Abstract
The development of durable and high-performance absorbents for in situ oil–water separation is of critical importance for addressing severe water pollution in daily life as well as for solving accidental large-scale oil spillages. Herein, we demonstrate a simple and scalable approach to fabricate magnetic-responsive superhydrophobic melamine sponges by in situ deposition of PDA coatings and Fe3O4 nanoparticles, followed by surface silanization with low surface energy 1H,1H,2H,2H-perfluorooctyltriethoxysilane (PTOS) layer. The prepared melamine sponge composite (PTOS-Fe3O4@PDA/MF) not only exhibits a very high water contact angle of 165 ± 1.5° and an excellent ability to uptake a variety of oils and organic solvents (e.g. up to 141.1 g/g for chloroform), but also shows robust durability and superior recyclability. The PTOS-Fe3O4@PDA/MF sponge can also efficiently separate oils (or organic solvents) and water, as demonstrated by different model systems including immiscible oil–water solution mixture and miscible water–oil (W/O) emulsion (stabilized by surfactants). Furthermore, the PTOS-Fe3O4@PDA/MF sponge is able to in situ recover organics from water using a peristaltic pump, which gives it significant advantages over other traditional batch processes for oil–water separation. We believe that the PTOS-Fe3O4@PDA/MF sponge provides a very promising material solution to address oil–water separation, especially for the large-scale problems that have been long-time challenges with conventional sorption methods.
Export citation and abstract BibTeX RIS
1. Introduction
Efficient oil–water mixture separation is important to address many occurrences of oil spill accidents and contamination/pollution from many industry sectors in recent years [1–5]. Conventional methods for solving this environmental challenge include gravity-assisted filtration and separation through rotary distillation and centrifugation [6–9], which typically involve long processing time, high cost, and tedious chemical separation procedures, and only exhibit a relatively low separation efficiency [10–12]. Therefore, a low-cost, simple, and scalable approach is required for enabling in situ oil–water separation, especially for addressing large-scale problems.
Utilization of oil absorbents has become a relatively common approach to separating oil from water, and has been demonstrated in various systems such as fibers, wool, activated carbon, and porous sponges [13–19]. Among all of these candidates, foam-based material is particularly attractive due to a high porosity (>99%) that is critical to yield high solvent uptake [20–23]. While several studies have demonstrated that commercial melamine formaldehyde (MF) foam could be useful for crude oil removal from sea and industrial wastewater [20, 24], the oil-absorbing performance of melamine sponges can be substantially improved through further chemical modifications when a superhydrophobic surface/framework is produced. Many functional superhydrophobic MF-based sponges from different preparation methods have been developed for selectively absorbing oil/organic solvents with excellent water-repelling property. For example, Wan et al reported a novel MoS2-coated melamine-formaldehyde (MF@MoS2) sponge created through a 'dipping-drying' process, enabling a high uptake of 157 g/g for chloroform [25]. Wang et al prepared a polydopamine (PDA)-coated sponge with sequential dodecanethiol modification, which absorbed up to 98.6 g g−1 of organic solvents relative to its original mass [26]. Dong et al fabricated a superhydrophobic porous composite sponge containing PDA, MIL-53(Fe), and 1-dodecanethiol, resulting in an absorption capacity of 54.1 g/g for petroleum chemicals and 120.2 g/g for chloroform [13]. Moreover, Lei et al fabricated a ZIF-8-embedding MF sponge by a solvothermal method, which absorbed oil from water rapidly with absorption capacity up to 38 g g−1 [27]. In a very recent seminal work, Liu et al introduced Fe3O4 magnetic nanoparticles (MNPs) during PDA coating onto the foam scaffold, followed by the growth of branched polydimethylsiloxane brush layers, and achieved a high oil uptake value (161.3 g g−1) [28].
All of these studies confirm the significance of producing a superhydrophobic surface by generating high roughness at hierarchical length scales (micro and nano), combined with the application of low surface energy materials. Generally, alkyl silane, fluorinated substances, and other materials containing alkyl groups are commonly employed as hydrophobic modifiers to reduce the surface energy [29–32], which can be paired with organic/inorganic particles for roughness enhancement including PDA coating, egg powders, metal organic frameworks (MOFs), titanium dioxide (TiO2), and silica (SiO2) [32–37]. However, most of these reported methods require either expensive chemical agents, a complex/tedious modification process, or potentially harsh reaction condition, hindering the practical applications of superhydrophobic MF sponges for addressing large-scale oil–water separation.
