Abstract
The purpose of this study was to generate hybrid micro/nano-structures on biomedical nickel–titanium alloy (NiTi). To achieve this, NiTi surfaces were firstly electrochemically etched and then anodized in fluoride-containing electrolyte. With the etching process, the NiTi surface was micro-roughened through the formation of micropits uniformly distributed over the entire surface. Following the subsequent anodizing process, self-organized nanotube structures enriched in TiO2 could be superimposed on the etched surface under specific conditions. Furthermore, the anodizing treatment significantly reduced water contact angles and increased the surface free energy compared to the surfaces prior to anodizing. The results of this study show for the first time that it is possible to create hybrid micro/nano-structures on biomedical NiTi alloys by combining electrochemical etching and anodizing under controlled conditions. These novel structures are expected to significantly enhance the surface biofunctionality of the material when compared to conventional implant devices with either micro- or nano-structured surfaces.
Export citation and abstract BibTeX RIS
1. Introduction
Among the titanium alloys, nickel–titanium (NiTi) has attracted increasing attention for biomedical applications due to its exceptional mechanical properties, i.e., superelastic properties and shape memory effects [1, 2]. To date, the biomedical applications of NiTi have shown a wide spectrum, including cardiovascular stents, endodontic instruments and orthopedic implants. However, despite the successful use of NiTi in the biomedical field, there is still concern over its risk of Ni release from the surface, which can cause toxicity and allergenicity to humans when its concentration exceeds a certain level [3–5]. In order to reduce Ni release, various types of surface modification have been reported, including chemical or electrochemical processes [6, 7], heat-treatment [7], ion implantation [8], etc. Among these methods, anodization has been proven to be efficient in forming a compact Ti-enriched oxide layer and therefore significantly reduces the Ni release from the material surface [9–11].
Another concern over the use of NiTi is its lack of surface bioactivity, which is in fact a common characteristic among metallic biomaterials, but has been less addressed so far in the case of NiTi. In general, the term bioactivity is related to the ability of the material to elicit or modulate biological activity [12]. On one hand, the bioactivity of NiTi alloy can be improved by the presence of a bioactive layer on the surface, intended to modify the surface chemistry of the substrate. For example, hydroxyapatite (Ca5(PO4)3OH) and other calcium phosphate coatings have been developed in order to promote the attachment of bone tissue and enhance the interface bonding of Nitinol surfaces for orthopedic applications [13, 14]. However, although these coatings can improve the surface bioactivity of NiTi alloys, low bonding strength with the underlying metal and deformation capacity are major concerns over its long-term usage [15, 16].
On the other hand, it may be possible to enhance the surface bioactivity by changing the surface topography into, for example, hybrid micro-to-nanoscale structures, which avoid the use of coatings with weak bonding strengths. Such a method is based on the concept of biomimetics, which suggests that the well-designed coexistence of micro- and nano-topographies would offer biologically relevant surface cues for implantable materials [17–19]. There have been several attempts to fabricate such hybrid micro/nano-textured structures on pure titanium for biomedical applications. Kubo et al have created TiO2 surfaces consisting of nanonodules within micropits, and the results of in vitro tests suggested improved osteoconductivity [20]. Moreover, Zhao et al showed that addition of nanotubes to the micro-structured Ti surface enhances multiple osteoblast behavior, which may result in better osseointegration in vivo [21]. These studies suggest that micro-to-nanoscale surface modification is an efficient and promising method which can enhance the surface biofunctionality of biomedical titanium alloys. It is, however, noticed that so far most of the studies aiming at improving the biofunctionality of NiTi have been focused either on enhancing the micro-scale surface roughness [22, 23] or on changing the nanoscale texture [24], with a combination of both being rare.
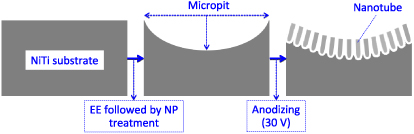
Therefore, in this study, our working hypothesis is that hybrid micro/nano surface topographies can also be created on NiTi substrates, given that the methods of surface treatment are carefully selected and optimized. Our strategy consists of two main steps. Firstly, micropits are formed by electrochemical etching to enhance the micro-scale surface roughness of the NiTi substrate. Subsequently, the superimposed nano-structures are created by anodization. The relationship between the processing parameters and the micro/nano hybrid topographies has been investigated. To the best of our knowledge, this paper addresses for the first time the hybrid micro/nano surface modification of NiTi alloy.
