Abstract
The search for novel nanomaterials driving the development of high-performance electrodes in lithium ion batteries (LIBs) is at the cutting edge of research in the field of energy storage. Here, we report on the synthesis of single wall carbon nanotube (SWNT)-bridged molybdenum trioxide (MoO3) nanosheets as anode material for LIBs. We exploit liquid phase exfoliation of layered MoO3 crystallites to produce multilayer MoO3 nanosheets dispersed in isopropanol, which are then mixed with solution processed SWNTs in the same solvent. The addition of SWNTs to the MoO3 nanosheets provides the conductive framework for electron transport, as well as a bridge structure, which buffers the volume expansion upon lithiation/de-lithiation. We demonstrate that the hybrid SWNT-bridged MoO3 structure is beneficial for both the mechanical stability and the electrochemical characteristics of the anodes leading to a specific capacity of 865 mAh g−1 at 100 mA g−1 after 100 cycles, with a columbic efficiency approaching 100% and a capacity fading of 0.02% per cycle. The low-cost, non-toxic, binder-free hybrid MoO3/SWNT here developed represents a step forward for the applicability of exfoliated MoO3 in LIB anodes, delivering high energy and power densities as well as long lifetime.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The worldwide lithium-ion batteries (LIBs) market is increasing with a relevant growth rate of more than 20% per year [1], with an estimated global market reaching $130 billion in 2025 [2]. Today, LIBs find the main application in portable electronic devices [3], while the increasing demand in the transport sector, i.e. full electric vehicles (EVs) and hybrid electric vehicles (HEVs) [4], will drive the growth of LIBs market towards automotive and industrial applications [1]. Moreover, energy storage for grid balancing and micro-grids, e.g. residential storage battery in conjunction with photovoltaic systems, will also play a role for the growth of LIBs market [5]. Current LIBs [6], based on the capture and release of lithium ions, are usually composed by an intercalated lithium compound cathode (e.g. LiCoO2 or LiFePO4), a graphitic-based anode and an electrolyte [6, 7], yielding a theoretical specific energy density of 387 Wh kg−1 (with LiCoO2 as cathode) [7] and a measured energy density of 120–150 Wh kg−1 [7].
Crucial to the performance of these rechargeable batteries is the specific capacity (or gravimetric capacity, i.e. total ampere-hours -Ah- available when the battery is discharged at a certain current density, per unit weight) to evaluate the capability of lithium ions storage in the active materials [4]. In this regard, LIBs are still limited by the specific capacity of the commercial active materials, e.g. graphite anode with a theoretical specific capacity of 372 mAh g−1 [8]. For this reason, a current research effort focuses on the exploitation of alternative anodic materials with high theoretical specific capacity such as Si (3579 mAh g−1) [9], Sn (994 mAh g−1) [10], and SnO2 (782 mAh g−1) [11]. However, the full development of such high-performance anode materials has been hindered by the significant capacity fading owing to the large volume contraction/expansion (100–300% with respect to the initial volume) [12] during charge–discharge cycling [13].
Another promising strategy that is emerging in the last few years is to replace graphite with other layered anode materials, i.e. transition metal oxides [14–18] and graphene-based materials [19–30], which are expected to improve the energy storage of LIBs. In this context, graphene and its derivatives, i.e. reduced graphene oxide (RGO) [19, 20] and graphene nanoplatelets (GNPs) [21], have been already largely explored for the realization of LIB anodes [22]. The aforementioned graphene-based materials display larger theoretical specific capacity (i.e. 744 mAh g−1 assuming lithium adsorbed on both sides of graphene to form Li2C6) [23] when compared to graphite, electrochemical and thermal stability within the LIBs operational temperature range (−20/60 °C) [24], offering additional properties of flexibility and/or stretchability [25]. However, they also suffer severe limitations. In fact, in single-layer graphene (SLG), Li storage is not thermodynamically favoured, with low value of Li uptake [26–28]. So an hypothetical SLG anode will not be an efficient solution. Moreover, the morphology (i.e. lateral size and thickness) of the flakes strongly influences the Li+ storage capability of few- (FLG) and multi-layer graphene (MLG) flakes [29]. Indeed more capacity is delivered at high potentials upon flake size reduction, resulting in reduced and non-constant voltage output from the battery, a detrimental factor on the voltage efficiency [24]. Finally, RGO and GNPs experience critical issues for LIB applications. In fact, RGO show large irreversible capacity [19, 20] and voltage hysteresis between lithiation and de-lithiation [30], while GNPs have not yet demonstrated considerable gain in maximum specific capacity with respect to graphite [21, 25].