Furthermore, durability is another very important property for enabling excellent recyclability of these sponge absorbents in order to further strengthen their economic competitiveness. PDA is selected as an ideal candidate for modifying MF sponges to produce superhydrophobic surface not only because it can improve the roughness via spontaneously polymerizing dopamine monomer under mild reaction conditions [3, 6], and also can strongly adhere/attach to the surface of various particles due to the abundant catechol hydroxyl and amino groups [13, 32]. Moreover, magnetic nanoparticles have often been introduced to the sponge surface to providing the ability of these absorbents to be magnetically separated/retrieved [23, 38, 39]. However, most of previous studies require the introduction of pre-synthesized MNPs on the surface of the sponge through dip-coating or a secondary coating approach, which increases the complexity of the preparation method and limits the durability of the sponge due to poor interactions between NPs and polymeric coating layers [40].
Here, we successfully prepare a highly durable, magnetic-responsive superhydrophobic MF sponge for in situ oil–water separation through a facile and scalable method. These sponges are composed of PDA particles and Fe3O4 nanoparticles (NPs) for increasing hierarchical roughness and 1H,1H,2H,2H-Perfluorooctyltriethoxysilane (PTOS) for producing low energy surface. The as-prepared PTOS-Fe3O4@PDA/MF sponges exhibit excellent water repellence with a water contact angle (WCA) above 160°, regardless of the presence of high acidity or alkalinity (pH value ranging from 1 to 14) water droplets. The employment of Fe3O4 results in excellent magnetic properties of foams, enabling the simple retrieval of PTOS-Fe3O4@PDA/MF by a low-cost magnet (0.2 T). The PTOS-Fe3O4@PDA/MF sponge is able to absorb a high amount of various oils and organic solvents, such as 59.3 g/g for hexane and 141.1 g/g for chloroform compared to its original weight. Furthermore, the PTOS-Fe3O4@PDA/MF sponge also has an outstanding oil uptake performance that can efficiently separate oils from different types of oil–water mixtures with a remarkable recycling performance (less than 2% performance deterioration after 15 cycles). Finally, we demonstrate the ability of the PTOS-Fe3O4@PDA/MF sponge to rapidly and continuously separate oil–water mixtures using a peristaltic pump, suggesting potential applications for addressing practical large-scale oil spill problems.
2. Results and discussion
2.1. Characterization and surface wettability of the sponges
Scheme
Scheme 1. Schematic illustration of the fabrication process of PTOS-Fe3O4 @PDA/MF sponge.
Download figure:
Standard image High-resolution imageSurface roughness is a key parameter for controlling surface hydrophobicity. According to Cassie–Baxter equations, the water contact angle of a surface relies on the volume fraction of the water/solid contact surface area, namely the ratio of the liquid/solid contact area to the total projected area of the drop basement. Therefore, producing roughness on the surface could effectively introduce 'air pockets' to achieve a superhydrophobic surface [46, 47]. As shown in figures 1, S1, and S2 (available online at stacks.iop.org/NANO/32/275705/mmedia), the surface morphology of sponges after each processing step was characterized by scanning electron microscopy (SEM). Compared to pristine MF (smooth surface from figures 1(a), (e)), the PDA/MF sponge shows a significantly increased roughness (figures 1(b), (f)) from the deposition of PDA layer. Introduction of Fe3O4 onto PDA/MF sponge further improves the surface roughness as illustrated in figures 1(c), (g), where NP aggregates are clearly visible. After the growth of low-surface-energy PTOS layer, PTOS-Fe3O4@PDA/MF sponge exhibits very limited changes in surface morphology (figures 1(d), (h), (i)). The elemental mapping images of PTOS-Fe3O4@PDA/MF sponge, from figures 1(j) to (p), confirm the homogeneous distributions of C, N, Fe, O, F, and Si elements onto the struts at a macro-scale, indicating the successful decoration of PDA layers, Fe3O4 NPs and PTOS, onto MF sponge surfaces. The heterogenous structures of different particles and layers at a nanoscale were confirmed by SEM images in figure S2.