2. Materials and methods
All the chemicals used in this study were purchased from Sigma-Aldrich Co. The NiTi alloy had a composition of 50.6 at.% Ni. Rectangular samples of 10 mm × 10 mm × 1 mm were cut from a NiTi sheet and the sharp corners were rounded by grinding with 320 grit SiC paper. The samples were ultrasonically cleaned in isopropanol and deionized water for 10 min, respectively. No grinding or mechanical polishing was performed in order to avoid any change in the surface chemistry and the induced martensitic relief of the exposed surface grains, as previously reported [3].
2.1. Formation of micropits on NiTi substrate
To create micropits on the surface, the NiTi samples underwent a sequence of treatment steps consisting of electrochemical etching (EE) and nitric acid passivation (NP). The electrochemical etching was conducted in a two component electrolyte: nitric acid (HNO3, 65%) and methanol (CH3OH) with a volume ratio of 33:67 (HNO3:CH3OH) [25]. The samples were made to be the anode in the electrolytic cell and surrounded by a stainless steel cylindrical cathode. The experiments were performed at a constant voltage of 5.5 V applied for various durations at room temperature, and the electrolyte was stirred during the process. After the treatment, the samples (series EE) were ultrasonically cleaned in deionized water for 10 min, and then dried with compressed air. Selected specimens from series EE were passivated in 20% HNO3 acid at 80 ° C for 20 min (series EE–NP) and finally ultrasonically cleaned in deionized water for 5 min. For each group, after EE and EE–NP treatment, the sample's mass loss was evaluated with a high accuracy balance and expressed as a percentage of the initial value.
2.2. Creation of nanoscale structures on the micro-pitted surfaces
To create nano-structures on the etched NiTi substrates, the samples were potentiostatically anodized in ethylene glycol solution supplemented with 0.5 wt% NH4F and 1.0 wt% H2O at room temperature, which was adapted from other reports [24]. The anodization was performed in a two-electrode cell configuration with the NiTi alloy as the working electrode and platinum mesh as the counter electrode. The two electrodes were clamped parallel at a fixed distance of 20 mm and immersed directly in the electrolyte. The potential was supplied by a program-controlled Agilent DC source, and the current was manually recorded during anodization. After anodization, the NiTi samples were rinsed with deionized water and then dried at room temperature.
2.3. Surface analyses
After sputter-coating for enhanced conductivity, the surface and cross-section morphology of the oxidized samples was examined by scanning electron microscopy (SEM, JSM-6500F, JEOL). In addition, the elemental composition was estimated using an energy dispersive x-ray spectrometer (EDS, INCA Energy, Oxford Instruments) coupled with the SEM equipment. For each surface, spectra from three different locations were acquired. A Taylor-Hobson Surtronic 3+ surface texture-meter was used to determine the average surface roughness (Ra) of the samples. Ten random measurements were taken for each sample followed by statistical analysis to determine the mean Ra value.
2.4. Contact angles and surface free energy
The dynamic advancing contact angles were determined with a Krüss DSA 100 drop shape analyzer using deionized water and diiodomethane. A volume of 10 μl of liquid was placed automatically on the tested surface using a microliter syringe. Upon contact with the surface the increasing droplet profile was measured at 1 s intervals for 33 s. For every sample, triplicate measurements were performed in the two different wetting liquids. The surface free energy (SFE) was calculated according to the Fowkes theory:
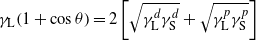

The values reported represent the average and standard deviations for contact angles in water and total surface free energy.