Metal oxides are another class of layered materials that can be used as anode materials in LIBs [31, 32]. Amongst them, MoO3, which has already shown promise for various applications, including optical devices [33], catalysts [34], sensors [35] and field emission devices [36, 37], is a promising candidate for electrochemical storage applications [38–44]. In particular, the intrinsic structural anisotropy of α-MoO3, i.e. the thermodynamically stable orthorhombic phase [45, 46], offers the possibility of reversible electrochemical insertion of lithium [47]. In LIB applications, α-MoO3 has been firstly proposed as a cathode material [48, 49], but its low voltage plateaus (3.2–2.0 V versus Li+/Li) [14, 50] has hindered its practical exploitation [51–54]. Contrariwise, α-MoO3 has been demonstrated a very promising anode material thanks to its high theoretical specific capacity of 1117 mAh g−1 [55, 56]. Additionally, the interlayer spacing as large as 0.69 nm of α-MoO3 [57, 58], compared to 0.34 nm of graphite, guarantees its Li host capability [59]. Moreover, its higher intercalation voltages (1.5–2.3 V versus Li/Li+), with respect to that of graphite (<0.4 V versus Li/Li+), could reduce the safety problems caused by the decomposition of electrolyte, especially for the utilization in HEVs [60].
The electrochemical performances of bulk MoO3 are limited by its own poor ionic [61] and electrical (i.e. ~10−5 S m−1) [62] conductivity, as well as volume expansion during charge–discharge processes [47, 60, 63, 64]. To overcome the aforementioned problems, one of the most encouraging strategies is to reduce the lateral size of bulk MoO3 by nanostructuring [47, 60–68], and mix the resulting MoO3 nanostructures with carbon-based nanomaterials, such as amorphous carbon [44], carbon nanotubes (CNTs) [63] and graphene [69], to form hybrid materials. The exploitation of these various nanostructured MoO3 hybrids as anode materials have demonstrated significant improvement, with respect to the bulk MoO3, in term of specific capacity [69, 70] and electrochemical stability [47, 60, 63, 64]. Nevertheless, the costly production processes used [47, 60–64, 68], e.g. chemical vapor deposition (CVD) [56] or hydrothermal methods [47, 61, 64, 71], pose scalability challenges towards practical industrial application [56, 57]. Additionally, the use of toxic materials [72, 73] as well as binders [74, 75] are also limiting factors for practical use.
Recently, liquid phase exfoliation (LPE) [76, 77] has attracted increasing attention to obtain nanoscale graphene and other 2D layered materials, which have strong covalent intra-layer bonding [78], but weak out-of-plane inter-layer interactions [79–81]. Henceforth, it is possible to peel off individual layers from the parent bulk crystal by supplying sufficient energy [82]. Thus, this process has become a widely used method for the production of a range of 2D flakes [74] (e.g. MoO3) [83] from their parent bulk crystals [84]. During the LPE process, the inter-sheet forces are broken by the input of either shear or ultrasonic energy in the presence of a stabilizing liquid [73, 85]. The resultant exfoliated flakes are generally defect-free and un-functionalized [86, 87].
