Abstract
Investigation employing bronchoalveolar lavage supports both increased and decreased iron concentrations in the epithelial lining fluid (ELF) of smokers. Exhaled breath condensate (EBC) is an alternative approach to sampling the ELF. We evaluated for an association between iron homeostasis and both smoking and a diagnosis of chronic obstructive pulmonary disease (COPD) by measuring metal concentrations in EBC samples from non-smoker controls, smoker controls, and individuals diagnosed with COPD. The total number of EBC specimens was 194. EBC iron and zinc concentrations (mean ± standard error) in the total study population were 0.610 ± 0.025 and 40.73 ± 1.79 ppb respectively. In linear regressions, total cigarette smoking in pack years showed a significant (negative) relationship with EBC iron concentration but not with EBC zinc concentration. Iron concentrations in EBC from GOLD stage II, III, and IV patients were all significantly decreased relative to those from non-smoker and smoker controls. In contrast to iron, zinc concentrations in EBC were not significantly different than those from non-smoker and smoker controls. It is concluded that smoking decreases EBC iron concentrations and patients diagnosed with COPD have significantly lower EBC iron concentrations. These results likely reflect an increased burden of cigarette smoke particles in the lower respiratory tract of ever-smokers and patients with COPD and the capacity of components in this particle to complex iron.
Export citation and abstract BibTeX RIS
Introduction
Chronic obstructive pulmonary disease (COPD) most frequently refers to some combination of chronic bronchitis and emphysema, a pair of commonly co-existing lung diseases [1, 2]. COPD is characterized by a physiologic limitation of the expiratory flow of air from the lungs which is irreversible. The natural course of the disease can include a gradual worsening of obstruction over years with exacerbations of airflow obstruction often caused by infections and air pollution. The vast majority of COPD in the United States is the result of cigarette smoking.
A disruption in iron homeostasis with concomitant accumulation of the metal has been proposed to contribute to COPD associated with cigarette smoking [3, 4]. Smoking a single cigarette exposes the human respiratory tract to between 150 00 and 400 00 μg particulate matter with a high rate of deposition in the human lung [5]. Incomplete oxidation of tobacco leaves produces oxygen-containing functional groups (e.g. carboxylates and phenolic hydroxides) at the surface of the retained cigarette smoke particles [6]. Following dissociation of protons at the physiological pH level, these functional groups introduce a negatively charged solid–liquid interface into the lung tissue. Iron (III) has a high affinity toward oxygen-donor ligands due to its electropositivity, and thus, it forms complexes with the particulate matter deposited from cigarette smoking [7]. Subsequently, these complexes of iron with cigarette smoke particle accumulate in the lungs of smokers [8, 9]. Elevated iron concentrations can be observed not only in the lower respiratory tract but systemically as well [10]. Reflecting this disruption in the homeostasis of iron and its accumulation, serum concentrations of ferritin, a metal storage protein, also increase in cigarette smokers [8].
Exhaled breath condensate (EBC) is an alternative approach to sampling the epithelial lining fluid (ELF). Investigation employing bronchoalveolar lavage supports both increased and decreased iron concentrations in the ELF (i.e. the alveolar and airway lining fluids) of smokers [10–12]. A prior EBC study has suggested lower levels of iron among patients with COPD; methodology employed to measure iron in this study used a bleomycin assay which reflects pro-oxidant levels rather than an absolute concentration of the metal [13]. We evaluated for an association between iron homeostasis in both ever-smokers and the diagnosis of COPD by measuring metal concentrations in EBC samples from non-smoker controls, smoker controls, and patients diagnosed to have COPD.
Methods
Samples of EBC
EBC was collected in the 'Evaluation of COPD longitudinally to identify predictive surrogate end-points' (ECLIPSE) study, a 3 year longitudinal investigation with the overall objective of identifying the parameters predictive of disease progression in individuals with COPD. These samples included non-smoker controls, smoker controls, and GOLD stage II, III, and IV COPD patients. Non-smoker controls were male/female subjects aged 40–75 years who (1) were free from significant disease as determined by history and physical examination, (2) had baseline post-bronchodilator FEV1 > 85% of the reference value and FEV1/FVC > 0.7, and (3) had a total smoking history of <1 pack-year. Smoker controls were male/female subjects aged 40–75 years who (1) were free from significant disease as determined by history, physical examination and screening investigations, (2) had baseline post-bronchodilator FEV1 > 85% of the reference value and FEV1/FVC > 0.7, and (3) were current or ex-smokers with a smoking history ≥10 pack-years. Non-smoker controls and smoker controls were recruited through site databases and other methods (advertisements in local newspapers and television/radio stations). COPD patients were male/female subjects aged 40–75 years who (1) had baseline post-bronchodilator FEV1 < 80% of the reference value and FEV1/FVC ≤ 0.7, and (2) were current or ex-smokers with a smoking history of ≥10 pack-years. GOLD stage II, III, and IV were defined as: 50% ≤ FEV1 < 80%, 30% ≤ FEV1 < 50%, and FEV1 < 30% predicted respectively. COPD patients were recruited from the outpatient clinics of the 46 participating centers.