3. Results and discussion
3.1. Formation of micropits on NiTi substrate
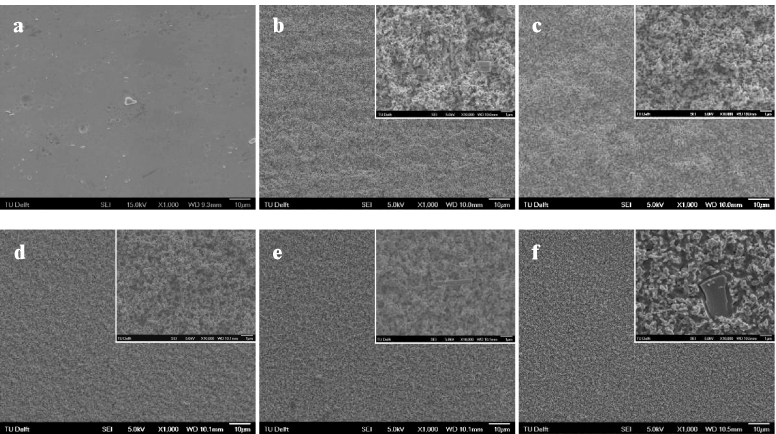
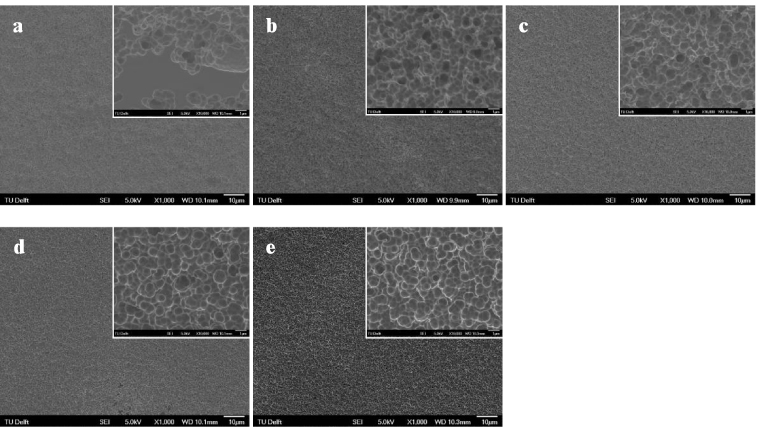
After EE treatment, the surface color of the as-prepared NiTi substrate was black, and no discernable difference could be seen after ultrasonic cleaning. Figure 1 shows the surface topography by SEM of the NiTi substrate before and after EE treatment with various durations. The NiTi substrate presented a smooth surface (figure 1(a)), and the typical hard inclusions were visible. After EE treatment, the initial smooth surface of the NiTi was converted into a rough topography with a fine structure composed of submicron features (figures 1(b)–(f)) which became more pronounced at extended treatment durations. It was noticed that the inclusions were still present on the surface regardless of the treatment duration. It is worth noting that the surface layer as prepared by EE treatment showed a weak bonding to the substrate, as black drop-off particles were observed during the ultrasonic cleaning. In order to remove the surface layer and expose the underlying micropits, the as-prepared samples with EE treatment were subjected to immersion treatment in nitric acid at 80 ° C for 20 min, and the surface topographies of the resultant samples are presented in figure 2. It can be seen that the morphology of the electrolytically etched surfaces changed significantly. For the samples with a relatively short EE treatment duration (e.g. 0.5 min in this study), non-etched areas were visible on the surface after NP treatment (figure 2(a)), whereas for those with a prolonged EE treatment (figures 2(b)–(e)), a typical etched structure with round cavities was revealed with uniformly distributed peaks and valleys on the entire surface. The diameter of the surface pits seemed to increase with the duration of the EE step, reaching about 1 μm after EE for 30 min (figure 2(e)). Furthermore, the inclusions were not present any more on the surface.
Figure 1. Surface morphologies of NiTi surfaces (a) before and after EE treatment for (b) 0.5 min, (c) 1 min, (d) 7 min, (e) 15 min, and (f) 30 min. The insets represent the treated surfaces at enhanced magnification.
Download figure:
Standard image High-resolution imageFigure 2. Surface morphologies of NiTi surfaces with EE treatment of (a) 0.5 min, (b) 1 min, (c) 7 min, (d) 15 min, and (e) 30 min after NP treatment. The insets represent the treated surfaces at enhanced magnification.
Download figure:
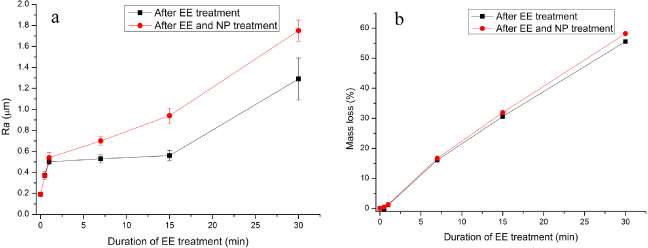
Standard image High-resolution imageThe changes in surface roughness (Ra) and mass loss of the NiTi after the EE and NP treatment are included in figure 3. It is clear that the EE step increased the surface roughness at short duration (i.e. 0.5 and 1 min) and then above 10 min (figure 3(a)). Further, an almost linear increase in mass loss with EE time was observed resulting in more than 50% mass loss after 30 min treatment (figure 3(b)). The additional nitric acid passivation performed at 80 ° C for 20 min did not change the trend of surface roughness and mass loss of the EE-treated specimens significantly while revealing the clear etched topography.