Figure 1. SEM micrographs of different foams including (a), (e) MF; (b), (f) PDA/MF; (c), (g) Fe3O4@PDA/MF; and (d), (h), (i) PTOS-Fe3O4@PDA/MF sponge. (j)–(p) Element mapping images of PTOS-Fe3O4@MF sponge, in which the element of interest is labeled in each micrograph.
Download figure:
Standard image High-resolution imageThe surface wetting behaviors of the as-prepared sponges to water droplets (applied on their surface) were changed after each processing step/modification, as shown in figure 2. The inclusion of PDA into the sponge changes its color from white (pristine MF, figure 2(a)) to black-brownish color (figure 2(b)), which is retained after sequential modification steps, including adding Fe3O4 (figure 2(c)) and PTOS layer (figure 2(d)). For pristine MF sponge (figure 2(a)), the water droplets were completely wetted on the surface after being deposited onto the sponge surface, leading to a nearly 0° WCA (representing highly hydrophilic nature). However, this water-wetting behavior was significantly altered for PDA/MF, Fe3O4@PDA/MF, and PTOS- Fe3O4@PDA/MF sponges as shown in figures 2(b)–(d), with all results confirming that these sponges are highly hydrophobic (WCA all above 130°). After coating PDA on the MF sponge surface, the WCA increased to 134 ± 2.0° after being completely dried. This substantial increase in hydrophobicity cannot be simply explained by the physical natural of PDA layer since PDA is a hydrophilic polymer. Instead, this phenomenon is due to the synergistic effects of (1) selective exposure of benzene moieties of PDA instead of its hydroxyl groups to air, and (2) significantly improved surface roughness, which is also consistent with previous reports associated with 'unexpected' superhydrophobic behavior of PDA layers on other substrates [48]. The introduction of Fe3O4 NPs from in situ deposition onto the PDA layer leads to an increase of WCA to 154 ± 1.8°. After the modification of PTOS, the WCA of the PTOS-Fe3O4@PDA/MF sponge further increases to 165 ± 1.5°, showing excellent superhydrophobic property owing to the significantly increased surface roughness (attributed to PDA and Fe3O4) and reduced surface energy (attributed to PTOS), as previously discussed using Cassie–Baxter theory.
Figure 2. Methyl blue dye labeled water droplets on the (a) MF, (b) PDA/MF, (c) Fe3O4@PDA/MF, and (d) PTOS-Fe3O4@PDA/MF sponges. The water contact angle measurements are included in the inset of each figure. (e) The contact angle from water with different pH values on the PTOS-Fe3O4@PDA/MF sponge. Photographs of water droplets on PTOS-Fe3O4@PDA/MF sponge surface are shown in the figure inset. (f) Photograph of water bouncing from PTOS-Fe3O4@PDA/MF sponge surface. (g) Photograph of MF (white) and PTOS-Fe3O4@PDA/MF (brown/black) sponges after being soaked in water and (h) after immersing the sponge in water.
Download figure:
Standard image High-resolution imageThe superhydrophobicity of the PTOS-Fe3O4@PDA/MF sponge is very stable even with highly acid and alkaline water (figure 2(e), pH value ranges from 1 to 14), which can fully meet the needs for some potentially harsh applications. When water was spilled onto the surface of PTOS-Fe3O4@PDA/MF sponge, it immediately bounced from the sponge surface with no residues left (figure 2(f)), which further confirms the superhydrophobicity of the PTOS-Fe3O4@PDA/MF sponge surface. The water-repelling property of pristine MF and PTOS-Fe3O4@PDA/MF sponge is shown in figure 2(g). The pristine MF sponge can completely sink into the water, while the PTOS-Fe3O4@PDA/MF sponge is only able to float on the water surface. Furthermore, when PTOS-Fe3O4@PDA/MF sponge is forced to be immersed into the water, air bubbles surrounding functionalized MF sponge surface were formed (figure 2(h)), and the sponge floats on the water surface again, immediately after the external force is removed, confirming the superhydrophobicity of MF sponge composite, attributed to the construction of micro/nanostructure (as observed from SEM, figure 1(d)) and the modification of low-surface-energy PTOS layers.