In this paper, we demonstrate that multi-layer MoO3 nanosheets produced by LPE of layered MoO3 crystallites combined with single wall carbon nanotubes (SWNTs) can be used as an active binder-free material for LIBs. The hybrid anodes are produced by mixing multilayer MoO3 nanosheets with solution processed SWNTs. Both the MoO3 exfoliation process and the SWNTs de-bundling are carried out in isopropanol, allowing a simple deposition onto the copper substrate. The designed binder-free solution processed hybrid MoO3/SWNT anode displays a specific capacity of 865 mAh g−1 at 100 mA g−1 after 100 cycles, with a columbic efficiency of 99.7%. The SWNTs addition determines a network structure with the MoO3 providing (1) long channels for electronic charge transport; (2) an active anode material, instead of polymeric binder, offering extra capacity for Li ions storage: (3) a buffer frame in the electrode, which reduce the capacity fading caused by the volume expansion of MoO3 flakes during the lithiation process. To further confirm the essential roles of SWNTs, we also tested multilayers MoO3 combined with carbon black (CB) nanoparticles, which are not able to create the network structure seen with SWNTs, yielding a significant capacity fading of the resulting battery. These results set the basis for the exploitation of exfoliated 2D MoO3 sheets as anodic materials in Li-ion batteries.
2. Experimental
2.1. Preparation of MoO3 flakes and MoO3/SWNT hybrid structure
2.1.1. Materials
MoO3 powder is purchased from Sigma Aldrich (99.98%, CAS 1313-27-5) and SWNTs are purchased from Carbon Solutions inc. All materials are used without any further purification.
2.1.2. LPE MoO3 nanosheets preparation
Molybdenum trioxide powder (240 mg) is added to 2-propanol (IPA) (80 ml) in a 100 ml open top, flat bottomed beaker. The dispersion is ultrasonicated using a horn probe sonic tip (VibraCell CVX, 750 W, 25% amplitude) for 5 h. The sonic tip is pulsed for 6 s on and 2 s off to avoid damage to the processor and reduce any solvent heating. To minimize heating effects, an external cooling system circulated cooled water (5 °C) around the beaker during ultrasonication. To remove any un-exfoliated material the ultrasonicated dispersion is filled in glass vials (~30 ml) and ultracentrifuged at 1 krpm (~100 g) for 30 min. The supernatant is decanted (~20 ml) and further ultracentrifuged at 5 krpm for 105 min to remove small flakes. The supernatant is decanted (containing small flakes) and discarded while the sediment is re-dispersed in fresh IPA.
2.1.3. SWNTs dispersion preparation
P3 SWNTs are dispersed in IPA at a concentration of 0.1 gl−1 and ultra sonicated in both a horn sonic probe and a sonic bath to achieve a completely homogeneous dispersion. The procedure involves horn probe ultrasonication (30 min) followed by 1 h in a sonic bath and an additional 30 min in the horn probe tip.
2.1.4. CB dispersion preparation
Carbon black is dispersed in IPA at a known concentration (0.25 gl−1) and bath ultrasonicated for 2 h.
2.1.5. MoO3/SWNT/CB hybrid
The as produced MoO3, SWNTs and CB dispersions are then mixed, without centrifugation, to form hybrid structures of known wt%. Accurate weighing of an alumina membrane (pore size 25 nm) before and after filtration of MoO3 dispersion allowed determining the concentration.
2.2. Characterization of SWNTs, CB, MoO3 flakes and MoO3/SWNT hybrid structure
Transmission electron microscopy (TEM) images of MoO3, SWNT, CB and MoO3/SWNT hybrid are taken with a JOEL JEM 1011 transmission electron microscope, operated at 100 kV. The samples are then diluted 1:10 with pure IPA associated with 10 min ultra-sonication. 100 µl of the resulting dispersions are drop-cast at room temperature onto carbon coated copper TEM grids (300 mesh), and subsequently dried under vacuum overnight. Raman measurements are carried out with a Renishaw 1000 using a 50× objective, a laser with a wavelength of 532 nm and an incident power of ~1 mW. The samples are drop-cast onto a Si wafer (LDB Technologies Ltd), with 300 nm thermally grown SiO2.