Figure 3. Variation in (a) average surface roughness and (b) mass loss of a NiTi plate after EE treatment with and without subsequent NP treatment.
Download figure:
Standard image High-resolution imageEtching is a commonly used surface modification technique for titanium in order to enhance its biological performance by forming micropits and thus increasing the surface roughness [26]. However, application of the method is rather scarce in the case of NiTi, which may be due to the presence of Ni which has a high dissolution potential during the treatment and thus may damage the as-formed micropits. This study shows that a micro-pitted structure similar to that on improved titanium surfaces is achievable on a NiTi surface by combining electrochemical etching with a subsequent acid passivation step under controlled conditions.
3.2. Creation of hybrid micro/nano-structure
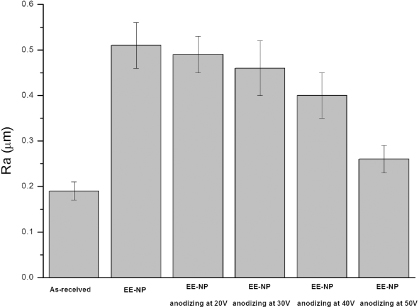
Considering the mass loss of the NiTi plates after etching and acid passivation procedures, samples with 1 min EE treatment and subsequently 20 min acid passivation were selected and subjected to further anodization treatment in order to create hybrid micro/nano-structures. Figure 4 shows representative surface morphologies by SEM of the treated surfaces after anodizing at different working voltages for 10 min. It can be seen that the as-modified surface presents various hybrid structures depending on the working voltage. After anodizing at 20 V for 10 min, the micro-roughness of the surface was maintained but the sharp edge of the micropits was smoothened by anodizing (figures 4(a) and (b)). Cracks were observed on the edges, which are probably due to the higher reactivity of the sharp edge during anodizing. At even higher magnification, nano-porous textures were observed in anodized micropits. When the working voltage was increased to 30 V (figures 4(c) and (d)), the micro-roughness of the surface was maintained, and the cracks as formed on the edges were wider than those formed after anodizing at 20 V. It can be seen at higher magnification that clusters of nanotubes of several tens of nanometers in diameter were formed within the micropits. As the applied voltage was increased to 40 V, some micropits disappeared after anodizing (figures 4(e) and (f)). SEM images with higher magnification showed that some nanotubes were discernable on micropits, but these nanotubes showed a damaged morphology and scattered distribution. When the applied voltage was 50 V (figures 4(g) and (h)), the surface became smoother, showing large and shallow micropits, and the presence of inclusions. A closer view of the surface revealed that no nano-porous/tubular structure was formed. The variation of surface roughness with anodizing voltage is presented in figure 5 and compared with those of the non-modified and etched surfaces. It can be seen that, corresponding to the surface topographies as shown in figure 4, the surface roughness of the samples decreased with increasing anodizing voltage. It is, however, noticed that when the applied voltage was relatively low (e.g. 20 and 30 V), the surface roughness did not sharply decrease after anodizing.
Figure 4. Surface morphologies of NiTi surfaces with EE and NP treatment after anodizing at (a), (b) 20 V, (c), (d) 30 V, (e), (f) 40 V and (g), (h) 50 V for 10 min. The arrows point out the clusters of nanotubes on the surface after anodizing.
Download figure:
Standard image High-resolution imageFigure 5. The effect of anodizing treatment with various applied voltages on the surface roughness of a NiTi plate with EE (1 min) and NP treatment.