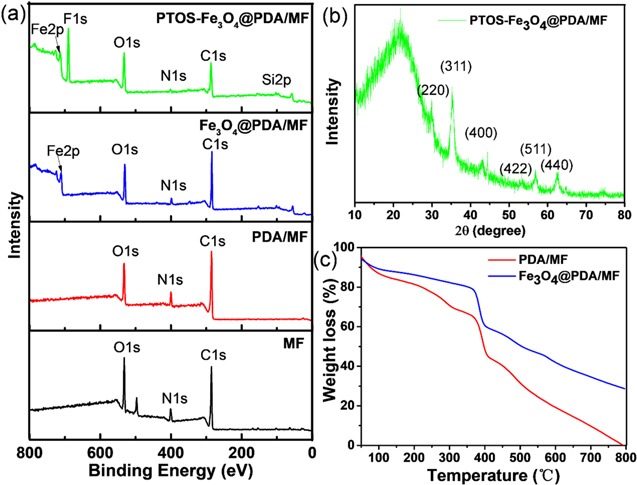
The surface elemental composition of the prepared sponges after each processing stage was investigated by XPS (figure 3(a)). The peaks at 286.9, 400.3, and 532.1 eV are attributed to the binding energy of C 1s, N 1s, and O 1s (from MF), respectively, which can be observed in the MF, PDA/MF, Fe3O4 @PDA/MF, and PTOS-Fe3O4 @PDA/MF sponges [49]. After Fe3O4 NPs were in situ co-precipitated onto the surface of PDA/MF sponges, additional peaks observed at 711.3 eV were ascribed to the binding energy of Fe 2p [50]. For PTOS-Fe3O4 @PDA/MF sponge, the new peaks of F 1s (689.0 eV) and Si 2p (102.3 eV) were observed [51, 52], corresponding to the introduction of PTOS layer. To further investigate the crystal structure of Fe3O4 within the PTOS-Fe3O4@PDA/MF sponge, X-ray diffraction (XRD, figure 3(b)) characterization was performed. Characteristics peaks of (220), (311), (400), (422), (511), and (440) were observed, corresponding to the cubic spinel structure of Fe3O4 (JCPDS card no. 19-0629) [41]. The thermal stability of the Fe3O4 @PDA/MF sponge and the loading amount of Fe3O4 NPs were determined by TGA, as shown in figure 3(c). For both PDA/MF and Fe3O4 @PDA/MF sponges, the weight loss from 375 to 400 °C is associated with the decomposition of methylene bridges from melamine sponge [28]. As a comparison, the total weight loss ratio of PDA-Fe3O4@MF decreases nearly by 28.1% at 800 °C, indicating a high loading content of Fe3O4 NPs by in situ co-precipitation method.
Figure 3. (a) XPS spectra of MF sponges after each processing step. (b) XRD pattern of PTOS-Fe3O4@PDA/MF sponge and (c) TGA of PDA/MF and Fe3O4@PDA/MF sponges.
Download figure:
Standard image High-resolution image2.2. Oil absorption and oil–water separation
The loading of Fe3O4 NPs not only improves hierarchical surface roughness of the PTOS-Fe3O4@PDA/MF sponge, but also enables the magnetic properties for easy retrieval. As shown in figures 4(a)–(c), the movement of the superhydrophobic PTOS-Fe3O4@PDA/MF sponge can be easily controlled by a magnet (0.2 T) to effectively absorb n-hexane (red) at desired locations in water solution (Video S1). The magnetic performance of Fe3O4 @PDA/MF and PTOS-Fe3O4 @PDA/MF sponges were both investigated by VSM (figure 4(d)), which showed them to be superparamagnetic at room temperature. The saturation magnetization (Ms) is 8.5 and 5.9 emu/g for the Fe3O4@PDA/MF and PTOS-Fe3O4@PDA/MF sponge, respectively. The slight decrease of the Ms of the PTOS-Fe3O4 @PDA/MF sponge was due to the introduction of PTOS, but it does not diminish the ability of retrieve these foams by a magnet as shown in figure 4(e).
