Abstract
The adhesion generated by a gecko's foot is realised by a structural hierarchy that is also present inside the cortex of a wool fibre. Both structures are based on the same fibril building blocks that belong to the α-keratin family. We show here that this hierarchical structure can be released from a Merino wool fibre with a combination of formic acid refluxing with agitation and trypsin digestion with ultrasonication. Thus, the cuticle scales are shown to be removed from wool yarns by mass-loss, FTIR spectroscopy and SEM followed by the breakdown of the cortex to release macrofibrils at the surface of the remaining yarn. SEM and AFM evidence are presented for the exposure of macrofibrils at the surface of cross-sections of descaled, fibrillated wool fibres. Adhesion measurements in the AFM show that regions of the treated wool have high adhesion, up to 58 nN, consistent with exposure of nanoscale macrofibrils. This exposure is not however homogeneous across the entirety of the cross-sectioned surface of a yarn and further digestion is required to optimise the depth profile of the exposure for direct comparison with the macroscale compliance and adhesion of a gecko's foot. Nonetheless, the current work has developed an experimental route to reserve engineer wool back to sub-unit macrofibrils, in order to replicate the format and to some extent the adhesive properties of a gecko's hierarchal foot structure.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The gecko's foot employs a five tier hierarchical system to achieve consistent adhesion to a surface; ranging from mm sized lamellae, seta, branch, spatula to nanoscaled spatula tips [1–5] and figure 1(a). The spatula tips provide the final contact with a surface, such that a non-muscular action is required for adhesion. This was proven in a rather unconventional way, by attaching a dead tokay gecko by a single digit to the ceiling of its glass tank; the digit was able to support its own body for three days where upon it was removed due to the smell becoming unbearable [1]. There have been several attempts to replicate the gecko's multi-levelled foot structure, and various new techniques and novel materials have been studied (because traditional machining methods do not replicate the appropriate macro to micro structures) ranging from microelectromechanical systems to multi walled carbon nanotube arrays [2–6]. Thus, synthetic dry adhesives have been produced consisting of adhesive elements with gecko like properties such as reusability, directionality, self-cleaning ability, rough surface adhesion and high adhesive stress however, scale-up of the systems has proved more of a challenge. Geckos increase adhesion with animal size by increasing toepad area and decreasing compliance of the toepad in the loading direction [7]. Scaling efficiency is provided by the hierarchical structure of the gecko's foot as a whole; with controllable adhesion at the spatula tip (by van der Waals forces), discrete adhesive elements (lamellae) and compliance normal to the adhering surface while remaining relatively stiff in the loading direction (close to parallel to the adhering surface). These key hierarchical features have been exploited to demonstrate a gecko-inspired adhesive system based on polydimethylsiloxane (PDMS) slanted micro-wedge adhesive elements, that was scaled to enable a 70 kg human to climb vertical glass with 140 cm2 of adhesive per hand [8]. Further work to enhance compliance control with respect to biomimetic, structured dry-adhesives continues [9].
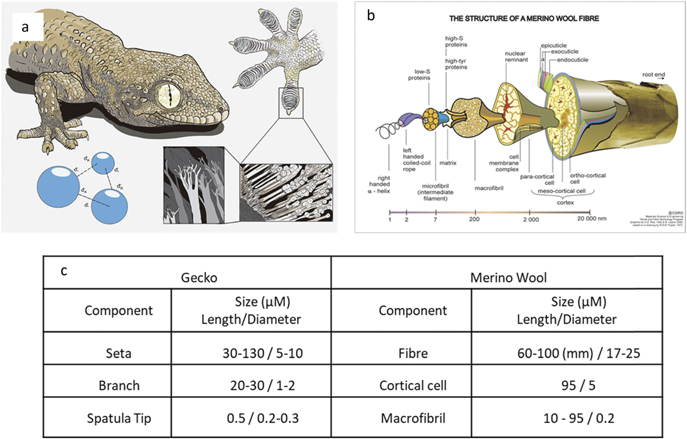
Figure 1. Physical comparison between a gecko's foot hierarchical hair structure and that of the sub components of a wool fibre. (a) Illustration showing each level of the hierarchical structure involved in a gecko's foot attachment system. Reproduced from (https://commons.wikimedia.org/wiki/File:Gecko%27s_secret_power_-_Matteo_Gabaglio.jpg). CC BY 3.0. (b) Illustration of a wool fibre broken down to its protein structure, revealing the sub components of the wool fibre. Reproduced from (https://scienceimage.csiro.au/image/7663). CC BY 3.0. (c) Size comparison of both wool fibre and its components, to the hierarchal structure of a gecko's foot hair.
