forces
Successful locomotion requires friction as well as adhesion, transducing forces perpendicular and parallel to the walked-on surface, respectively. Friction forces between two surfaces can, in a simplified manner, be attributed to two basic principles: the action of surface tension and the laws of hydrodynamic lubrication [97, 98].
The contribution of the surface tension of a fluid to the generation of friction is rather limited, as conclusively demonstrated by the fluids secreted by Indian stick insects [99]. The calculated maximal shear stress attributed to surface-tension effects and the observed shear stresses of stick insects, cockroaches or ants differed by several orders of magnitude [32, 47, 85]. Hence, surface tension alone appears implausible to explain the high-friction forces observed in these studies. Therefore, the second basic principle, namely hydrodynamic lubrication, has to play an important role.
Hydrodynamic lubrication focuses on friction forces based on the viscosity of the mediating fluid layer. Contrary to earlier studies, where Newtonian behaviour of the fluids was assumed, later studies suggest that biphasic adhesive fluids with shear-thinning properties help the insects to generate both sufficient adhesion and friction forces [1, 32, 58, 95, 100].
Yet other investigations demonstrated that the secreted film thickness is rather low and may thus not affect insect adhesion through hydrodynamic lubrication, but rather by boundary friction [23, 101]. In the case of very thin fluid films ( nm, corresponding to boundary friction), interactions via van der Waals forces (vdW) between two substrates become dominant [102].
nm, corresponding to boundary friction), interactions via van der Waals forces (vdW) between two substrates become dominant [102].
Recently, the model of a continuous fluid film between adhesive organs and contacted surfaces in insect adhesion was questioned altogether and instead dewetted areas that give rise to enhanced friction forces were proposed, dominating insect adhesion forces [103, 104]. Clearly, further studies are needed to uncover the exact mechanisms underlying the influence of the fluid [1], particularly as direct experimental evidence for the occurrence of dewetting or direct contact between the adhesive organ and the substrate is missing [36].
Despite many years of research, fundamental physical aspects regarding insect adhesion still warrant further research efforts. Although new studies and experimental data suggest that the classic microscopic attachment models cannot fully explain the observed adhesion and friction forces, it is still quite common to employ simple fluid-mediated models based on capillary and viscous forces. Recent results hint towards more complex models, incorporating non-Newtonian properties of the adhesive fluid or the deformation of the adhesive organ and thus increased contact areas [1]. Possible nano-tribological models as well as boundary lubrication mechanisms will likely replace the 'classic' models explaining the observed friction forces [85, 105]. With substantial research efforts in this field, there are still several open questions, including the exact physics of adhesion underlying the locomotion of the insects.
2.3. Investigated insects
Over the course of the past four decades, a number of insects have been chosen as models for adhesion studies, which are by now well-established [106–110]. This section provides an overview of the most commonly employed insects and their importance.
A large number of insects are commercially available from specialised companies or can be locally sourced, making studies cost-effective and allowing statistical sampling across multiple animals. Another benefit of working with insects compared to other lab animals is that approval from an institutional animal care and use committee (IACUC) is usually not required [111]. Their fast proliferation further enables the establishment of laboratory colonies, which has several advantages compared to wild populations since controlled colonies can provide a certain level of uniformity, which may not be granted by field-collected animals. 'Wild' animals are hard to unify as they might differ in age, health, genetic diversity and/or nutritional conditions. Established colonies under laboratory conditions, on the other hand, may consistently provide disease-free, high-quality samples and thus minimise performance variations [112]. Furthermore, laboratory colonies supply the studies with research model organisms year-round, while most insects are highly seasonal with short lifespans in their natural environments [112]. Model insects are classified into two categories, according to their attachment organs, namely soft adhesion and hairy adhesion pads.
Soft adhesion pad role models frequently include stick insects (e.g. Carausius morosus), ants (e.g. Atta cephalotes), cockroaches (e.g. Gromphadorhina portentosa) and orthopterans (e.g. Tettigonia viridissima) [106, 107, 113, 114], as they have demonstrated great adaptability to rough surfaces. Studies addressing hairy pad adhesion often employ leaf beetles such as the Colorado potato beetle (e.g. Leptinotarsa decemlineata), green dock beetles (e.g. Gastrophysa viridula) and ladybirds (e.g. Coccinella septempunctata) [110, 115, 116]. A recent comparative study between Colorado potato beetles and green dock beetles demonstrated that adhesion mechanisms were comparable in both beetle species, despite different body dimensions and widths of the terminal endings of the adhesive hairs [117]. It was, however, also shown that the bigger potato beetles were able to generate stronger absolute adhesion forces while the smaller green dock beetles displayed two to five times larger force per body weight (often termed the 'safety factor') [117]. Nevertheless, the similarity in size and form of adhesive setae allow studies to compare the adhesive mechanisms of these insects and thus develop effective countermeasures for a range of similar insects.
Nature already provides several examples in the plant kingdom that offer efficient methods to reduce insect adhesion on their surfaces. The following section discusses these natural countermeasures.
3. Plant leaves displaying natural insect anti-adhesion properties
Plants and insects share the planet and one cannot survive without the other, displaying intricate plant–insect interactions. These can be classically divided into mutualistic (beneficial for both parties), antagonistic (one party is harmed, one benefits), or commensalistic (one party benefits, but the other is not harmed) interactions. Antagonistic relationships between plants and insects, in particular with regard to adhesion, are most relevant to this review, as plants have developed multiple ways to minimise them.
Over the course of approximately 500 million years, plants have evolved in the most diverse habitats on the planet [27, 118], bringing forth numerous adaptations to niche pressures and extreme environmental conditions that are reflected in the large variety of biological structures. Adaptations to the structure and morphology of plant leaf surfaces provides them with a range of multifunctional properties that are summarised in figure 4.
Figure 3. Schematic of the close contact of smooth and hairy pads to a rough substrate. Note that, in both cases, the contact zone is mediated by an adhesive fluid (blue). Adapted from [85]. CC BY 4.0.
Download figure:
Standard image High-resolution imageFigure 4. Schematic of the most prominent functions of the plant boundary layer on a hydrophobic micro-structured surface: (A) transport barrier, (B) surface wettability, (C) anti-adhesive, self-cleaning properties, (D) signalling, (E) optical properties, (F) mechanical properties and (G) reduction of surface temperature. Adapted with permission from [119].
Download figure:
Standard image High-resolution imageSimilar to the exoskeleton of insects, the outermost layer of a leaf is known as the cuticle. The cuticle of leaves governs protective properties, in addition to stabilising the plant tissue (figure 4(F)). One of the most important features is its function as a transpiration barrier that prevents the plant from drying out (figure 4(A)). There are only very few examples, such as algae or the bark of trees, that can survive without the cuticle and its protective barrier [27]. Other traits include surface wettability and self-cleaning properties (figures 4(B) and (C)), as most famously known from lotus leaves [120]. Furthermore, the cuticle of plants can modify the heat signature of the surface [121] (figure 4(G)), form photonic structures [122] and provide both signalling as well as traction for pollinating insects [123] (figures 4(D) and (C)).