Figure 4. (a)–(c) Cleaning of n-hexane (labeled by oil red O) from water using a piece of PTOS-Fe3O4 @PDA/MF sponge retrieved by a magnet. (d) VSM patterns of the Fe3O4 @PDA/MF and PTOS-Fe3O4 @PDA/MF sponge, and (e) retrieving a PTOS-Fe3O4 @PDA/MF sponge with a low-cost magnet.
Download figure:
Standard image High-resolution imageThe PTOS-Fe3O4@PDA/MF sponge exhibits high potential for selectively absorbing organic contaminants in water solution, which is attributed to its superhydrophobicity and high porosity. Figure 5 and Video S(2–3) show that PTOS-Fe3O4@PDA/MF sponge can absorb both low-density n-hexane (figures 5(a)–(d)) and high-density chloroform (figures 5(e)–(h)) from aqueous solution. The saturated absorption capacity and recyclable performance of the PTOS-Fe3O4@PDA/MF sponge for various oils and organic solvents were also investigated, which are shown in figures 5(i), (j). The PTOS-Fe3O4@PDA/MF sponge can absorb nearly 59.3 g/g for n-hexane and 141.1 g/g for chloroform, which is higher than most of the previously reported MF sponge materials, as shown in table 1. For example, most of previous sorbents shown in the table have a capacity less than 100 g/g for organic solvents, while our MF sponge can absorb 141 g/g for chloroform. The WCA of this MF composite sponge is also among the highest in the table. Furthermore, compared to conventional dip-coating/blending methods, our in situ growth of NPs on the MF scaffold leads to a more robust sponge system. In order to confirm this advantage, a recycling experiment has been performed. As a result, only a very limited decease (from 141.1 to 136.7 g g−1) in absorption capacity is observed after 30 cycles of absorption–desorption for chloroform, indicating the excellent durability of the PTOS-Fe3O4@PDA/MF sponge for recycled use.
Figure 5. Pictures of PTOS-Fe3O4@PDA/MF sponge for absorbing different oils (dyed with Oil Red O): (a)–(d) n-hexane (lighter than water), and (e)–(h) chloroform (heavier than water). (i) Oil absorption capacities of the PTOS-Fe3O4@PDA/MF sponge. (j) Variations in absorption capacity for chloroform with increasing of the recycle time.
Download figure:
Standard image High-resolution imageTable 1. Comparison of various adsorbent materials for oil uptake.
| Absorbent Sponges | Oils | Q (g/g) | WAC (°) | Methods | References |
|---|---|---|---|---|---|
| HDTMS/rGO-MF sponge | Chloroform | 20.85 | 153.2 | Dip-coating method | [53] |
| Fe3O4/PDMS@MF sponge | DMF | 38 | 158 | Dip-coating method | [54] |
| PVA/MF sponge | Chloroform | 85.33 | 157 | Dip-coating method | [55] |
| MF/PPy/Ag/F sponge | Chloroform | 96 | 156.1 | Dip-coating method | [56] |
| PDA/Fe3O4/PU sponge | Chloroform | 72.68 | 153.7 ± 1.6 | Dip-coating method | [57] |
| MF-CMC-HPU-13 | Chloroform | 130 | 127.1 | Dip-coating method | [43] |
| DLC/TiO2@MF sponge | Chloroform | 130 | 153 ± 1 | Dip-coating method | [35] |
| Spiropyran methacrylate modified MF sponge | Chloroform | 154 | 155.5 | Radical copolymerization | [58] |
| PMS@Fe3O4-PDMS MF sponge | Chloroform | 91.52 | 152 | Chemical synthesis method | [20] |
| DDT-PDA/MF sponge | Chloroform | 98.6 | 158 | Chemical synthesis method | [26] |
| Silica nanorods modified MF sponge | Chloroform | 154.9 | 148 | Chemical vapor deposition (CVD) | [59] |
| MF-ZIF-8 sponge | Chloroform | 38 | 140 | In situ growth method | [27] |
| MIL-DDT@MF sponge | Chloroform | 120.2 | 151.8 | In situ growth method | [13] |
