Abstract
Tropical peatlands are a globally important source of methane, a potent greenhouse gas. Vegetation is critical in regulating fluxes, providing a conduit for emissions and regular carbon inputs. However, plant roots also release oxygen, which might mitigate methane efflux through oxidation prior to emission from the peat surface. Here we show, using in situ mesocosms, that root exclusion can reduce methane fluxes by a maximum of 92% depending on species, likely driven by the significant decrease in root inputs of oxygen and changes in the balance of methane transport pathways. Methanotroph abundance decreased with reduced oxygen input, demonstrating a likely mechanism for the observed response. These first methane oxidation estimates for a tropical peatland demonstrate that although plants provide an important pathway for methane loss, this can be balanced by the influence of root oxygen inputs that mitigate peat surface methane emissions.
Export citation and abstract BibTeX RIS
1. Introduction
Tropical peatlands are an important part of the global carbon cycle, containing 104.7 Gt C, and constituting a significant source of methane (CH4) and carbon dioxide (CO2) emissions [1, 2]. CH4 fluxes alone are estimated at up to 0.23 Gt CH4 yr−1, equivalent to 17%–40% of global emissions [3, 4]. However, tropical peatlands are threatened by agricultural expansion and climate change, underlining the importance of understanding processes regulating decomposition and greenhouse gas (GHG) fluxes.
Vegetation exerts a strong control on GHG emissions through several pathways: first, autotrophic root respiration can represent a substantial contributor (c. two-thirds) to net CO2 emissions in tropical peatlands [5]. Second, tropical peats are predominantly composed of decaying leaf, root and stem material [6], which results in organic matter properties varying substantially with peat botanical origin [7]. Third, roots can release substantial amounts of carbon as root exudates, which can drive significant GHG emissions depending on the composition and concentration of root exudate profiles [8, 9]. Fourth, roots are also well-adapted to waterlogged, anoxic conditions with aerial roots, pneumatophores and aerenchymateous tissue. These adaptations represent a substantial pathway for GHG transport, with root aerenchyma mediating up to half of net CH4 emissions in the Amazon floodplain [10]. Finally, roots can also be a significant source of oxygen in otherwise anoxic conditions, due to diffusion from root tissue into the peat. These localised oxic conditions can suppress CH4 fluxes by inhibiting methanogenesis and/or driving methanotrophy [11]. However, the importance of this process in regulating peat surface GHG fluxes in tropical peatlands represents a critical knowledge gap, with evidence from temperate and boreal peatlands, as well as wetland ecosystems more widely, indicating substantial potential for oxygen to limit net CH4 fluxes [12, 13]. The relative importance of this process may also differ in tropical peatlands versus northern climes, due to differences in vegetation type (generally tree and palm dominated versus moss dominated), higher temperatures and associated changes in rates of productivity and decomposition of organic matter [4].
Here, we use in situ in-growth mesocosms to study the role of roots and dissolved oxygen inputs in regulating GHG production under two dominant and contrasting plant species, Campnosperma panamensis, a broadleaved evergreen tree, and Raphia taedigera, a canopy palm, located in a mixed forest stand in a lowland tropical peatland in Panama. We hypothesised that root-derived oxygen controls carbon dynamics and consequently predicted that (i) root exclusion would significantly reduce concentrations of dissolved oxygen; (ii) plants with different root structures would be associated with contrasting oxygen inputs and resultant GHG emissions; (iii) root oxygen inputs alter the balance of aerobic versus anaerobic decomposition; (iv) changes in organic matter chemistry and reduced root inputs of oxygen increase CH4 fluxes, mediated through altered microbial community structure.
2. Methods
2.1. Site description
This study was conducted from January–May 2016 in San San Pond Sak, a large freshwater and marine wetland in Bocas del Toro Province of Panama that includes an 80 km2 ombrotrophic peatland at Changuinola. The site features a central peat dome (>8 m deep, 5000 years old), and distinct vegetation and nutrient gradients, ranging from Rhizophora mangle mangrove swamp on the coastal margins, mixed and monodominant palm and broadleaved evergreen tree swamp and a bog-plain [14]. This study was conducted in a mixed forest stand featuring both C. panamensis broadleaved evergreen trees and R. taedigera palms. C. panamensis develops approximately 1 m tall buttress roots t, with lenticels for oxygen transport to roots (stacks.iop.org/ERL/15/064013/mmedia). In contrast, R. taedigera forms a dense surface root mat with pneumatophores [15] (supplementary figure 2).