A closer look at a plant's protective membrane shows that the cuticle is in fact a composite material consisting of several layers of cross-linked networks of cutin and hydrophobic waxes [124, 125]. The cuticle can be generally subdivided into a thick underlying and a thinner top layer [119]. Both layers are formed by a network of the biopolymers cutin and cutan. Cutin is a polyester made of hydroxyl and hydroxy-epoxy fatty acids, while cutan is mainly composed of ether-linked long alkyl chains with lengths ranging from C22 to C34 [119, 126, 127]. While the cuticle is the main component of the plant boundary layer, many of the functional properties of leaves arise from cuticular waxes, which are both integrated (intracuticular) as well as on the surface (epicuticular) of the cutin network.
The chemical composition of plant waxes differs from species to species and even from organ to organ of the same species [128]. Chemically, the waxes are a mixture of long-chain and cyclic hydrocarbons with a range of functional groups (hydroxyl, carboxyl, ketyl) [33, 125, 128–130]. Intracuticular waxes regulate water loss (figure 4(A)) as well as the outwards elution of molecules [131–134]. Epicuticular waxes form an interface layer to the surrounding environment. The exterior waxes (partially) regulate properties such as wettability and self-cleaning (figures 4(B) and (C)), solar protection and, notably, anti-adhesive effects against particles, pathogens or insects (figure 4(E)) [120, 135–141].
Barthlott established a classification system describing 23 different wax types based on their chemical and morphological features [33, 142]. Epicuticular waxes form crystalline structures [143, 144]. Studies showed that waxes that were isolated from plant surfaces recrystallised into their original morphology as grown on the plant [144–147]. These structures can be both two-dimensional films or three-dimensional surface structures with various morphologies, such as tubules, platelets, filaments, rods and convex sculptures with a broad size range of 0.5–100 μm [33, 125]. Additionally, the wax crystal morphology and orientation also depends on the substrate material on which they crystallise [148].
Some of the most prominent properties of the structured surfaces of plants is superhydrophobicity (enabling self-cleaning) and the reduction of insect adhesion. Tracing the physical origins of reduced insect adhesion on plants is complex due to the variety of structural features that have been observed. A first classification differentiates between waxy plant surfaces, featuring a protective layer of three-dimensional (3D) epicuticular wax crystals, and non-waxy plant surfaces, which may or may not possess a thin layer of wax.
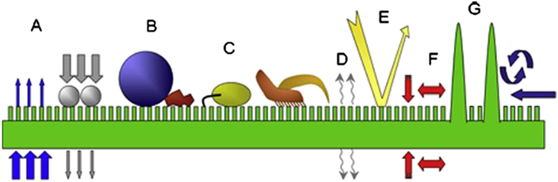
While many studies have shown that both waxy and non-waxy plant surfaces demonstrate anti-adhesive properties, the physical concepts underlying anti-adhesion are very different [120]. Gorb and Gorb first demonstrated that waxy plant surfaces cause a reduction in insect adhesion and attributed this to four different contributing factors [34, 149], schematically shown in figure 5:
- (a)Contamination: wax crystals detach from the surface of the plant cuticle contaminating the adhesive pads of the insects, thus impairing their ability to adhere.
- (b)Wax dissolution: adhesive fluids secreted by insects dissolve wax crystals, thus increasing the film thickness of the mediating fluid layer, rendering the surface slippery.
- (c)Fluid adsorption: waxy surfaces may both adsorb and absorb the mediating adhesive fluid secreted by the adhesive organs of the insects, thus reducing capillary forces responsible for adhesion.
- (d)Roughness: wax crystals on the plant cuticle form a micro-rough surface, thus decreasing the contact area between the substrate and the adhesive organ of the insect.
Figure 5. Schematic representation of the contributing factors giving rise to anti-adhesive properties of plant cuticles covered with wax crystals. (A) A seta contacting a waxy surface; (B) contamination of adhesive organs; (C) wax dissolution to render the surface slippery; (D) fluid adsorption to lower capillary forces; and (E) impact of roughness (inspired by [149]).
Download figure:
Standard image High-resolution image3.1. Contamination
Contamination lowers adhesion based on the impairment of the adhesive organs of the insects due to the adsorption of loose wax crystals (figure 5(B)). This can be observed in several plant species, such as Brassica or Nepenthes, both of which feature wax crystals that are easily detachable from their surface [150, 151]. Gorb et al observed detached wax crystals that function as a separation layer between the insect pad and plant surface, thus minimising capillary adhesion as well as adhesion caused by van der Waals (vdW) forces [152]. This effect has been observed for flies, ants and beetles [150, 153]. Furthermore, the contamination effect has shown enhanced effectiveness against adhesive pads that employ fluid secretion [149].
3.2. Wax dissolution
Wax-dissolving assumes that the secreted adhesive fluids of insects are capable of dissolving wax crystals, leading to an increased thickness of the fluid layer. This, in turn, promotes reduced adhesion and friction forces and the surfaces become slippery for insects (see figure 5(C)). When studying pad secretions left behind by insects, the secretions were found to contain non-volatile lipid-like components [154]. In the pad secretions of ladybird beetles (Coccinellidae), hydrocarbons, waxes, fatty acids and alcohols were found [86, 89, 155], which indicate that the adhesive fluid has the capability of dissolving plant wax crystals.
Despite these preliminary results, there is only indirect evidence that adhesive fluids of insects indeed dissolve waxes [156, 157]. While many studies suggest that non-polar organic solvents such as benzene, hexane and some polar solvents such as chloroform are appropriate choices to dissolve most epicuticular waxes [158, 159], it has been shown that solvents of intermediate polarity, such as mixtures of chloroform and methanol (3:1) or chloroform and ether, are more efficient [160]. Indeed, solvent mixtures of polar and non-polar solvents are more efficient in dissolving all wax constituents, such as the hydrophobic hydrocarbons and the much more polar compounds containing functional groups [160–162]. Clearly, more work in this interesting field is necessary to determine whether most adhesive secretions are able to dissolve epicuticular waxes.