Download figure:
Standard image High-resolution imageThe hair of the gecko's foot belongs to the keratin protein family as do the fibres of wool. Wool fibres are in fact composite materials that have highly complex physical and chemical composition (figure 1(b)), that has evolved over millions of years in order to protect sheep from the extremes of hot and cold. Wool fibres contain more than 170 different proteins [10]; each type is located in a specific region of the fibre. There are two main structures in the wool matrix; the outer cuticle, or scales, and the inner core called the cortex. The cortex is composed of cortical cells, in turn these cortical cells contain keratin based macrofibrils that run along the length of the mature wool fibre. The macrofibrils have been shown to consist of bundles of microfibrils held together by interfibrillar cement to form bundles that are ≈200 nm in diameter [10]. The cuticle scales, which act as armour plating for the wool, are comprised of three layers; epicuticle, exocuticle and the endocuticle, each have a specific role to protect the fibre from moisture penetration and mechanical abrasion [11–13]. Bradbury however showed complete cuticle removal is possible by submersion of wool fibres in boiling formic acid [14]. Furthermore it is well established that the cortex of wool is susceptible to further breakdown by enzymatic digestion, as well as reduction via thiol groups [15]. Recent work has reported how stretching of single wool fibres and bundles of fibres can be used to explore hierarchal structure transformation mechanisms to produce 230% strains in single fibres as the disulphide bonds and peptide chains were taken apart or reconstructed under load by new crosslinking bonds inside the fibres [16].
The physical dimensions of the hierarchical wool fibre components and that of the gecko's foot hair have been summarised and compared in the table in figure 1(c). It is immediately apparent that the diameter of each structural component of the gecko hair is of comparable size to those of a wool fibre, especially the spatula tip and macrofibril which share a diameter of 200 nm. The wool fibre has a further sub division of the macrofibril, the microfibril (which is in the region of 10 nm in diameter). We suggest that if wool is processed to release these subcomponents at a fibre end then the similarity of these protein structures to those of the gecko should make it possible to replicate the compliance and adhesive power of the gecko's foot. This would require complete removal of the cuticle scales of a cross-sectioned wool fibre and then controlled removal of the cortical matrix to fibrillate and release the component macrofibrils. It is clear from the work of Bradbury that a combined chemical and physical attack is required to achieve such fibrillation. If successful, exposed macrofibril ends (themselves composed of many microfibrils) could provide adhesive surfaces (similar to the spatula tip of the gecko), while the amount of retained cortical matrix could provide control over surface compliance (as per the seta to branch ratio of the gecko foot). If undertaken on an array of wool fibre ends presented at the surface of a textile it may be possible to produce an adhesive fabric with each single wool fibre end acting as a discrete adhesive element (as per the lamella of the gecko's foot).
We report here on the release of the hierarchical structure of Merino wool fibres using a combination of formic acid refluxing with agitation and trypsin digestion with ultrasonication and on subsequent adhesion measurements of exposed macrofibrils at the surface of cross-sections of descaled, fibrillated wool fibres.