EBC collection was carried out as described previously [14]. All subjects were asked to refrain from eating, drinking, and smoking for at least 3 h prior to sample collection. EBC samples were collected during tidal breathing for 10 min using the RTubeTM (Respiratory Research Inc., Charlottesville, Virginia) and aliquoted for storage at 70 °C. Available data did not include either EBC volume or duration of collection.
Measurement of iron and zinc concentrations in EBC
The concentration of 56Fe (iron) and 64Zn (zinc) in EBC was determined by inductively coupled plasma-mass spectrometry (ICP-MS; ELAN DRC II, PerkinElmer, Waltham, MA). The analyses were carried out using nickel cones, a quartz cyclonic spray chamber, and a Meinhard Type A quartz nebulizer. Instrument conditions were: 1400W RF power, nebulizer gas flow of 1.08 l min−1, pump sample flowrate of 1 ml min−1, 1000 ms dwell time per isotope, and 5 replicates per measurement.
EBC samples (500 μl) were diluted with 2.0 ml nitric acid (Fisher Optima A467), and manually spiked with 6.25 ppb indium (In) internal standard (Part number LIS6020, VHG Labs, Manchester, NH). To minimize polyatomic interferences, 56Fe was determined using dynamic reaction cell (DRC) mode. The DRC was operated with 0.9 ml min−1 of ammonia gas and cell settings of RPq = 0.75 and RPa = 0.00. 64Zn was determined in standard analysis mode. Analysis closely followed EPA Method 200.8rev5.4 [15]. Calibration (Catalog number CL-CAL-2, Spex CertiPrep, Metuchen, NJ) and quality control (Catalog numbers 44CS1Y and 44CS2Z, VHG Labs) stock standard solutions were diluted with a 2% acid blank solution that was matrix-matched with the diluted EBC samples. Data were collected, processed and analyzed with ELAN software v3.4. Calibration curve r2 values were 0.9996 for 56Fe and 0.9999 for 64Zn. The instrument detection limits for 56Fe and 64Zn were 0.7 and 0.03 ppt while the method detection limits were 11 and 2 ppt.
Metal concentrations are expressed in units of absolute concentration and not normalized to any index to account for dilution. It was elected to communicate results in parts per billion without normalization for purposes of comparison to other studies focused on EBC. In addition, measurements of protein, albumin, and creatine to normalize the metal concentration were of uncertain value.
Statistics
Data are expressed as mean values ± standard deviation (SD) unless specified otherwise. Differences between two and multiple groups were compared using T-tests of independent means and one-way analysis of variance respectively; the post-hoc test employed was Duncan's multiple range test. Pearson product-moment correlation coefficients were calculated. Two-tailed tests of significance were employed. Significance was assumed at p < .05.
Results
The total number of EBC specimens was 194. The individuals with COPD (GOLD stage II, III, and IV) were significantly older than the non-smoker controls and the smoker controls (table 1). The percentage of females in each group varied widely. All but three patients were Caucasian. Non-smoker controls and smoker controls smoked less than the COPD patients (F = 4.04, p = 0.004; table 2). There were no significant differences in pack years smoking among GOLD stage II, III, and IV COPD patients.
Table 1. Characterization of the study population.
| Group | N | Age (years) | Gender | Ethnicity |
|---|---|---|---|---|
| Non-smoker controls | 30 | 59.5 ± 10.0 | 22 females | 29 Caucasian |
| 1 African-American | ||||
| Smoker controls | 16 | 59.1 ± 6.8 | 2 females | 16 Caucasian |
| Gold stage II | 52 | 65.4 ± 6.2 | 22 females | 52 Caucasian |
| Gold stage III | 50 | 63.3 ± 6.0 | 26 females | 48 Caucasian |
| 1 Asian-American | ||||
| 1 mixed race | ||||
| Gold stage IV | 46 | 63.3 ± 5.9 | 4 females | 46 Caucasian |
Table 2. Smoking histories of the study population.