3.3. Fluid adsorption
The basis of fluid adsorption lies in the high porosity of the wax surface present on the cuticle. As a result, lipid-bearing fluids of insect attachment pads may be absorbed (figure 5(D)) and, as a consequence, the contact area and capillary forces in the contact zone between the adhesive pad and the porous wax surface are reduced. Previous work demonstrated that adhesive fluids secreted by insects have a major impact on their adhesion forces on both smooth and rough surfaces [100, 163]. For rough surfaces (below the critical roughness range), a general increase in adhesion forces was observed, since the adhesive fluids occupy the gaps between the wax crystals or grooves of the cuticular folds (see section 3.4), thus increasing the contact area. However, thick porous substrates can drain the adhesive fluid [164], thereby reducing the true contact area between the adhesive organ and the substrate, leading to reduced adhesion. Gorb et al investigated the fluid adsorption hypothesis by measuring applied traction forces of seven-spot ladybirds Coccinella septempunctata on nanoporous substrates with the same pore diameters but different porosities [165].
Beyond artificially porous substrates, previous work has hypothesized that three-dimensional extracuticular wax crystals on plant leaves may adsorb the insect-secreted adhesion fluids. However, experimental support for this hypothesis is missing [166]. Gorb and colleagues recently showed that wax coverage in the pitcher of the carnivorous plant Nepenthes alata demonstrates selective adsorption properties [166]. Following this example, Barthlott et al investigated five different plants (Salvinia, Pistia, Fibigia, Helinathemum & Cistus), which possess different extracuticular structures, such as hairs or wax crystals, demonstrating selective surface properties such as hydrophobicity, superhydrophobicity or oleophilicity [167]. They concluded that Salvinia and Cistus developed wax crystals that have the highest and lowest adsorption capacity towards (high) viscous oils, respectively [167]. This discovery may be argued to present a potential tuning of surface adhesion to the adhesion fluids of specific insects.
3.4. Surface roughness
It is well known that the surface roughness of leaves strongly influences adhesion forces, in a manner similar to the challenging task of fixing adhesive tape onto a rough patch of wall. The interaction of an insect with a plant is not different in this regard. The main principle underlying this effect is that surface roughness reduces the usable contact area between the plant surface and the adhesive organ of the insect, thereby reducing the resulting contact forces [168, 169]. The contact area and surface topography of the plant leaf is therefore strongly influenced by the form and size of the wax crystals. The dimensions of extruded epicuticular waxes can range from the nm scale to several µm. Consequently, these wax crystals form micro-rough surfaces that have been shown to reduce the adhesive properties of various insects species [170, 171] (figure 5(E)).
The term 'roughness' is frequently employed in surface studies and describes the deviation from an ideal surface in the normal direction of the plane. The term 'critical roughness' has been coined as the substrate roughness where the attachment forces generated for a specific investigated insect species is the lowest. For stick insects, the critical roughness is 3 μm [172]. For flies, e.g. Musca domestica, or beetles, e.g. Gastrophysa viridula, Leptionatarsa decemlineata and Coccinella septempunctata, the lowest attachment forces were measured for roughnesses of 0.3 to 1 μm [44, 170, 171, 173].
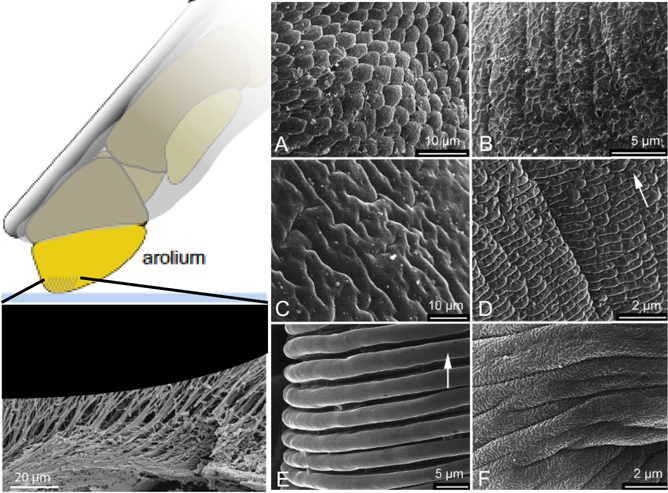
Roughness in plant leaves may also result from cuticular folding, which takes various shapes. Hence, hierarchical sculpting of the cuticle does not only entail the growth of 3D-wax structures, but many plant surfaces also display a combination of cuticular folding and wax crystals [149]. Unsurprisingly, there is a vast variety in shape, size and orientation for both structures [175]. The shapes can vary from tubular over convex to papillate epidermal cells, ranging in size from as small as a few nm (figure 6(A)) to several µm both in diameter and height (figure 6(C)) [176].
Figure 6. Plant surfaces showing different nano- and microstructures. The top-row schematics show the cross-sectional shape of the epidermal cells: tabular cells (i), convex cells (ii) and papillate cells (iii). The pictograms on the left illustrate the level of superimposed microstructures: thin films of wax (o), epicuticular wax crystals (wc) and cuticular folds (cf). Scanning electron microscopy micrographs of plant surfaces of (A) Magnolia grandiflora, (B) Paeonia officinalis, (C) Calathea zebrina, (D) Diospyros kaki, (E) Paeonia suffruticosa, (F) Colocasia esculenta, (G) Hevea brasiliensis, (H) Vitis vinifera and (I) Rosa hybrid Floribunda cv. 'Sarabande'. Reproduced with permission from [174]. © 2011 The Royal Society.
Download figure:
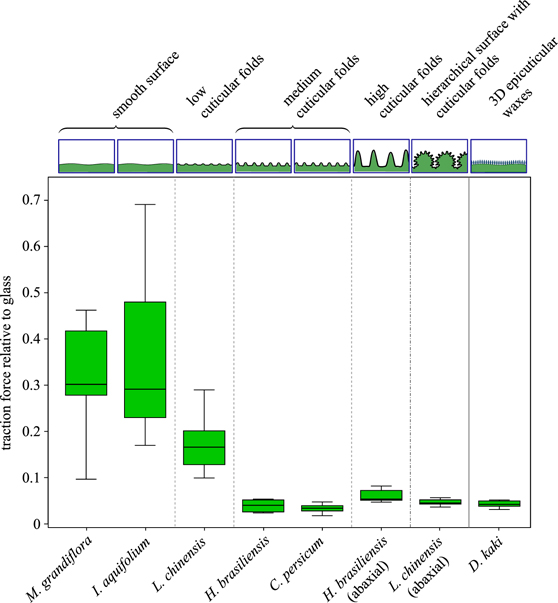
Standard image High-resolution imagePrüm et al demonstrated that plant surfaces with cuticular folds have the same effect on adhesion reduction as epicuticular waxes (figure 7 [174]). These results show that an additional level of superimposed microstructuring had a big effect on the reduction of traction forces, independent of the cell shape. The reduced insect adhesion was ascribed to the micro-roughness created by the superimposition of cuticular folds and epicuticular waxes.