2.3. Fabrication and characterization of MoO3 and MoO3/SWNT electrodes
50 mg of MoO3 nanosheet and MoO3/SWNT hybrid samples are dried and re-dispersed in 5 ml ethanol via ultra-sonication for 15 min. Subsequently, the aforementioned samples are drop-cast on the Cu foils as supporting substrates in a circular shape with a diameter of 15 mm at 40 °C in air. Then, the as deposited films are dried at 120 °C and 10−3 bar pressure for 12 h in oven (BÜCHI, B-585). The mass loading of the active materials are calculated by subtracting the average weight (obtained with balance of Mettler Toledo XSE104) of bare Cu foil with the same area, from the total weight of the electrodes. Scanning electron microscopy (SEM) images of the MoO3, MoO3/CB and MoO3/SWNT films are taken by a field-emission scanning electron microscope FE-SEM (Jeol JSM-7500 FA), without any conductive coating. Raman measurements of the as-prepared MoO3, SWNT, CB and MoO3/SWNT films are carried out in the same experimental conditions reported for the characterization of the dispersions, see section 2.2.
2.4. Assembling and electrochemical tests of MoO3 based anode
MoO3 and MoO3/SWNT based half-cell assembling. The MoO3, MoO3/CB and MoO3/SWNT based anodes are performed in half-cells configuration against Li foils (Sigma-Aldrich) as the counter electrodes. Half cells are assembled in coin cell (2032, MTI) in an argon filled glove box (O2 and H2O < 0.1 ppm) at 25 °C, using 1M LiPF6 in a mixed solvent of ethylene carbonate/dimethylcarbonate (EC/DMC, 1:1 volume ratio) as electrolyte (LP30, BASF), as well as glass-fiber as separator (Whatman GF/D).
Electrochemical tests. The cyclic voltammetries (CVs) are performed at a scan rate of 50 µV s−1 between 1 V and 5 mV versus Li+/Li with a Biologic, MPG2 potentiostat/galvanostat. The electrochemical impedance spectroscopies are collected with a VMP3 (BioLogic). All the electrochemical measurements are performed at room temperature. Constant current charge/discharge galvanostatic cycles are performed for the as prepared binder-free anodes in half-cell and in full battery configuration at a different current density, using a battery analyzer (MTI, BST8-WA). The charge/discharge cycles are performed at different rates at room temperature.
3. Results and discussion
3.1. Properties of MoO3, MoO3/CB and MoO3/SWNT dispersions
The synthesis of the MoO3/SWNT hybrid anode for LIBs starts with the solution processing of the two nanomaterials. The MoO3 flakes in dispersions are obtained by LPE of pristine MoO3, while the SWNTs are prepared in IPA by tip ultrasonication, see Experimental for detailed information about their preparation, as well as for the processing of the CB nanoparticles.
The morphology of the as-produced MoO3 and SWNTs are analysed by TEM. Figure 1(a) shows MoO3 flakes with lateral sizes ranging from 50 to 300 nm, while figure 1(b) shows that the SWNTs are in bundles of ~10 nm in diameter, forming a spider web-like network onto the TEM grid. The TEM image of the hybrid MoO3/SWNTs sample (see figure 1(c)), clearly shows the bundles of SWNTs acting as bridges to connect isolated MoO3 flakes, forming an interconnected network in the hybrid MoO3/SWNTs. The TEM of CB, reported in the supplementary information -S.I.- (see figure S3(b) (stacks.iop.org/TDM/5/015024/mmedia)), shows a particle size distribution of ~50 nm. We further carried out the characterization of the morphological properties of the dispersed materials by Raman spectroscopy, which is a fast and non-destructive technique commonly used to identify type of defects, doping, disorder and chemical modifications of nanomaterials [88]. In figure 1(d) we show the Raman spectra of MoO3, in blue, and SWNTs, in red. The full spectroscopy characterization, i.e. optical absorption and Raman, of the as-prepared MoO3, SWNTs and CB nanoparticles are discussed in the S.I.