2. Results
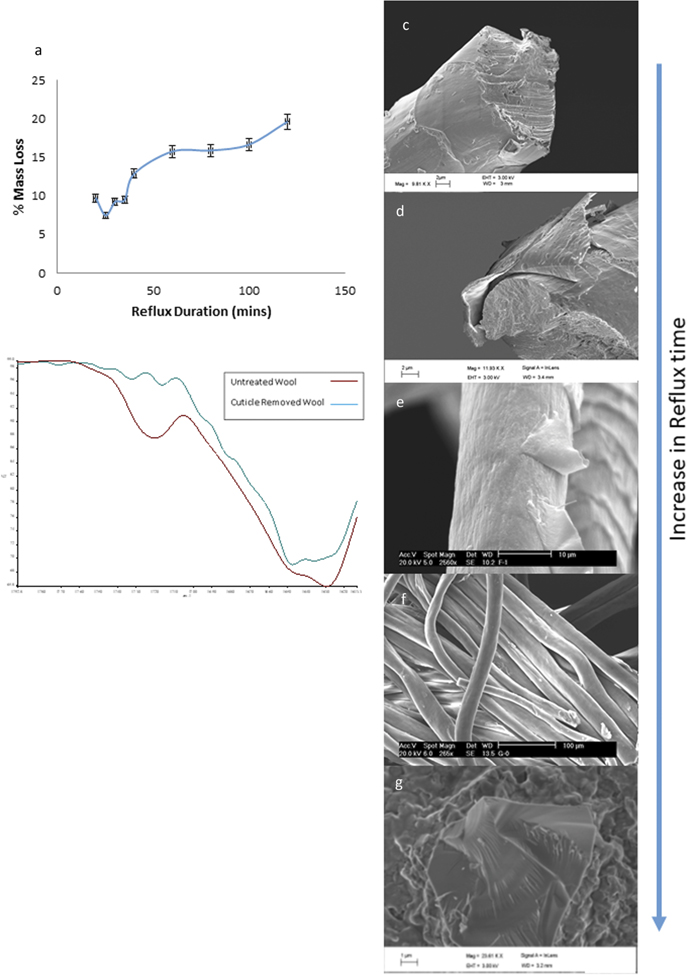
Removal of cuticle scales from individual merino wool fibres has been previously demonstrated by a process of agitation in formic acid [14]. Transferring this technique to Merino wool yarns required modification to the methodology; including increases in the duration of the reflux time and a subsequent agitation. Confirmation that complete removal of cuticles scales could be achieved is demonstrated by a combination of measured mass loss, FTIR spectroscopy and scanning electron microscopy (SEM) (figure 2). The mass loss data show that after an initial increase a plateau is reached after 60 min of reflux (figure 2(a)). FTIR spectroscopy of untreated and refluxed (for 60 min) fibres showed that there was a shift to higher wavenumber of the amide I absorption band (figure 2(b)). This observation is consistent with prior work that shows a shift in the peak of the amide I absorption band occurs upon the loss of cuticle cells, because the cuticle cells contain more cysteine, proline, serine, and valine residues, which are non-α forming amino acids [17–20]. The final piece of evidence for cuticle removal is that from SEM, which shows near elimination of cuticle with increasing reflux time, (figures 2(c)–(f) show progressive removal of surface scales or cuticle, figure 2(f) shows smooth fibres consistent with complete cuticle removal). Furthermore, SEM suggests that the formic acid is delaminating the cuticle scales i.e. favourably attacking the cell membrane complex that holds the cuticles to the cortex (figure 2(d)), as previously reported [14]. The resultant solution was passed through a 2 μm pore sized filter, upon which the delaminated cuticle scales were retained and subsequently imaged (figure 2(g)).
Figure 2. Controlled removal of cuticle scales from a wool fibre. (a) Mass loss of wool fibres as formic acid reflux duration is increased. (b) Fourier transform infrared (FT-ir) spectroscopy of as received wool and cuticle stripped fibres; there is a shift in the peak of the amide 1 absorption band of the refluxed wool that is consistent with the removal of cuticle proteins. SEM of untreated fibre (c) after (d) 10 min, (e) 30 min and (f) 80 min reflux. (g) Detached cuticle scale retained after filtering the 80 min reflux and agitated solution.