Figure 7. Traction forces of actively walking male Colorado potato beetles, Leptinotarsa decemlineata, on plant surfaces relative to traction forces on glass. Comparison of the influence on traction forces due to different surface structures. These natural surface structures included cuticular folds of different heights, as well as plant surfaces showing 3D epicuticular waxes and smooth plant surfaces devoid of cuticular folds. Reproduced with permission from [174]. © 2011 The Royal Society.
Download figure:
Standard image High-resolution imageVarious studies have confirmed that micro-roughness dramatically reduces insect adhesion [40, 171, 177, 178]. It describes fluctuations in the surface for short wavelengths (peak-to-peak distance) and is characterised by local maxima (asperities) and local minima (valleys) of differing amplitudes [179]. While roughness is a commonly used term, it can be expressed through a multitude of different parameters and can mean different things from one scientific field to another [180]. The amplitude is considered to be the key roughness parameter since it describes the vertical characteristics of the surface deviations. RMS is a frequently employed parameter used to describe the surface topography of samples. RMS is the root-mean-square average of the profile height deviations from the mean line, recorded within the evaluation length [180]. Insects are able to adhere relatively well to smooth surfaces (asperity size  0.3 μm) or micro-rough surfaces (asperity size
0.3 μm) or micro-rough surfaces (asperity size  3 μm) [44, 170, 173]. In contrast, for substrates with a roughness of 0.3 to 1 μm (RMS of 90.0 nm and 238.4 nm, respectively) the attachment forces of the investigated insect species were significantly reduced [181]. Mechanical modelling has shown that these asperities are in a size range that leads to loss of contact area between the tips of the insect setae and the substrate, effectively reducing contact area and adhesion forces [83]. Likewise, it has been shown that both the frequency as well as the spacing in between asperities contribute to the slipperiness of the micro-structured surface [24].
3 μm) [44, 170, 173]. In contrast, for substrates with a roughness of 0.3 to 1 μm (RMS of 90.0 nm and 238.4 nm, respectively) the attachment forces of the investigated insect species were significantly reduced [181]. Mechanical modelling has shown that these asperities are in a size range that leads to loss of contact area between the tips of the insect setae and the substrate, effectively reducing contact area and adhesion forces [83]. Likewise, it has been shown that both the frequency as well as the spacing in between asperities contribute to the slipperiness of the micro-structured surface [24].
While for some plants the micro-rough surfaces on their leaves portray a passive form of protection, other plants employ these actively to capture prey. Carnivorous plant species belonging to pitcher plants Nepenthes developed a fascinating system that exploits the anti-adhesive properties of its surface. A combination of cuticular folding and a water film creates an effective aquaplaning effect leading to the demise of insects [182]. The rim of the pitcher plant leaves feature a micro-structured surface of smooth anisotropic overlapping cells pointing inwards. The micro-roughness of the surface renders the epidermal surface completely wettable, leading to the formation of homogeneous thin liquid films caused by rain, condensation or nectar secretion [183]. This liquid thin film renders the surface slippery for insects since the additional liquid film reduces their adhesion. Poppinga et al investigated the surface morphologies of 53 carnivorous plant species. Their study resulted in a classification of 12 types of anti-adhesive surfaces based on different combinations of epidermal cell curvatures with cuticular folds and/or 3D wax crystals [184].
Nature bestowed a wide range of plants with numerous ways to successfully reduce insect attachment to increase survival. The scientific basis of these methods, namely contamination, wax dissolution, fluid adsorption and surface roughness, forms the groundwork for industrial approaches through bio-mimicry. These industrial advancements come in various forms, sizes and intended uses.
4. Bio-inspired insect-repelling materials based on adhesion reduction
As reviewed in the preceding sections, nature endowed animals and plants with a plethora of astounding functional properties based on surface structures that are important for their everyday survival [27, 185, 186]. The interaction of adhesive mechanisms of insects and the means to counter these mechanisms by plants have substantial potential for industrial and technological applications that also often rely on controlling the adhesion of insects and other 'sticky' materials.
Put into a global context, the demand for multi-functional adhesive systems is constantly rising: according to recent market research, the adhesive market size is projected to grow from 60.4 billion USD in 2020 to 79.9 billion USD by 2025 [187]. This projection includes the demand for adhesives in sub-markets, such as healthcare for biomedical applications that are looking for non-toxic, quick-to-bond, bio-inspired adhesives [188]. This market alone is estimated to grow to a total volume of 10.6 billion USD by 2024 [189]. Other sectors, such as electronics as well as automotive industries, are investing in research for adhesive products with improved properties, such as weather resistance, quick to de/bond properties and re-usability [190]. Controlled anti-adhesion based on surface microstructuring shows promise to substantially contribute to two rapidly growing markets: anti-bacterial coatings and anti-adhesive surfaces for pest control [26, 191], to name just a few.
A further important research area that employs controlled anti-adhesion is pest control. There is high demand for better control and prevention of insect pests, since more than a fifth of globally stored food grains is lost every year [192]. Furthermore, many pathogens, i.e. those causing dengue, rift valley fever or malaria, are transmitted by insects and pose a danger to humans, livestock and the environment [3, 193], highlighting the need for new materials that eliminate them. Current approaches to limit insect adhesion are presented below; some are commercial while others are still in the experimental stage. Following this, ongoing research work focussed on mimicking the diversity of nature's adhesion-reducing structures is highlighted.
4.1. Current commercial approaches to insect management
Current state-of-the-art strategies to tackle insect pests are mostly chemical-based and include insecticides and insect-repellents. Since many of these materials pose toxic dangers to the environment, alternative eco-friendly strategies are sought [26]. Other pest-control approaches exist, such as biological pest control and containment [194], but many of these are difficult to implement on the local scale and incur additional ecological and environmental issues [195].
Recently, substantial progress was made by developing insecticides that target selected pests only [196, 197]. However, even natural solutions with a specialised focus, such as the use of entomopathogenic fungi, may be dangerous to beneficial insects and influence other animals [198, 199].
Plant-based essential oils and their derivatives are significant sources of insecticides. These oils comprise volatile components, featuring generally a lower density than water [200]. They conventionally consist of terpenes, benzene derivatives or hydrocarbons [201]. While mono-terpenoids represent the biggest fraction of these essential oils (up to 90%), displaying a diversity of structures and functions, most mono-terpenes are also the cause of cytotoxic damage to both plants and animal tissue [202, 203]. Due to their volatility, many also act as chemical messenger molecules (pheromone) for both insects and other animals [204]. The physiological interaction between essential oils and insects is still not fully understood, but symptoms of neurotoxic effects have already been observed [205]. Hence, effective pest control is reliant on both sufficient quantities of material as well as efficient functional pathways, which in turn play a very important role for the safety of humans, plants and other vertebrates and therefore need to be carefully identified [206].