| Group | Ex-smokers | Current smokers | Pack years smoking |
|---|---|---|---|
| Non-smoker controls | 0 | 0 | 0 |
| Smoker controls | 16 | 0 | 27.6 ± 19.5 |
| Gold stage II | 34 | 18 | 53.8 ± 34.9 |
| Gold stage III | 30 | 20 | 47.7 ± 28.2 |
| Gold stage IV | 12 | 34 | 54.4 ± 28.2 |
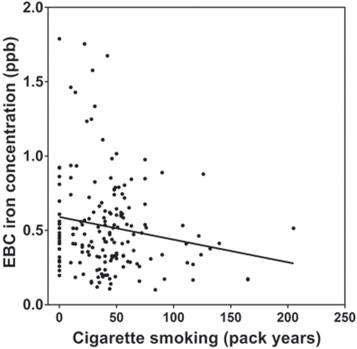
EBC iron and zinc concentrations (mean ± standard error) in the total study population were 0.610 ± 0.025 and 40.73 ± 1.79 ppb respectively. The percentage relative standard deviation for measurements of 56Fe and 64Zn were ≤ 20% in 94.6% and 100% of the samples respectively. Linear regressions between (1) age and iron concentrations and (2) age and zinc concentrations showed no significant relationships (r = 0.093; p = 0.24 and r = 0.024; p = 0.81 respectively). Similarly, gender did not impact EBC iron and zinc concentrations (T = 0.285 and p = 0.78 and T = 0.514 and p = 0.61 respectively). Total cigarette smoking in pack years did show a negative relationship with EBC iron (r = 0.201; p = 0.009) but not with EBC zinc concentration (r = 0.088; p = 0.270) (figure 1).
Figure 1. Correlations of pack years cigarette smoking with EBC concentration of iron. There was a significant decrease in EBC iron with pack years cigarette smoking (p = .03).
Download figure:
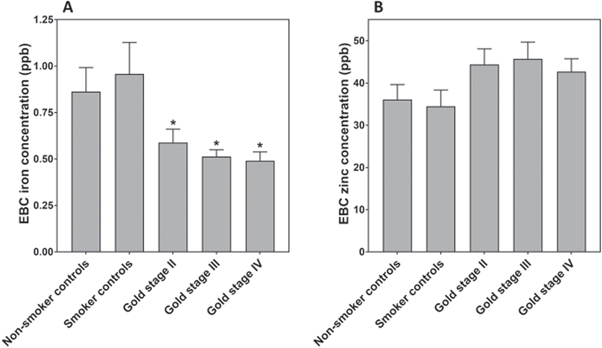
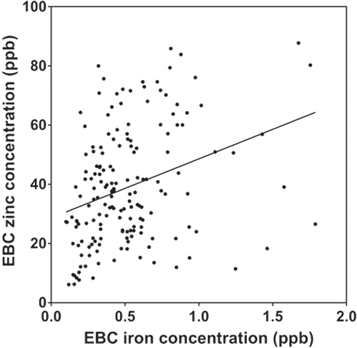
Standard image High-resolution imageIron concentrations in the EBC from GOLD stage II, III, and IV patients were all significantly decreased relative to those from non-smoker and smoker controls (F = 3.594, p = 0.007; figure 2(A)) Zinc concentrations were greater than iron with the ratio of zinc to iron concentrations varying from 34 to 95 among the groups (figure 1(B)). In contrast to iron, zinc concentrations in EBC from non-smoker controls were not significantly different than those from smoker controls, and Gold stage II, III, and IV patients (F = 1.488, p = 0.2076; figure 2(B)). EBC iron and zinc concentrations correlated positively (r = 0.32; p < .0001) (figure 3).
Figure 2. EBC concentrations of iron (A) and zinc (B) in non-smoker controls, smoker controls, Gold stage II, Gold stage III, and Gold stage IV subjects. There are decreased concentrations of EBC iron among those individuals with Gold stage II, Gold stage III, and Gold stage IV disease relative to non-smoker controls and smoker controls. *decreased relative to non-smoker controls.
Download figure:
Standard image High-resolution imageFigure 3. Correlations of EBC concentrations of iron and zinc. There was a significant increase in EBC zinc with EBC iron (p < .0001).
Download figure:
Standard image High-resolution imageDiscussion
In prior investigation, levels of metals (i.e. manganese, chromium, nickel and chromium) in EBC were affected by gender [16]. These disparities between genders, and those proposed to occur with aging, can reflect the volume of collected condensate which is proportional to the ventilatory volume (pulmonary function is determined by gender, age, and height/weight). However, gender and age did not impact EBC iron and zinc concentrations in this study of ELIPSE subjects.