Download figure:
Standard image High-resolution imageOur results showed that the anodizing behavior in fluoride-containing electrolyte of an etched NiTi surface is quite analogous to that of a smooth surface [24, 27], during which nano-porous or nanotubular structure can be formed by adjusting the applied voltage. The dependence of the surface morphology of the micro-pitted NiTi substrate on the anodizing voltage can be explained with the mechanism that has been established for the anodization process of titanium in F-containing electrolytes. It is known that the anodizing voltage plays an important role in the anodization of titanium, and the formation of the nano-porous/nanotubular layer is essentially the result of competition between the electrochemical formation and the chemical dissolution of titania in fluoride-containing solution during anodizing [28, 29]. Under low anodizing voltages (i.e., 20 V in this study), the chemical dissolution reaction dominated the process and the formation of porous structure occurred randomly. In this process, the sharp edges of the micropits suffered from more chemical attack and electric field, and thus cracks appeared under stress. As the anodizing voltage was increased to 30 V, equilibrium between the electrochemical formation and the chemical dissolution of titania was reached, which resulted in the formation of nanotubes [24]. However, as the working voltage further increased, the nanotubular structure within micropits on the NiTi substrate was diminished or not formed, whereas it was reported that increased voltage would increase the diameter of the nanotubes rather than diminish the integrity of the structure [30]. It is supposed that this difference in anodizing behavior is due to the presence of Ni in a large amount on the surface. In the case of titanium, the growth of the film formed by the anodic oxidization process is based on the migration of Ti4+ from the underlying metal to the metal–electrolyte interface under electric field. In contrast, when NiTi is subjected to anodization, such movement not only happens to Ti4+, but also to Ni2+ or Ni3+. Since Ni does not belong to the class of 'valve metals' that can form stable oxide layers under electric field [31], these ions would be driven from the metal surface to the electrolyte, which is strengthened at higher voltage. A higher anodizing voltage (i.e., 50 V in our study) did not support the growth of an anodic oxide layer on NiTi. The surface became smooth as the edges of micropits suffered more mass loss due to the higher local current density through the sharp points. It is clear that the anodizing conditions should be finely controlled in order to obtain a nano-structure on a micro-pitted NiTi substrate, similar to that on a smooth NiTi surface [24, 27].
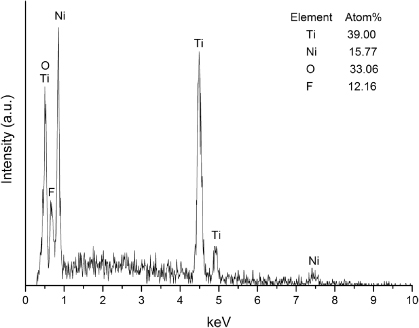
It is noted that the samples after anodizing at 30 V are of special interest as nanotube clusters were uniformly formed on the surface [32]. To confirm formation of the hybrid surface structure, the samples' cross-sections were observed with SEM and the results are presented in figure 6. It can be seen that the micropits were retained after anodizing. Combined with the surface SEM images in figures 4(c), (d), the findings point out the formation of a hybrid structure consisting of nanotubes superimposed on micropits. Figure 7 shows schematically the formation of the hybrid structure on NiTi by electrochemical etching, nitric acid passivation and anodizing under the specific conditions used in this study. Figure 8 presents the elemental composition of the resultant surface as measured with EDS. It can be seen that the oxide showed the presence of O and F from the electrolyte, as well as Ti (39.00 at%) and Ni (15.77 at%) from the substrate with a Ni/Ti ratio of 0.40. According to previous reports, the main composition of the nanotubes is supposed to be Ni- or Ni2O3-doped TiO2 [24, 27]. The significant decrease of the Ni/Ti ratio for oxides produced by anodizing as compared with the original NiTi substrate can be attributed to preferential oxidation of titanium which is thermodynamically favored [3]. Figure 9 includes the results of contact angle measurements in water and the as-calculated surface free energy (SFE) after etching and anodizing treatment. Deionized water on original NiTi samples formed a regular drop, with a contact angle of 73.5°. After EE and acid passivation treatment, the contact angle was reduced to 42.0°, indicating a change to more hydrophilic state. The final anodizing step further reduced the contact angle to 14.5°, which suggests that the sample possessed the highest hydrophilicity among all groups. The total SFE of the surface after various treatments showed a reverse trend to that of the contact angle. The marked lowering of contact angle and higher SFE as the surface undergoes the series of treatments may be determined by the increase in surface area after micro-roughening and the transition from metal to oxide structures as well as the presence of nanotubes after anodizing [33, 34]. The increased hydrophilicity of the surface after etching and anodizing is considered favorable for both orthopedic and cardiovascular applications [35, 36]. Related to the former, it has been previously shown [35] that hydrophilic surfaces induce homogeneous spatial growth of osteoblast-like cells (hFOB, MC3T3-E1, MG63 and SAOS-2) with beneficial effects on later cellular events including extracellular matrix deposition and mineralization. One of the important biocompatibility issues related to blood-contacting biomaterials is their resistance to thrombus formation. Thrombus is formed through protein adsorption from blood (e.g., fibrinogen) followed by platelet adhesion and activation. Systematic studies on the effects of surface wettability on protein adsorption using self-assembling monolayers [36] indicated a linear decrease in fibrinogen adsorption with increase in surface hydrophilicity. This was related to reduced platelet adhesion and activation. The effect could be explained by diminished hydrophobic interactions with increasingly hydrophilic surfaces.