Due to the phytotoxic effects of essential oils, products derived from these oils are carefully evaluated for agricultural use [206]. Nevertheless, demand for natural-based products for pest reduction in agricultural use is rapidly growing [207, 208]. Various essential oil constituents are already employed as substitutes for traditional (toxic) insecticides. For instance, isolates such as d-limonene, derived from the essential oils of oranges, lemons and grapefruits, form an essential ingredient in flea shampoos, although the safety of these products is still being discussed [209, 210]. Other isolates, like pulegone or citronella, have found uses as fumigants against mosquitoes and fruit flies [211–213].
While some constituents of essential oils have been reported to be moderately phyto- or cytotoxic, most are still considered as environmentally friendly and safe insect-control agents [214]. A multitude of essential oils are used as culinary herbs and spices, such as rosemary and lavender, featuring properties such as biodegradability and low mammalian toxicity [215]. Pesticide products that are majorly based on these herbs are frequently cleared from toxicity data requirements by the US environmental protection agency [216]. Although there are already various reviews discussing the chemical composition and insect-repellent efficiencies of essential oils, further eco-friendly alternatives need to be developed to cover a bigger range of insect pests [200, 217]. Alternative insect-repellent methods are often coating mechanisms that aim at the reduction of adhesion rather than intoxicating the insect.
4.2. Bio-inspired coatings to control insect adhesion
With the current upsurge in environmental awareness, pest-control solutions based on anti-adhesive surface designs offer the design benefit of not requiring as frequent applications as commercial pesticides. However, the fabrication of bio-inspired coatings that mimic the reduced adhesion properties of plant leaves towards insects forms a tremendous challenge to both scientists and interested manufacturers. While the science investigating anti-adhesive surfaces has made significant progress over the past 20 years, open questions regarding the synthesis and precise function mechanisms are still unanswered [26]. Copying hierarchical rough structures from nature poses important limits on the fabrication of these surfaces (films or particles) that ultimately needs to reflect an accurate and reliable way to synthesize morphologically complex materials. Despite these limitations, a range of different anti-adhesive or insect-repellent products with the potential for real-life applications have recently been developed.
Most of these approaches are based on polymeric materials. Despite their unpopularity in the media, polymers offer unique opportunities in terms of synthesis and adaptability that ultimately benefit the synthesis of environmentally friendly, economical and long-lasting pest control, particularly with the development of novel, bio-degradable polymers [218].
Countermeasures against insects, in the form of adhesion-reduction coatings that are applied to plants or other surfaces, are presented below. Systems that mimic surface features on plant leaves are of particular interest. These are categorised by their adhesion-reduction mechanisms, namely contamination, dissolution, absorption or roughness.
4.2.1. Contaminating surfaces
As discussed above, one way to minimise or prevent insect adhesion is by contaminating the adhesive organs. Inspired by a range of plants that allow the detachment of wax crystals to 'clog or impair' the adhesive organs of the insect [75, 219–221], recent studies have reported similar bio-inspired anti-adhesion material concepts.
Particle size has a major influence on the cleaning efficiency of insects with hairy attachment systems. Particles 10–20 µm in size were removed more slowly than smaller or larger particles [75, 221, 222]. This is due to the fact that the 10 μm particles can be arrested between single setae, as their size coincides with the inter-seta distance. This aggravates the removal of particles, immobilises individual setae and thus inhibits lateral locomotion (figure 8). Particles larger than the inter-setae distance cannot infiltrate the setal arrays and can be cleaned or removed faster, therefore not efficiently restricting adhesion.
Figure 8. Scanning electron microscopy images of adhesive pads of G. viridula after contamination with differently sized particles, followed by eight consecutive steps allowing the self-cleaning of the pads. (A) and (B) 1 µm-diameter particles; (C) and (D) 10 µm-diameter particles; (E) and (F) 45 µm-diameter particles. As pads contaminated with 45 μm particles did not contain any beads after self-cleaning, (E) and (F) show a freshly contaminated pad. Reproduced with permission from [75]. © 2010.
Download figure:
Standard image High-resolution imageFollowing this principle, coatings based on loose particles with tailored sizes were employed as an effective repellent against specifically chosen types of insects [26]. Two studies focussed on this approach using glass and poly(tetrafluoroethylene) (PTFE)-based particles that were found to strongly impair the adhesion of ants, coccinellids, dock beetles and stick insects [75, 221]. While the attachment of both hairy and soft adhesion pads were reduced in equal measure, self-cleaning was much quicker for insects possessing smooth pad organs [75].
Powders that are already employed in modern agriculture demonstrate tremendous potential as eco-friendly alternatives to toxic insecticides [223]. Studies have shown that loose particles are able to form a protective layer against Colorado potato beetles on potato plants [224]. Other studies investigated the efficiency of dust coatings or particle coatings using alumina silicate (kaolin) against a range of arthropod pests, including codling moths [225]. Kaolin (Al4Si4O10(OH)8) is a white, non-swelling, non-abrasive, fine-grained mineral, commonly forming nano-platelets [223]. This chemically inert mineral is sprayed onto plants in the form of an aqueous dispersion [226, 227]. Kaolin particle films were applied to a range of agricultural plants, including pears, apples, olives and potatoes [225, 228–230]. All studies reported a significant decrease in plant penetration rate by the local insect pests, ascribing it to a combination of adverse effects, including impeding movement and lower rates of feeding and egg-laying [223]. Salerno and colleagues recently reported the first detailed study describing the effect of kaolin particle-containing films on insect tarsal attachment [231]. They found a significant reduction in insect adhesion caused by the surface roughness that was created by the kaolin nano-platelets. They also discussed that the observed adhesion reduction may be partially attributed to fluid-adsorption, given the high hydrophilicity and adsorption ability of kaolin [231]. Nanoparticle coatings based on aluminium silicates already comply with the need for economically sustainable and environmentally friendly surrogates to synthetically produced pesticides [223, 232]. Nonetheless, more alternatives to the currently employed systems are required for effective pest-management strategies.
4.2.2. Wax-dissolving slippery surfaces
While the bio-inspired anti-adhesive surfaces described above are based on local contamination, other solutions employ the dissolution of waxes. This strategy relies on the formation of a fluid layer upon interfacial contact, reducing insect adhesion. The systems described below merely demonstrate the potential of this approach as a bio-inspired countermeasure against insects, but unfortunately still lack experimental evidence.