Tobacco smoke includes numerous metals in significant concentrations [17]. Subsequently, smoking can impact metals concentrations in EBC [12, 16, 18–21]. Healthy smokers were previously demonstrated to have higher EBC concentrations of lead and cadmium relative to healthy non-smokers [22]. Cadmium, lead, and aluminum levels in EBC were also observed to be higher among both smokers and smokers with COPD [23]. When patients with COPD were subdivided into smokers versus ex-smokers and non-smokers, smokers were shown to have higher EBC lead, cadmium, and aluminum levels [12]. Ex-smokers with COPD who had quit smoking for more than 2 years continued to show elevations in EBC metals relative to non-smokers [22]. Regarding iron, both tobacco leaf and tobacco smoke include this specific metal and smoking can be predicted to introduce some quantity into the respiratory tract [17, 24]. Iron concentrations in EBC, measured using a bleomycin assay, were previously observed to be significantly increased in smokers when compared to healthy controls [25]. However, reduced concentrations of iron (as well as nickel) have also been observed in the EBC of smokers [12, 19]. In our cohort of 194 EBC samples obtained from the ECLIPSE study, regression showed that smoking was associated with decreased iron concentration in the EBC. This effect of smoking on EBC iron concentration is attributed to coordination of the metal by retained smoke particle. There is a large mass of particle which is retained in the lung with smoking. The incomplete combustion of tobacco leaf produces humic-like substances (HULIS) [9]. As a result of possessing a variety of oxygen-containing functional groups (e.g. phenolic and carboxylate groups), HULIS complex metal cations [26–28]. The high content of oxygen-containing functional groups in HULIS favors the formation of stable complexes with metals but the sorption ability of iron is greatest among all of them [29]. Accordingly, cigarette smoking introduces HULIS into the human lung where it disrupts iron homeostasis. Despite an accumulation of iron by the particle, an intracellular iron deficiency can be evident with increased cell import of this metal following uptake of the particle by macrophages and epithelial cells [8]. Accordingly, the ELF iron is proposed to be diminished in smokers as it functions as a source of the metal for cells in the lower respiratory tract with endocytosed particle. However, when the data was evaluated categorically, smoker controls were not demonstrated to have a significantly lower EBC iron concentration relative to that concentration in non-smoker controls. This seeming contradiction can be attributed to decreased total smoking in the smoker controls group relative to Gold stage II, III, and IV groups. In addition, the lack of a significant decrease in EBC iron can possibly reflect the smaller number in this group relative to the total number of smokers in the study (i.e. the number included in smoker controls and Gold II, III, and IV groups).
Results from this and other studies support a decreased level of iron in the ELF in smokers. This contrasts with other approaches which have revealed increased iron concentrations among smokers. Digestion of lung tissue from smokers demonstrated elevated concentrations of non-heme iron [30]. Prior investigation employing bronchoalveolar lavage supported increased iron concentrations in the ELF (i.e. the alveolar and airway lining fluids) of smokers [10, 11]. Lung tissue includes iron complexed by the particle. While total tissue and cell iron concentrations are increased, metal concentrations in the ELF appear to be diminished through the same pathway of iron complexation by the endocytosed particle. This is comparable to exposures to numerous compounds which demonstrate a capacity to complex metal and subsequently total cell iron can be increased but available metal concentrations are decreased [31].
In previous investigation, there were lower levels of iron (and copper) in EBC obtained from patients with stable COPD patients relative to samples collected from healthy subjects and healthy smokers while lead, cadmium, and aluminum were higher [12]. Using a bleomycin assay, another study revealed that EBC iron concentrations were decreased among patients diagnosed to have COPD relative to smokers with no obstruction [13]. These decrements in EBC iron were attributed to a failure of the diseased lung to excrete iron [13]. In our cohort of samples, COPD was associated with decreased iron concentration in the EBC comparable to these other studies. While the impact of smoking on EBC iron varies among prior studies, the diagnosis of COPD has repeatedly been associated with lower EBC iron concentrations. Rather than a failure to excrete iron, these results likely reflect an increased burden of cigarette smoke particle, including HULIS, in the lower respiratory tract of patients with COPD [13]. As the particle accumulates with greater smoking, host iron homeostasis is disrupted and, despite an accumulation of this metal in the lung, a functional iron deficiency develops. Loss of pulmonary function among smokers has been shown to correlate with the disruption in iron homeostasis (e.g. FEV1/FVC decreases as serum ferritin increases) supporting such a pathway [32].
A limitation of this investigation is that the metal concentrations are expressed in units of absolute concentration and not normalized to some index to account for dilution (e.g. protein). However, it was elected to communicate results in parts per billion without normalization for purposes of comparison to other studies focused on EBC metal levels in COPD which had reported the same end-points as absolute concentrations.
It is concluded that smoking and a diagnosis of COPD both decrease EBC iron concentrations. These results likely reflect an increased burden of cigarette smoke particle in the lower respiratory of smokers and patients with COPD and the capacity of components of this particle to complex iron.
Acknowledgments
We thank John Sullivan of the US Environmental Protection Agency for technical excellence and assistance in measuring metal concentrations in the specimens.
Footnotes
- *
Disclaimer: This report has been reviewed by the National Health and Environmental Effects Research Laboratory, United States Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendations for use.