| PDVB–PDMS modified MF sponge | Chloroform | 123 | 158 | In situ growth method | [60] |
| PTOS-Fe3O4@PDA/MF sponge | Chloroform | 141.1 | 165 | In situ growth method | This work |
The prepared PTOS-Fe3O4@PDA/MF sponge (1 cm × 2 cm × 2 cm) was utilized as an efficient filter layer to separate the immiscible oil–water mixtures within a long neck funnel. As shown in figures 6(a)–(d), low-density n-hexane and high-density chloroform were red and water was blue. When the immiscible oil-water mixtures were introduced through the funnel containing PTOS-Fe3O4@PDA/MF sponge, oil was immediately absorbed in the PTOS-Fe3O4@PDA/MF sponge filter. As a result, water was effectively retained while oil was passed through the sponge (Video S4–5). Furthermore, other immiscible oil-water mixtures, including diesel oil, soybean oil, dodecane, n-hexadecane, mineral oil and toluene, can also be successfully separated by above method using this melamine sponge composite. According to figure 6(f), the Es values for all immiscible oil–water mixtures using PTOS-Fe3O4@PDA/MF sponge are higher than 99.9%, demonstrating its excellent oil-water separation performance under the gravity filtration. In order to study the recyclability of the PTOS-Fe3O4@PDA/MF sponge for practical applications, separation efficiencies were measured repeatedly after each absorption-desorption cycle. As shown in figure 6(f), the PTOS-Fe3O4@PDA/MF sponge shows a very stable performance for oil–water separation performance, with only less than 2% performance deterioration after 15 cycles. This result confirms its robust mechanical durability and chemical stability.
Figure 6. Photograph of oil–water separation experiment of PTOS-Fe3O4@PDA/MF sponge, for (a)–(d) chloroform/water and (e)–(h) n-hexane/water mixtures. Organics solvents are red and water is blue. (i) Separation efficiency as a function of various selections of organics in immiscible oil–water mixtures. (j) The separation efficiency is stable with after 15 cycles (less than 2% decay).
Download figure:
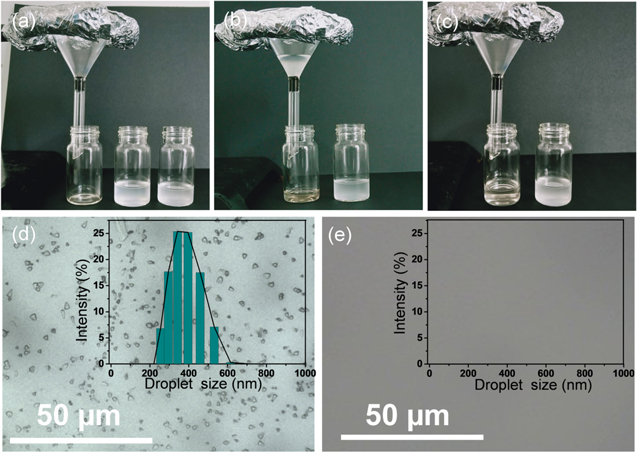
Standard image High-resolution imageWe also performed separation experiments with surfactant-stabilized water/oil (W/O) emulsions, which typically involve significantly more difficulty compared with immiscible oil–water mixtures. Herein, W/O type emulsions were prepared by the assistance of span 80 surfactant (1.0 mg mL−1), in which water droplets are dispersed in the n-hexane phase (1/99, v/v). It can be observed from the optical images (figures 7(a)–(c)) that white cloudy span-80-stabilized emulsion became clear and transparent (indicative of no microphase separation) after passing through PTOS-Fe3O4@PDA/MF filtration layer under gravity-driven separation. Interestingly, even though the highly emulsified water droplets (ranging from 200 to 800 nm) are much smaller than the typical pore size of PTOS-Fe3O4@PDA/MF (>100 μm) from SEM images, successful oil–water separation was confirmed by both optical microscopy images and DLS measurement (figures 7(d), (e)). The excellent ability of this melamine sponge composite to remove water from oil matrix can be explained by the significantly decreased pore size upon squeezing into the funnel, which can effectively reject water molecules due to its superhydrophobic surface [61]. This result also indicates the broad applications of our sponge for separation of bulk oil–water emulsions.