Figure 1. Bright-field TEM images of (a) MoO3 flakes, (b) SWNTs and (c) MoO3/SWNT hybrid dispersed in IPA. (d) Raman spectra of MoO3 (in red) and SWNTs (in blue) taken at an excitation wavelength of 514.5 nm.
Download figure:
Standard image High-resolution imageThe as-prepared samples are then exploited for the realization of electrodes, i.e. anodes, for LIBs. In particular, solution processed MoO3 flakes and the MoO3/SWNT hybrid (9:1 ratio) is deposited onto Cu substrates. A reference sample, i.e. MoO3 mixed with 10% CB, is also prepared by using the same process. The mass loading of MoO3, MoO3/SWNT and MoO3/CB in the corresponding electrodes has been calculated as 0.80 mg, 0.74 mg and 0.75 mg, respectively. The morphology of the MoO3/SWNT and MoO3/CB electrodes is characterized by SEM. The SEM image of the MoO3 electrode (figure 2(a)) shows MoO3 flakes with regular polygonal shapes, which are homogenously distributed onto the Cu substrate.
Figure 2. Scanning electron microscope images of (a) MoO3, (b) MoO3/SWNT and (c) MoO3/CB deposited onto copper substrates. (d) Raman spectra of the MoO3/SWNT (green), MoO3/CB (red) and MoO3 (blue) electrodes, deposited onto copper substrates.
Download figure:
Standard image High-resolution imageFigure 2(b) shows how the morphology of the hybrid MoO3/SWNT is dominated by the MoO3 flakes inserted in the mesoporous network of SWNT bundles. In contrast, the morphology of the MoO3/CB electrode is dominated by large aggregates of CB, ~400 nm in diameter, with a few MoO3 flakes observed in figure 2(c).
3.2. Electrochemical properties of MoO3, MoO3/CB and MoO3/SWNT electrodes
In order to fully understand the optimal SWNTs/MoO3 weight ratio on the electrochemical performances of the MoO3/SWNT hybrid anodes, we prepared other two samples with different SWNTs/MoO3 weight ratio of 2:8 and 3:7, respectively, see Experimental. The Raman and SEM characterization of the electrodes are presented and in depth discussed in the S.I. As shown in figure S4, the three MoO3/SWNT hybrid anodes show a homogenous coverage of SWNTs and MoO3 nanoflakes onto the Cu substrates.
The CVs (figure 3(a)) of the three samples, i.e. MoO3, MoO3/CB and MoO3/SWNT are collected at a scan rate of 50 µV s−1 starting from 5 mV versus Li+/Li potential, to cover the lithiation processes in both MoO3 and SWNT [89]. In the first reduction sweep, the MoO3 exhibits two peaks at 2.3 and 2.7 V, which can be linked with the insertion of Li ions into the interlayers of the MoO3 structure to form LixMoO3, and another peak at 0.4 V, which corresponds to the conversion reaction of LixMoO3 into Mo and Li2O [42, 54, 93].
Figure 3. (a) Cyclic voltammograms and (b) charge/discharge voltage profiles of MoO3, MoO3/CB and MoO3/SWNT as anodes against Li foil in half cell configuration.
Download figure:
Standard image High-resolution imageThe two processes determine, the accommodation of six Li ions in each MoO3, reaching theoretical specific capacity of 1117 mAh g−1 [56, 57], as summarized by equations (1) and (2) [54, 93],
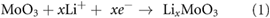
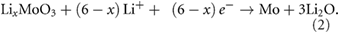
In the reverse oxidation process, metallic molybdenum is converted into amorphous MoO2, in the 1.0 V–2.2 V range [90]. From the 2nd cycle onward, a clear shift is observed in the conversion reaction peak at 0.4 V, which is consistent with the formation, upon oxidation, of MoO2 with lower reactivity with respect to MoO3 [97]. Furthermore, in the second cycle a new peak, at 1.5 V, appears which can be assigned to the lithium insertion into amorphous MoO2 [91, 97]. For the MoO3 and the hybrid MoO3/CB samples, the reduction peaks at 1.5 V and 0.4 V rapidly disappear during the following 8 cycles. On the contrary, in the case of MoO3/SWNT sample the intensity of these two peaks is maintained from cycle 2 to cycle 10, leading to a remarkable improvement on its electrochemical stability with respect to the other two samples.