Download figure:
Standard image High-resolution imageControl of cellular breakdown at the fibres' end was achieved through a combination enzymatic digestion and mechanical attack of resin embedded and diamond knife, cross sectioned, refluxed yarns (as described in the control of digestion section of the methods and shown in figure 3(a)). SEM shows how the combination of trypsin immersion and ultrasonics breakdown the cellular structure of the cortex (figure 3(b)). Optimum conditions to achieve this breakdown were found to be 0.2 g trypsin per 100 ml of disodium hydrogen phosphate solution combined with 180 s of 40 kHz ultrasonics. Higher magnification SEM images show that this treatment successfully releases fibrils at the surface of the cross-sectioned yarns (figure 3(c)). Image analysis of the exposed fibril ends reveals a mean diameter 260 +/− 58 nm s, (table (d) in figure 3).
Figure 3. Tryspin digestion cortical cells to release macrofibrils in formic acid refluxed wool fibres. (a) Schematic drawing showing how the resin embedded and cross-sectioned fibre was subjected totryspin digestion. (b) and (c) SEM micrographs of the fibre end after digestion and ultrasonic action. (d) Physical dimensions of the macro fibrils observed at the other fibre end.
Download figure:
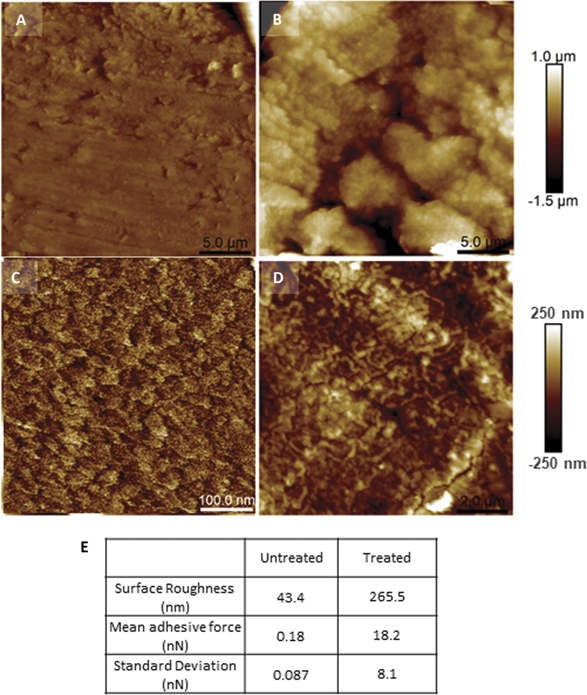
Standard image High-resolution imageAtomic force microscopy (AFM) analysis on the resin embedded yarn cross-sections shows the effect digestion has on the fibre end (figure 4). The tapping mode images, of the resin mounted and cross-sectioned, non-digested and digested fibre ends clearly show a difference in the surface structure and topography. We can see that there is a clean and smooth surface present in the non digested cross section (figure 4(a)) whereas in the digested cross-section there is clear evidence that the structure has under gone morphological changes (figure 4(b)). Higher magnification of the untreated sample is shown in figure 4(c). We can see at higher magnifications that in the treated sample potential macrofibrils are exposed within the cells (figure 4(d)). The areal density of the macrofibrial ends (260 nm across) exposed within a cortical cell is estimated to 6.9 × 106 per mm2. The digested cross section has a mean roughness 6 times greater than that of the as received sample (table in figure 4(e)). An increase in the surface roughness is a key mechanism used by several biological attachments systems, not just geckos [21]. AFM was further utilised here to perform adhesion measurements between the tip and the surface of the digested and non-digested yarn cross-sections. AFM tip retraction or adhesive force was made as a measure of local adhesion on both the non-digested and digested cross-sections (with 100 repetitions for each characteristic region). The non-digested cross-section had a mean adhesive force of 0.18 nN (and a standard deviation, SD of 0.09 nN) whereas the adhesive force in the digested cross sections increased by a factor of 100 to a mean values of 18.2, nN (with a significantly greater range or SD of 8.1 nN) (table in figure 4(e)).