Bio-inspired low insect adhesion coatings include the use of waxes, silicones and polymers for the development of anti-adhesive paints. A particularly elegant and versatile approach are slippery liquid-infused porous surfaces (SLIPS; figure 9) developed by Aizenberg and colleagues. SLIPS demonstrate repelling properties to liquids as well as to organisms such as bacteria, fungi and potentially insects [233–235] by making use of the 'aquaplaning' effect that can be observed in the carnivorous pitcher plants (section 3.4). SLIPS are nanoporous networks, prepared from or integrated into a range of substrate materials, including a variety of polymers (e.g. poly(vinyl chloride) (PVC) or PTFE), metals (e.g. titanium or magnesium) and nonmetallic substances (e.g. wood, glass and silicon) [236–242]. These porous solids are imbued with a lubricant that fills the hollow space of the network. The lubricant overcoat and its chemical properties are critical for the functioning of SLIPS. They determine the immiscibility against intruding liquids and thus the repellency of the surface, as well as the drop mobility of the system [243]. Popular fabrication methods include templating, lithography or layer-by-layer assembly [244, 245]. After hydrophobisation by means of silanisation or fluorination, a liquid overcoat is generated by applying a perfluorinated lubricant (figure 9)[234, 246].
Figure 9. Process of preparing SLIPS by femtosecond laser direct writing. (a) Photo of a pitcher plant. (b) Formation of 3D porous microstructures through femtosecond laser ablation. (c) Modification of the porous substrate with a fluorine layer. (d) Infusion of a silicone oil. (e) Schematic illustration of a liquid droplet sliding down the as-prepared SLIPS. [247] John Wiley & Sons. © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution imagePerfluorinated liquids are, however, expensive and the perfluorinated overcoat poses detrimental effects to the environment and diminishes over time [248]. Therefore, alternative, more durable non-fluorinated lubricants are already being investigated, including siloxanes, ionic liquids, coconut oil and almond oil [248–251].
While SLIPS display an impressive array of features, such as anti-wetting, anti-adhesion, self-healing and anti-fouling properties, their applicability is currently limited to relatively small sample sizes and other concerns such as limited biocompatibility [246, 251–253]. Nevertheless, the presented concept bears tremendous potential as non-polluting countermeasures against insect infestations, if eco-friendly and biodegradable SLIPS variations can be developed on a larger scale. Further systems based on the wax-dissolving principle need to be developed, since their applicability to non-living objects may hold great potential as insect pest countermeasures.
4.2.3. Fluid-absorbing surfaces
Various studies have successfully demonstrated novel materials based on the fluid-adsorption concept. These materials possess a high affinity to adsorb or partially absorb either polar or non-polar solvents, in some cases even partially both. The adsorption of these solvents causes a reduction of the true contact area between the adhesive organ of the insect and the substrate, thus leading to diminished attachment abilities. A reduction of attachment on smooth glass surfaces was observed when treating the adhesive pads of Rhodinus prolixus with lipid solvents [254]. Similarly, aphids, Aphis fabae, temporarily lost traction when walking on silica gels [163]. This study demonstrated that the adhesive organs required a recovery period of approximately 2.4t, where t is the time the insect previously spent walking on a silica gel, until it was again able to walk up a vertical glass surface. This is attributed to the assumption that the reserve of adhesive fluids in the adhesive organs was depleted by the silica gel and needed to be restored [254]. A further study demonstrated a significant attachment reduction due to variations in the adhesive fluid of stick insects, Carausius morosus, which was first observed on smooth polyimide (PI) substrates [100]. The spin-coated polyimide substrates absorbed the watery components of the adhesive secretions of the stick insect and hence significantly reduced friction forces. Experiments using artificial smooth control pads based on poly(dimethylsiloxane) (PDMS) yielded results that supported that fluid-adsorption is the main mechanism at play.
Hydrophilic, and especially hygroscopic, polymer films, such as those made of polyimides or poly(vinyl alcohol) (PVA), are interesting candidate materials for attachment reduction [255] due to their straightforward preparation. While various smooth surfaces from selected materials, as previously shown, demonstrate excellent adsorption properties, several studies have shown that structured surfaces possess equally efficient properties. Previous studies established that nanoporous media, such as porous Al2O3 membranes, adsorb and absorb both polar and non-polar fluids [165]. Indeed, nanoporous Al2O3 surfaces were found to significantly reduce insect attachment (up to 90%), but it is unclear whether this was due to the fluid adsorption and absorption, or local roughness (figures 10(a)–(c)) [165]. In a follow-up study, Gorb and colleagues confirmed that the absorption of a mediating liquid layer between the adhesive organs of ladybird beetles and Al2O3 substrates with 200–250 nm diameter pores was the key factor underlying the observed adhesion force reduction [256].
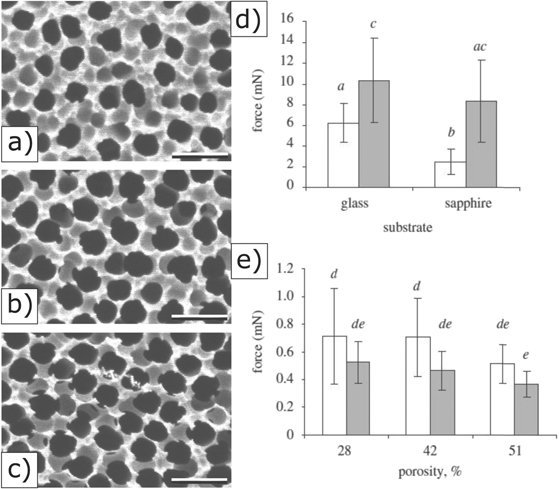
Figure 10. Left: Scanning electron microscopy micrographs of porous membranes with porosities of (a) 28%, (b) 42% and (c) 51%. Scale bars: 500 nm. Right: traction forces of male and female beetles Coccinella septempunctata on smooth solids (d) and porous surfaces (e). Reproduced with permission from [165]. © 2010 The Royal Society.
Download figure:
Standard image High-resolution imageHence, porous substrates may open the path towards the technological development of novel insect-repelling surfaces based on fluid absorption. Studies investigating numerical models for the most efficient substrates with highly efficient absorption properties have already been established [257]. These models revealed that pad fluid is taken up faster by surfaces that feature a fine roughness compared to surfaces with increased aspect ratios of the substrate irregularities [257]. Oleophilic nanoporous surfaces have been successfully fabricated from various materials, including biodegradable polymers and metal composites [258–260]. Modification of pore sizes and surface energies, the two key factors in fluid absorption of porous surfaces, has been achieved using various materials, including silicon and several polymers (e.g. polydivinylbenzene (PDVB)–PDMS and 1,3,5-triethynylbenzene) [261–263]. These materials have shown great absorption capacity for both polar and non-polar solvents as well as repeated reusability. Anti-adhesion experiments with insects have, however, yet to be performed in order to determine their usefulness in this field. Furthermore, the transfer of micro- or nanoporous substrates to large surface areas is still missing, which will be key to their potential use as surface coatings on buildings or other objects.