Figure 3(b) shows the charge–discharge voltage profiles of bulk MoO3 at 100 mA g−1, in order to get a complete electrochemical response for the Li ion transfer [92], during the lithiation/de-lithiation process at the anode. In the 1st charge (lithiation) process, two plateaus at 2.3 V and 0.4 V are observed in all the three samples. These two plateaus have already been attributed to the formation of LixMoO3 and its following conversion reaction into metallic Mo and LiO2, respectively [42, 54, 93]. These reactions have also contribution on the large initial specific capacity obtained in the three samples, i.e. 864 mAh g−1 for MoO3, 1332 mAh g−1 for MoO3/CB, and 1357 mAh g−1 for MoO3/SWNT. The higher initial specific capacity shown by both the MoO3/SWNT and MoO3/CB samples, compared with the MoO3 sample, can be associated to the enhanced electrical conductivity in the hybrid electrodes due to the presence of the carbon nanomaterials [65, 93]. On the contrary, significant differences of the three samples are shown by the specific capacities obtained at the 1st discharge (de-lithiation) process. In fact, initial specific capacities of 481, 625 and 962 mAh g−1 are achieved for the MoO3, MoO3/CB, and MoO3/SWNT samples, respectively. For all the 3 samples, the capacity drop between the 1st charge and discharge processes (see figure 4(a)), is caused by the combination of several irreversible processes, including: (1) the solid electrolyte interphase (SEI) formation [62]; (2) the structural modulation during Li+ insertion/extraction into the inter-layers and intra-layers of MoO3 [94]; (3) the conductivity loss caused by the electrode pulverization upon lithiation/de-lithiation [95].
Figure 4. (a) Specific capacity and coulombic efficiency over charge/discharge galvanostatic cycles, and (b) impedance spectroscopy of MoO3 (red), MoO3/SWNT (green) and MoO3/CB (black) as anodes against Li foil in half cell configuration.
Download figure:
Standard image High-resolution imageFor the MoO3 and MoO3/CB samples, we observed a coulombic efficiency (the ratio between discharge and charge capacities) at the 1st cycle as low as 55.7% and 46.9%, respectively, see figure 4(a). Moreover, both samples show capacity fading upon cycling, which is the main drawback of MoO3 anodes due to the pulverization of the electrode [65], with capacity loss of 84% and 64%, respectively, after 60 cycles. Alternatively, the MoO3/SWNT sample shows a coulombic efficiency as high as 70.9% at the 1st cycle, a value which is significantly enhanced compared to the MoO3 and MoO3/CB samples. Additionally, the MoO3/SWNT sample shows a tangible improvement on the stability of the electrochemical performance, delivering reversible capacity of 950 mAh g−1 at 50th cycle, with only a 1.2% capacity loss from the 1st cycle.
In order to further understand the different electrochemical performance of the three MoO3-based electrodes, we carried out electrochemistry impedance spectroscopy (EIS) for all the three samples at charged state, after 60 cycles. The Nyquist plots of the electrodes are presenting a semi-circle at high-to-medium frequency [60], demonstrating the different interface resistances in the three samples. The interface resistance occurring at high frequency is associated with phenomena such as Li+ ion diffusion through the SEI film and/or in the active material, and the contact layer between the electrode and current collector [96–98].