Figure 4. AFM, surface analysis and adhesion data of the resin embedded and cross-sectioned, trypsin digested and non digested wool fibres. AFM topographs of the embedded wool fibre cross-sections. (a) Pre-treatment and (b) trypsin digested at the same scale, 25 μm scan size and 2.5 μm height scale. Higher resolution views of different morphologies on the untreated (c), scan size 500 nm and digested wool fibre (d) surface (10 μm images and 1 μm height). Surface roughness and adhesion data are presented in table (e).
Download figure:
Standard image High-resolution image3. Discussion
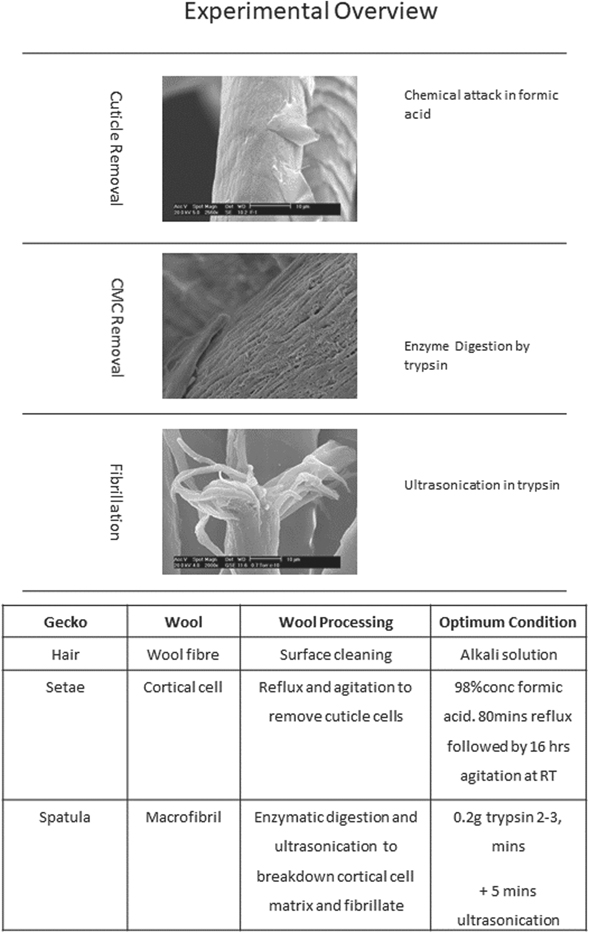
This research has developed a processing route to expose, layer by layer, the components in a Merino wool yarn down to the release of nanoscale macrofibrils at fibre ends. An overview of the experimental procedure and the optimum conditions for each processing step is presented in (and discussed below), and includes indication of which aspect of the gecko's hierarchical foot structure the processing is attempting to release and replicate on a wool fibre. Importantly the physical structure and shape of individual keratin-based macrofibrils can be maintained throughout (figure 5).
Figure 5. Overview of experimental procedure and optimum conditions for each wool processing step and equivalent scale sub-component of the gecko's foot.
Download figure:
Standard image High-resolution imageThe initial processing reported here expands on Bradbury's work for cuticle removal of wool fibres [13, 14]. Use of boiling formic acid to strip the wool fibre of cuticle was adapted in this instance to completely remove the cuticle from a yarn of wool. Using a combination of analytical techniques, a repeatable procedure for complete cuticle removal has been achieved without causing significant damage to the underlying structure of the cortex. This is an essential step as the cuticle protects the cortex of the wool and we had to be confident that all cuticle was removed before any further reverse engineering. The optimum conditions found for complete cuticle removal of a wool yarn are 80 min of reflux in boiling formic acid to attack the cell membrane complex that holds the cuticle to the cortex with an additional 16 h of laboratory shaking in the acid at room temperature to fully detach the cuticle cells.