Figure 7. (a)–(c) Photographs of experiment setup for showing how to remove oils from miscible water/oil emulsions. Optical microscope images of water/oil emulsion (d) prior to the separation and (e) after separation. The illustration is the DLS diagram of the emulsion before and after separation.
Download figure:
Standard image High-resolution imageIn order to address large-scale oil–water separation, in situ and continuous process is highly desired since other methods such as mechanical extrusion and gravity filtration are typically batch-process, time-consuming, and difficult to recover oils form water. Herein, we demonstrate the ability of our foam to in situ absorb large quantities of oils though a pump-driven process, as shown in figures 8(a)–(d) and Video S7. During this process, a PTOS-Fe3O4@PDA/MF sponge was embedded at the end of a water pipe (outlet) as a filter. The tubing was immersed into the oil–water mixture. Upon pumping, oil was continuously sucked into the pipe through the PTOS-Fe3O4@PDA/MF sponge and finally collected into the beaker. As shown in figure 8(c), oil can be completely removed and collected from the water/oil mixture, while the water remains in the solvent container. The recyclability was also investigated in figure 8(d), which shows an approximately 99.2% efficiency even after ten cycles. The mechanism for this oil–water separation can be explained by figure 8(e). When the PTOS-Fe3O4@PDA/MF sponge was introduced into oil/water mixture, oil was uptaken and collected rapidly by the peristaltic pump. When the oil on the water surface was absorbed, the sponge above the water was filled with air bubbles under the continuous suction of the pump [24, 62]. As suggested in figure S3, the introduction of air bubbles around the sponges allows the circulation of only the oil component, as the superhydrophobic surface of the PTOS-Fe3O4@PDA/MF sponge would reject water molecules under pressure. Furthermore, this high separation efficiency implies the stability of both structure and chemical composition of the as-prepared PTOS-Fe3O4@PDA/MF sponge, especially prepared through an effective and low-cost approach. We believe these results suggest the great potential of our highly durable, magnetic-responsive melamine sponge composite for high efficiency, in situ oil–water separation, especially for addressing large-scale oil spill applications.
Figure 8. (a)–c) Photographs of collecting n-hexane (red) from water through a peristaltic pump. (d) Separation efficiency and recyclability of n-hexane from water, using the PTOS-Fe3O4@PDA/MF sponge as filtration by a peristaltic pump. (e) Illustration showing the mechanism of in situ oil–water separation using PTOS-Fe3O4@PDA/MF sponge and a peristaltic pump.
Download figure:
Standard image High-resolution image3. Conclusion
Superhydrophobic, magnetic-responsive sponge composites were successfully prepared and applied to modifications of MF sponge substrate by PDA coating, in situ deposition of Fe3O4 NPs, and the introduction of PTOS. The as-prepared PTOS-Fe3O4@PDA/MF sponges exhibited excellent superhydrophobicity with a high WCA of 165 ± 1.5°, attributed to high surface roughness from PDA layer and Fe3O4 NPs, and low surface energy from the PTOS layer. The PTOS-Fe3O4@PDA/MF sponge can not only effectively uptake oils and organic solvents up to 141.1 g/g for chloroform, but also can in situ separate oil and water from both immiscible solutions and W/O emulsions. Furthermore, PTOS-Fe3O4@PDA/MF sponges can in situ and continuously remove oils and other organic solvents from both miscible and immiscible solutions through a peristaltic pump. The advantages of the PTOS-Fe3O4@PDA/MF sponge, including low-cost, magnetic retrievability, high durability, and superior recyclability, render it a potential candidate for in situ oil–water separation for various applications.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51203118), the Fundamental Research Funds for the Central Universities, and the Open Funds for Characterization of Tongji University. Z Q acknowledges generous financial support from the University of Southern Mississippi.
Data availability statement
The data generated and/or analyzed during the current study are not publicly available for legal/ethical reasons but are available from the corresponding author on reasonable request.