As obtained from figure 4(b), the interface resistance of the MoO3 sample is ~160 Ω, which is significantly reduced to ~80 Ω for the MoO3/CB sample. The MoO3/SWNT hybrid structure gives the lowest value of interface resistance, i.e. ~40 Ω, which is one fourth and one half with respect to the ones shown by the MoO3 and MoO3/CB-based electrodes, respectively. The reduction of the interface resistance upon the addition of carbon additives, especially SWNTs, compared with the MoO3, might be attributed to the different structural morphology of the electrodes after lithiation/de-lithiation processes [60, 61].
Thus, in order to understand the relation between the electrochemistry performance of the three samples and their structural morphology after charge-discharge cycles, we carried out post-mortem SEM measurements on MoO3, MoO3/CB and MoO3/SWNT electrodes after 60 charge/discharge cycles. As shown in figure 5(a), the MoO3 electrode clearly presents cracks and fractures with width of 200–400 nm, likely caused by the volume change during the charge/discharge cycles. These cracks determine a drop in the electrical conductivity, with consequent capacity fading [99, 100], as clearly presented in figure 4. As shown in figure 5(b), large cracks over 1 µm are observed in the MoO3/CB electrode as well. Even if, compared to free MoO3, the presence of CB seems able to furnish better electrical conductivity during the first cycles MoO3/CB electrodes still suffer a remarkable capacity fading upon cycling. This is likely due to the inability of CB to keep the anode material in continuous contact with the current collector [102, 103]. Although the MoO3/SWNT sample shows cracks after 60 cycles, the cracks are much narrower with respect to the ones presented by the MoO3 and MoO3/CB electrodes.
Figure 5. SEM images of (a) MoO3, (b) MoO3/CB and (c) MoO3/SWNT electrodes after 60 charge/discharge galvanostatic cycles.
Download figure:
Standard image High-resolution imageMoreover, the carbon network of nanotubes ensures high electrical conductivity upon the expansion/contraction processes of MoO3. This conductive framework is therefore beneficial for both mechanical stability [101] and the specific capacity of the anodes.
3.3. Electrochemical properties of MoO3/SWNT electrodes with different mix ratio
From the obtained results, it is clear that the SWNTs addition (10% with respect to the MoO3 flakes) is beneficial for the electrochemical properties of the electrodes. Thus, in order to further investigate the contribution of the SWNTs addition in the MoO3/SWNT hybrid anode, we designed other two electrodes, adding SWNTs to the MoO3 flakes at a weight ratio of 20% and 30%, with mass loading of 0.74 mg and 0.76 mg, respectively. As shown in figure 6(a), while the percentage of SWNTs in the MoO3/SWNT electrodes rises from 10% to 30%, the initial capacities of the three samples reach 1357, 1161 and 1044 mAh g−1, with corresponding discharge capacity at the first cycle of 927, 675, and 566 mAh g−1, respectively. The specific capacity and coulombic efficiency (see figure 6(a)) demonstrate that all the hybrid electrodes with different mixed ratios show remarkable stable cyclability up to 50 cycles, if compared with the MoO3 anode.
Figure 6. (a) Specific capacity and coulombic efficiency over charge/discharge galvanostatic cycles, and (b) impedance spectroscopy of MoO3/SWNT hybrid electrodes with different SWNT weight ratios as anodes against Li foil in half cell configuration. (c) Capacity retention of MoO3/SWNT hybrid anodes and (d) Specific capacity of MoO3/SWNT hybrid anode calculated with respect the weight of MoO3 (red) and the hybrid structures, respectively (green).
Download figure:
Standard image High-resolution imageThe post-mortem SEM images of the three MoO3/SWNT electrodes shown in figures S5(b)–(d) clearly demonstrate that the SWNTs in the MoO3/SWNT electrode create a network between the cracked 'islands' following the MoO3 volume change during charge/discharge cycles [65]. Notably, the width of the cracks is reducing with the percentage increase of SWNT in the MoO3/SWNT hybrids. A possible explanation could be linked with the fact that the increasing amount of SWNTs, as buffer between the MoO3 flakes, can efficiently attenuate the volume change during charge/discharge cycles, reducing the mechanical degradation of the electrodes.