Once the cuticle was successfully removed it is then possible to further break down wool into macrofibril subcomponents. Immersion of refluxed yarns in a trypsin solution and application of an ultrasonic environment supply the appropriate driving force for the trypsin to digest the matrix that binds the cortical cells and release sub unit macrofibrils. The optimum condition for digestion was found to be a solution of 0.1 M Na2HPO4 containing 0.2 g of trypsin per 100 ml. The refluxed and partially digested wool was submersed and subjected to 3 min of ultrasonic agitation (at 40 kHz) in this solution. This indicates that replication of gecko adhesion by fibrillation of wool is potentially viable, however the mechanical stability of the resulting macrofibrils within the wool fibres is not optimal; it became apparent on this digestion that the released macrofibrils are unable to self-support in a manner similar to a gecko spatula (data not shown, see Caven PhD thesis 2011 [22]). So resin embedding and cross-sectioning of refluxed yarns was essential here to achieve greater control over the fibrillation process.
Resin embedding the fibrillated yarn is an unwanted step as it means that the transfer from yarn to fabric will be a major obstacle however, it was necessary at this stage in order to demonstrate digestion for fibrillation into a hierarchical structure. Digestion at the surface of cross-sectioned, embedded yarns exposes macrofibrils (figure 3) which are measured to occupy an areal density of 6.9 × 106 per mm2, comparing favourably to that of the gecko's foot which has been reported to have a spatula density of 1–14 × 106 per mm2 [2–6].
Mechanical testing to analyse the surface structure of the digested wool by AFM revealed differences in the surface structures of the non-digested and digested or fibrillated cross sections of the wool yarns. Topography images show clear cortical cell boundaries; furthermore roughness analysis shows that fibrillation increased the surface roughness of the digested cross sections by a factor of 6 suggesting macrofibril ends have been exposed (figure 4). The key result of the AFM work is the adhesive force measurements which show that fibrillation of refluxed and digested yarn cross-sections increases the retraction force required to detach an AFM cantilever from the wool surface by 100 from a mean of 0.18 up to 18 nN. The combined adhesive force of a gecko's seta, composed of multiple spatula tips, was reported to be 194 μN [1]. We have not be able to measure adhesion of bundles of macrofibril ends of the digested, fibrillated cross sections of the wool yarns because the force analysis technique used here differs to that of prior AFM work done on the gecko's foot [1]. Nonetheless, digestion of the wool fibre surface has shown a very significant and repeatable increase in adhesive force and if we have indeed exposed macrofibril ends, as suggested by the AFM imaging and surface roughness measurements, then a mean adhesive force of 18 nN is comparable to that reported for the gecko's foot spatula tip of 11 nN [1]. We are yet to confirm if the depth profile is optimised to meet the buckling (and therefore compliance) conditions reported for a gecko's foot, however we believe this could be achievable with further digestion analysis e.g. (prolonged ultrasonication in trypsin) [23, 24].
4. Conclusion
The current work has developed an experimental route to reserve engineer wool back to its sub unit macrofibrils, where each experimental step only targets the desired wool component that needs to be removed, and does not significantly damage the remaining structure. Thus, the format and to some extent the adhesive properties of the gecko's hierarchal foot structure have been successfully replicated in wool yarns in terms of structure and geometric conformation at a resin embedded, cross-sectioned surface of a formic acid refluxed and trypsin digested yarn. Further depth optimisation is required to fully mimic the hierarchical structure of a gecko foot to provide compliance and adhesion however, an optimal release of microfibrils from the exposed cortex of wool could potentially increase surface roughness further and generate a higher density of van der Waals forces on surface contact than that of other biological attachment systems.
5. Materials and methods
5.1. Material
Merino wool fibres were supplied in yarn format, from Godwin Associates Limited, UK. The purchased yarns had been worsted; the raw wool fibre was subject to cleaning in an alkali bath and scouring, before undergoing top marking, where the cleaned fibres are carded and combed so the fibres align. The aligned fibres are then spun into yarns. Spun yarns were used in this format unless otherwise stated.