While several studies investigated the fluid adsorption affinity of naturally occurring waxes, synthetic wax crystals have received less attention. Nonetheless, synthetic wax crystals have been successfully prepared via moulding, thermal deposition processes or through laser structuring [264–266]. In absorption experiments, these synthetic materials demonstrated comparable adhesive properties to their natural counterparts. Gorb and colleagues tested the attachment abilities of the seven-spot ladybird to synthetic wax surfaces, which were prepared through thermal evaporation and consecutive self-assembly based on four alkanes of varying chain lengths (C36H74, C40H82, C44H90, C50H102). The prepared micro-rough coatings comprised wax crystals of different sizes with similar plate-like morphologies (figures 10(d)–(g)) [267]. Traction experiments demonstrated an up to 30-fold reduction of insect attachment forces compared to glass reference samples. This reduction was, however, mainly attributed to the reduction of the real contact area between the tips of the adhesive hairs and the rough surfaces of the wax layers rather than fluid absorption alone [267]. It is thus evident that untangling the precise adhesion mechanism is difficult, especially as roughness is an important parameter, as discussed below.
4.2.4. Rough and slippery surfaces
Rough surfaces as a means to control insect adhesion have received increasing research attention over the last decade [24, 41, 45, 69]. Studies demonstrated that surface roughness, both on plants and artificially prepared surfaces, is a promising property that reduces insect adhesion [268, 269]. In a simple approach, it has been shown that the coarseness of sand paper influences the attachment and locomotion behaviour of various ants by decreasing the real contact area between adhesive organs and substrate surfaces [270–272]. Surface polarity was shown to have only a minor influence on adhesion reduction [268, 273]. Given the importance of roughness, a range of promising innovative surface designs with potential for use as anti-adhesive surfaces against insects from a range of materials have already been established.
Graf and colleagues fabricated insect repellent foils covered by ultraviolet (UV)-sensitive polymers, featuring a regular micro pattern with a 2 μm wavelength, which showed an adhesion reduction of cockroaches by 40% [24, 274]. The fabrication of microstructured surfaces by employing photolithography and nanoimprinting methods served as a model for the preparation of wrinkled polymer films for several anti-adhesive insect studies [24, 275, 276].
Recently, Bergmann and colleagues employed a similar polymer-based approach to fabricate films with controlled surface topographies [273]. Using plasma-induced polymerization, wrinkled polyacrylate films (tetra ethylene glycol diacrylate (TEGDA)) were produced that displayed wrinkling patterns resembling the leaf surface of the rubber tree Hevea brasiliensis. The amplitudes of these wrinkles were tuned in a range from 0.3 to 3.5 μm. Traction force experiments showed that low surface folds resulted in a traction force reduction of 22%, compared to the maximum traction force on glass (figure 11, green). With increasing amplitude, reductions in the traction forces down to relative traction values of 6% were observed, championing the natural role model Hevea brasiliensis (figure 11, green). This study demonstrated that the aspect ratio (AR) of the wrinkled surfaces (ratio of amplitude to wavelength) is a key parameter for the manipulation of insect attachment. Surfaces with ARs in the range of 0.3–1 proved to be the most effective for traction force reduction [273].
Figure 11. Observed traction forces of potato beetles on several surfaces relative to glass. From left to right: green and blue bars represent smooth and structured TEGDA polymer films [273]. Red bars represent micro-rough films consisting of various ethyl cellulose (EC) particles, differing in size and morphology [277]. Both systems (green and red) are compared to biological models, depicted in a light blue colour, namely Hevea brasiliensis and Litchi chinensis.
Download figure:
Standard image High-resolution imageAlternative methods focussing on the preparation of both simple and complex hierarchical micro-roughness include the transfer of surface structures onto arbitrary objects through replication techniques. While techniques such as electroforming, sol-gel techniques or physical vapour deposition have garnered considerable interest in recent years, replica moulding has also proven particularly useful [278]. In replica moulding, a liquid polymeric material is poured onto a master surface to create a negative replica. This negative replica is separated from the master and serves as a transfer medium to create the desired surface structure into a second material, the positive replica [264, 279]. The simplicity and precision of this replication method strongly depends on the desired length scale and the materials employed [280], a wide range of which have been successfully employed, including PDMS, poly(vinyl siloxane) (PVS), acrylonitrile-butadiene-styrene copolymer (ABS) and other UV-curable polymers [281–285]. Two studies demonstrated that accurate replicas of complex model structures, such as the leaf surfaces of Hevea brasiliensis, can be efficiently fabricated [279, 286]. The replication of biological anti-adhesive surfaces through moulding techniques represents a great opportunity for biomimetic applications since these techniques are straightforward and scalable. Furthermore, since surface roughness has a dominant impact on the adhesion mechanism of insects, rather than surface chemistry, it enables the potential use of a wide range of eco-friendly materials [268]. Moulding techniques enable the rapid reproduction of nanostructures as small as 4.5 nm on large areas in a small amount of time (as fast as 60 min per sample) [264] and may therefore be attractive for large objects, such as buildings.
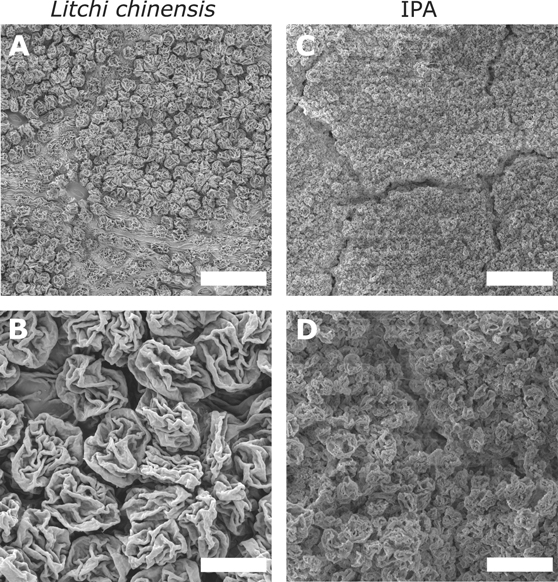
Other approaches to synthesize insect anti-adhesion surfaces involve the fabrication of waterborne organic-based slippery paints that incorporate microparticles with rough surfaces within the paint medium [287, 288]. These studies showed that tailoring the particle sizes and the particle volume concentration results in the loss of traction of insects including fire ants or leafcutter ants on coated surfaces [288]. In a recent study, bio-inspired anti-adhesive surfaces were fabricated via electrospraying. Ethyl cellulose particles with varying sizes and morphologies were deposited onto substrates by incorporating PVA as an adhesive. The resulting micro-rough surfaces featured a strong resemblance to the abaxial side of the leaves of the lychee tree (Litchi chinensis) (figure 12). This study demonstrated that both the particle size and the surface morphology of the particles are crucial for effectively reducing insect attachment [277].