The EIS results of the 3 samples shown in figure 6(b), demonstrates how the higher is the percentage of SWNTs in the hybrid MoO3/SWNT electrodes, the lower is their charge transfer resistance. In fact, a charge transfer resistances of ~40 Ω, ~30 Ω and ~17 Ω have been obtained for the sample with 10%, 20% and 30% of SWNTs with respect to the MoO3 flakes, respectively. However, although higher percentage of SWNTs (i.e. 20–30%) in the hybrid structure can provide better electrical conductivity (i.e. charge transfer resistances of 30 Ω and 17 Ω for the electrodes containing 20% and 30% of SWNTs with respect to the MoO3 flakes) this is not directly associated to an increase of the electrode specific capacity. In fact, the increasing percentage of SWNT has determined a tangible decrease of the specific capacity with respect to the total loading of MoO3/SWNT hybrid electrodes. This could be linked with the high irreversible capacity that affects CNTs-based anode for LIBs [102]. In fact, the irreversible capacity increases from 32% for the 10% MoO3/SWNTs sample to 46% in the case of 30% MoO3/SWNTs one. Moreover, the 10% MoO3/SWNTs sample shows the highest capacity retention (71.6% after 50 cycles) over charge/discharge cycles, obtained by dividing the charge-capacity to the initial capacity (see figure 4(a)), amongst the electrodes, i.e. the hybrids MoO3/SWNTs and the MoO3 one.
Moreover, we have also calculated the specific capacity of each electrode, as shown in figure 6(c), labeled by different SWNTs content from 0 to 30%. The specific capacities are calculated using the mass loading of MoO3 and MoO3/SWNT, respectively. In both cases, the 10% SWNT sample reaches the highest specific capacity of 1028  (926
(926  ), see figure 6(d), which represent the 92% of the theoretical specific capacity of MoO3 [55, 56]. As summarized in table S1 (see S.I.), the performance of our MoO3/SWNT binder-free anode favorably compare with state of the art MoO3-based LIBs [39, 42, 44, 49, 56, 60, 65, 72, 103, 104]. The reported electrochemical analysis indicates that the addition of 10% SWNT in the hybrid structure with MoO3 flakes represents the best compromise in term of mechanical and electrochemical properties of the as-produced anodes.
), see figure 6(d), which represent the 92% of the theoretical specific capacity of MoO3 [55, 56]. As summarized in table S1 (see S.I.), the performance of our MoO3/SWNT binder-free anode favorably compare with state of the art MoO3-based LIBs [39, 42, 44, 49, 56, 60, 65, 72, 103, 104]. The reported electrochemical analysis indicates that the addition of 10% SWNT in the hybrid structure with MoO3 flakes represents the best compromise in term of mechanical and electrochemical properties of the as-produced anodes.
4. Conclusion
By exploiting the combination of multilayer MoO3 nanosheets, produced by LPE of layered MoO3 crystallites, with solution processed SWNTs we demonstrated a high performance binder-free MoO3/SWNTs hybrid anode for LIBs. Contrary to CB nanoparticles, the SWNTs addition determines a network structure with the MoO3, which is beneficial for the mechanical and (electro)chemical performances of the as-produced anode by providing (1) long channels for electronic charge transport; (2) an active anode material, instead of polymeric binder, offering extra capacity for Li ions storage: (3) a buffer frame in the electrode, which reduce the capacity fading caused by the volume expansion of MoO3 flakes during the lithiation process. The designed binder-free solution processed hybrid MoO3/SWNT (90:10) anode has demonstrated a specific capacity of 865 mAh g−1 at 100 mA g−1 after 100 cycles, with a columbic efficiency of 99.7% and a capacity fading of 0.02% per cycle. We believe that the low-cost, non-toxic, binder-free hybrid MoO3/SWNT can boost the development of high-performance anodes for LIBs.
Acknowledgments
This project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 696656—GrapheneCore1.