5.2. Cuticle removal
100 ml of 100% conc. formic acid (Fisher Scientific) was placed in a 250 ml conical flask with 0.1 g of 8 mm sized glass anti-bumping granules (Fisher Scientific). The solution was heated using an electro-mantle to the boiling point of formic acid (97.3 °C), where upon the specimen was lowered into the boiling solution. A condenser was placed onto the conical flask creating reflux [14]. On termination of the reflux, the treated yarn was removed and placed in 100 mml of fresh formic acid at room temperature, and then agitated in a laboratory shaker, Fisher Scientific, at standard laboratory conditions. The optimum treatment for complete removal of the cuticle with minimal damage to the cortex, as defined by a combination of FTIR, mass loss, and microscopy, was found to be 80 min reflux duration in 98% formic acid followed by 16 h of agitation in a laboratory shaker at room temperature [25]. The fibres were then removed from the solution, where upon they were washed and neutralised with distilled water.
5.3. Cortical cell breakdown
Refluxed yarns were subsequently digested through enzymatic action in order to release the macrofibrils. 0.2 g of trypsin, supplied by Fischer Scientific, was dissolved in 100 ml solution of disodium hydrogen peroxide (Na2HPO4), supplied by VWR International. This solution was placed in an Ultrawave QS3 ultrasonic bath and the refluxed yarns then added to the solution. The suspended yarns were subjected to ultrasonic action of 40 kHz, at 23 °C +/− 5 °C, up to a duration of 5 min.
5.4. Control of digestion
Refluxed wool yarns were resin embedded using a JB-4 resin embedding kit (Fisher Scientific). Cross sections of the embedded yarns were prepared by a Reichart Ultracut L5 ultramicrotome using a glass knife for bulk resin removal and a diamond knife for the final section cut. The digestive solution was prepared as above, the resin block was held vertically and lowered into the solution such that the cross sectioned yarns were facing down and were immersed (figure 3(a)).
5.5. Microscopy
Two types of scanning electron microscope techniques were used to analyse the results of the experiments. A Phiips XL30 environmental scanning electron microscope with tungsten electron source and a Carl Zeiss Gemini 1530 field emitting gun scanning electron microscope (FEGSEM). Specimens were mounted on aluminium stubs with a carbon conductive adhesive pad. This secured the specimen in place and allowed conduction to occur, and to create better conductivity graphite dag was painted on the stub and one edge of the specimen. Sample preparation for SEM was as follows, sputter coating with gold and the machine was operated at a working distance of 10 mm with an accelerating voltage of 20 kV. The specimens observed under the FEGSEM were prepared in the same way as the above method, with the exception of a platinum coated being used and the machine was operated using an accelerating voltage of 3 kV with a working distance of 4 mm.
5.6. Atomic force microscopy
The resin embedded and sectioned yarns were examined under the multimode AFM on a Nanoscope Iva controller, equipped with a 150 μm range J-scanner, and for imaging it was operated in tapping mode with a TESPA-V2 silicon probe (Bruker). The samples were cut down to 2–3 mm thickness so that they could physically fit into the sample stage of the AFM. A cut off device with a diamond cutting wheel was used to perform this sectioning, Struers Accutom. Adhesion maps were obtained in force volume mode using SNP probes (Bruker) with a nominal spring constant of 0.32 N m−1. Trigger (max) force was 50 nN (around 150 nm deflection) and the z-ramp was 600 nm at a velocity of 20 μm s−1. 100 adhesion measurements were extracted from the force volume maps, across multiple fibres for both treated and untreated fibres. Several probes were used across multiple experiments, and each was calibrated individually using the thermal tune method.
Acknowledgments
The authors gratefully acknowledge the Engineering and Physical Sciences Research Council (UKRI-EPSRC) for funding the research as a Doctoral Training Partnership. We also acknowledge support from the Leeds EPSRC Nanoscience and Nanoequipment User Facility Grant EP/R02863X/1 at the University of Leeds. The study was also partly supported by the Bergrettung Tirol (Austria) and the Comet K-Project 'Sports Textiles' (Grant No. 820494), funded by the Austrian Research Promotion Company (FFG), Standortagentur Tirol (Austria), and region Vorarlberg (Austria).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).