Figure 12. Scanning electron miscroscopy images of the surfaces of (A) and (B) the abaxial side of a lychee plant (Litchi chinensis) and (C) and (D) a surface covered by electrosprayed ethyl cellulose particles (obtained from an isopropyl alcohol solution). Scale bars: (A) and (C) 50 μm; (B) and (D) 10 μm. Reprinted with permission from [277]. Copyright (2021) American Chemical Society.
Download figure:
Standard image High-resolution imageThe fabrication of wrinkled microspheres with controlled variations of particle diameter, morphology and surface roughness enabled the optimization of the overall insect adhesion to values below those observed in nature with reductions up to 96% compared to a glass reference surface. Rough surfaces created by bigger particles (22 μm EC particles, figure 11) reduced the relative traction forces by 81%. Furthermore, the study demonstrated that particle sizes below 10 μm, wrinkle widths below 2 μm and wrinkle-to-wrinkle distance in the µm range are essential for effectively lowering beetle adhesion below relative values of 10% (5 μm and 2.7 μm EC particles, figure 11). The best investigated system lowered beetle adhesion by a factor of 20 below values that were measured on smooth surfaces, comparable to the forces measured on the biological role model, i.e. the abaxial side of the leaves of the lychee tree (Litchi chinensis) (figure 11). The efficiency of the displayed systems is due to the combination of narrowly spaced wrinkles on the particle surface (in the micrometer range or smaller) and the overall particle size (smaller than the claw diameter), which defeats the adhesion mechanism of both components of insect adhesive organs.
Fundamentally, all these studies show that, when manufacturing anti-adhesive surfaces, several size aspects need to be considered: the size of individual elements, such as spheres or films, and the sub-micron morphology, which is the most crucial. Particle systems with tailored size and morphology have enormous potential for the manufacture of surfaces that defeat insect infestations and present applicable opportunities in agriculture or in the building sector due to their straightforward scalability.
While the different studies and methods have shown a variability in possibilities to reduce insect attachment, there is an ongoing discussion about the efficiency of chemical methods in comparison to mechanical ones. Previous studies hold the opinion that surface roughness has a more dominant effect on insect adhesion than surface chemistry [268, 289]. Nevertheless, there are other contributing factors that are still being researched and fiercely discussed. The safety factor (shear force per body weight) is a commonly employed value in combination with surface roughness and insect mass in order to evaluate and determine the anti-adhesion properties of surfaces [290]. The measured safety factors were divided into two categories, namely gripping and slipping. Gripping safety factors describe the shear forces per body weight that can be produced when the insects attach to the surface through the employment of their claws. Research has shown that, for this safety factor category, there was a scaling effect with body mass [40]. On the other hand, the second category, slipping safety factor, does not show a scaling tendency with the insect body mass [290]. The slipping safety factors for various insect species involve smaller forces in comparison to gripping safety factors. Yet for both safety factor categories it was shown that, in most cases, the safety factor decreased with increasing surface roughness. Hence, attachment performances on rough surfaces can be assessed by exploring the correlation between the safety factors (grip and/or slip) and body mass as well as claw diameters [290]. This information may vary from species to species and hence it is difficult to state generic numbers at which safety factors an anti-adhesive surface becomes efficient in repelling insects. Nevertheless, there are several reported examples, such as 1.10 for G. portentosa or 1–3 for N. viridula [231, 290].
5. Concluding remarks
This review has highlighted important topics related to the adhesion of insects to surfaces and biological and bio-inspired ways to counteract insect adhesion. Despite an increasing research activity in this field that is often particularly focussed on bio-inspired materials, many questions remain to be answered. From a biological viewpoint, for example, the adhesive organs of insects have been characterised in detail, yet their precise bio-mechanical functions still need to be elucidated in further experiments. For instance, the non-alignment between the 'fraction mechanics', 'force scaling' and the 'work of adhesion' models need further research. Furthermore, additional research is needed to verify the hypothesis that there is a direct correlation between the specialization of friction pads and body size [53]. Experimental evidence showing the non-Newtonian nature of adhesive fluid has only recently been confirmed [105]. Current tribological models revealed that both the interfacial interactions between the fluid and substrate as well as the chemistry of the bulk play an equally important role for establishing shear-thinning properties [105, 291]. Therefore, further nano-tribological investigations are needed to gain additional insights into the origin of the observed friction forces of various insect species.
Answering these biological questions will undoubtedly lay the basis for potential adhesive and anti-adhesive applications in industry. Bio-inspired adhesive mechanisms, e.g. based on surface structures such as fibrillar interfaces that mimic the adhesive organs of insects or lizards [73, 292–296], as well as chemically different materials [188, 190, 297, 298], have recently gained increased attention. In addition to agricultural applications, other relevant technical sectors have shown increased interest in anti-adhesive products, improving weather resistance, rapid de/bonding properties and re-usability with the potential to be used in the automotive industry [190].
Nature is an excellent role model driving innovation. Plant leaves demonstrate nature's ability to create materials with excellent efficiency in reducing the attachment of insects. Plants such as the Nepenthes or Litchi chinensis developed anti-adhesive surfaces by using several structural and functional adaptions, including varying degrees of surface roughness, slippery infused surfaces or obstruction of adhesive organs of insects. Furthermore, the multitude of surfaces and strategies developed in the plant kingdom highlight the availability of natural solutions to modern-day problems.
As shown in the previous section, such natural materials have fuelled research into bio-inspired solutions for pest control. While several fascinating approaches for the prevention of insect adhesion on surfaces exist, such as slippery liquid-infused porous surfaces or slippery paints, there is still tremendous potential for improvements and large-scale production of these materials. Coatings inspired by slippery plant surfaces may provide an alternative to current toxic insecticides, but these approaches should be inexpensive, easy to produce and apply in order to become commercially feasible and to replace currently used insecticides. Therefore, micro-structured coatings with hierarchical morphologies are the centre of attention for insect countermeasures, aiming to control insect pests in a rapidly growing global market. Scientific research is required to develop further bio-inspired eco-friendly solutions. We envision that the next decade will bring forth a range of new approaches to create long-lasting eco-friendly materials for the production of sustainable insect-repelling surfaces.
Acknowledgments
We thank Professors T Speck (U. Freiburg) and W Federle (U. Cambridge) for helpful discussions. This work was financially supported by the European Commission through ITN 'PlaMatSu' (722842), the Swiss National Science Foundation through the NCCR Bio-inspired Materials (Grant No. 51NF40-182881) and the Adolphe Merkle Foundation.
Data availability statement
No new data were created or analysed in this study.