Figure 6. Cross-section morphologies of micro-pitted NiTi samples (a) before and (b) after anodizing at 30 V as observed by back-scattered SEM. The arrows point out the micropits before and after anodizing treatment.
Download figure:
Standard image High-resolution imageFigure 7. Schematic representation of the formation of a hybrid micropit/nanotube structure on a NiTi surface by combining EE–NP and anodizing treatments.
Download figure:
Standard image High-resolution imageFigure 8. Elemental composition of NiTi surfaces with EE (1 min) and NP treatment after anodizing at 30 V for 10 min.
Download figure:
Standard image High-resolution imageFigure 9. Effect of EE–NP treatment and EE–NP plus subsequent anodizing treatment (30 V) on the water contact angle and total surface free energy of NiTi.
Download figure:
Standard image High-resolution imageExtensive research on surface modification of titanium has shown that anodization can produce dense or porous oxide layers on the surface. Similarly, it is also possible to create various porous layers, from nano- [24, 27] to micro-scale [37], on a NiTi surface in order to facilitate surface biofunctionalization of the shape memory alloy. It is, however, noticed that all these surface modifications have been aimed at creating a certain texture on a flat NiTi surface. The results of our study indicate for the first time that it is possible to create hybrid micro/nano surfaces on a NiTi substrate, namely self-organized nanotubular structures superimposed on a micro-pitted surface, by combining etching and anodizing procedures under specific conditions. Based on the well-accepted concept that combination of nano- and micro-structured surfaces can lead to more balanced enhancement of multiple cell functions when used in orthopedic or cardiovascular applications [21, 38, 32], it is reasonable to believe that the newly developed hybrid micro/nano-structure on a NiTi surface, showing increased hydrophilicity and enrichment in TiO2, will enhance the surface biofunctionality of the material. Meanwhile, another benefit, related to the concern over adverse Ni release from the NiTi substrate, may derive from the use of anodizing technology. It has been reported that by forming a protective oxide layer as well as depleting Ni from the surface, anodizing could help to enhance the corrosion resistance of NiTi and hence suppress Ni release [24, 39]. Therefore, in addition to enhanced surface biofunctionality, the proposed hybrid micro/nano-structure may also help reduce Ni release from the surface. In addition to the effects of topography and chemistry on interface biology and Ni release, the biocompatibility of such surfaces will also depend on their structural integrity and mechanical properties under the specific conditions relevant for the application. Recent data suggest that nanotube structures created by anodic oxidation on titanium can resist shear forces associated with implant insertion in addition to promoting peri-implant bone formation in vivo [40]. On the other hand, annealing post-treatments under specific conditions of temperature and duration (e.g., 400–600 ° C for 1–2 h) have shown beneficial effects on nanotube adhesion to the underlying titanium, enhanced corrosion resistance and apatite forming ability or specific cell response [41]. However, since these post-anodizing thermal treatments affect the oxide crystallinity and possibly the morphology of the nanotubes, or even the original mechanical properties of the NiTi alloy, their effects on biocompatibility have to be thoroughly evaluated for each type of application and substrate.
4. Conclusions
In this study, a hybrid micro/nano-structure was created on the surface of biomedical nickel–titanium alloy (NiTi), in order to enhance the surface biofunctionality of the material. Uniform micropits were firstly formed on the flat NiTi surface by electrochemical etching followed by nitric acid passivation. During the subsequent anodizing process, nanotubular structures enriched in TiO2 could be superimposed on the etched surface under specific conditions. Furthermore, the anodizing treatment significantly reduced the water contact angle and increased the surface free energy of the etched surfaces. The results of this study show for the first time that it is possible to create superimposed nanotube structures on micro-etched surfaces on biomedical NiTi alloys by combining electrochemical etching and anodizing procedures under controlled conditions. These novel structures are expected to significantly enhance the surface biofunctionality of the material when compared to conventional implant devices with either micro- or nano-structured surfaces.
Acknowledgments
This study is part of the Project P1.02 NEXTREAM under the research program of the BioMedical Materials Institute, co-funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation. The financial contribution of the Nederlandse Hartstichting is gratefully acknowledged.