Between 2002 and 2016 mean annual air temperature was 25.7 °C, with low intra-annual variability. During sampling, mean temperature was 26.9 °C. Over the same period, mean annual rainfall was 3293 mm, with a monthly mean of 173 mm between January and May 2016. Mean sub-surface peat temperature was 25.0 °C. Water table height is variable, fluctuating from just above to just below the peat surface, with a range of 20 cm [16].
2.2. Mesocosm design
Two mesocosm designs and one control collar were used to assess the role of root oxygen n peat decomposition and CH4 fluxes. Mesocosms were constructed from 9 cm diameter PVC piping (Amanco, Mexichem Panama, S. A.) and cut to a length of 30 cm. To allow root regrowth, four 8 cm diameter holes were cut into the side of one mesocosm, 10 cm from the top and 5 cm from the bottom of the mesocosm (supplementary figure 2). The second treatment consisted of a solid 30 cm PVC tube to stop both root regrowth and inputs. Mesocosms were open at the top and bottom to allow changes in water table depth. Mesocosms were placed in holes excavated to a depth of 25 cm under five C. panemensis and five R. taedigera, within 25 cm of the trunk of each individual plant, within a mixed forest stand, and with 5 cm remaining above the surface. Excavated peat was extracted in a circular core using the diameter of the mesocosm as a template. Roots were cut using a machete and scissors. The core was then placed inside the mesocosm before insertion into the peat. Roots were not removed from the peat column to maintain structure, and because fully and partially decomposed roots are key contributors to peat formation at the site [17]. A depth of 25 cm was chosen as previously the majority of CH4 production has been identified as occurring with 30 cm of the peat surface for both species [18]. A 5 cm deep surface collar was installed beside the mesocosms as a control. An additional perforated PVC tube (1.8 cm internal diameter) was inserted down the side of each mesocosm to allow measurements of water table and dissolved oxygen profiles. The maximum distance between each mesocosm within a set was approximately 0.50 m, to ensure similar microtopography within each group. Mesocosms were installed in February 2016.
2.3. Greenhouse gas fluxes
In situ CH4 fluxes were measured weekly from each mesocosm using the closed-chamber technique [18], with sampling between 10 am and pm on five occasions between April and May 2016, following mesocosm installation and three months' recovery following installation [19]. Chambers (0.35 dm3) were placed on mesocosms following removal of fresh litterfall. Gases were mixed using a syringe and needle, and injected at over-pressure into pre-evacuated glass exetainers. If bubbling was observed, indicating an ebullition event, sampling was repeated. Three samples were collected over 20 min after fitting the chambers. CH4 concentrations were measured using gas chromatography (GC) and flame ionization. Calculations of gas fluxes assumed linear accumulation over time within the chamber and were calculated using the ideal gas law [20].
2.4. Plant and peat properties
Plant height and diameter at breast height (DBH) were measured for each tree associated with a mesocosm. Plants were of similar size: C. panamensis trees had a mean height of 16.2 m and DBH of 38.2 cm, with 1951 g m−2 of fine roots. R. taedigera palms were somewhat smaller with a mean height of 10.4 m and DBH of 25 cm and 1670 g m−2 of fine roots (table 1).
Table 1. Mean plant and peat properties for C. panamensis and R. taedigera in situ mesocosms. Means ± 1 SE (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001, ns = not significant, na = not applicable.
| C. panamensis | R. taedigera | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Root-accessible | Closed | Control | Root-accessible | Closed | Peat type | Treatment | Peat type × treatment |
| Height (m) | 16.2 ± 0.9 | — | — | 10.4 ± 0.9 | — | — | |||
| DBH (cm) | 38.2 ± 2.7 | — | — | 25.0 ± 4.1 | — | — | na | na | na |
| Bulk density (g cm−3) | 0.16 ± 0.01 | — | — | 0.15 ± 0.03 | — | — | ns | ns | ns |
| Fine roots (g m−2) | 1951 ± 490 | 798 ± 321 | 0 | 1670 ± 417 | 343 ± 171 | 0 | ns | *** | ns |
| Organic matter (%) | 92 ± 2.0 | 95 ± 0.5 | 93 ± 1.4 | 88 ± 2.4 | 89 ± 1.4 | 87 ± 1.1 | ** | ns | ns |
| pH | 4.3 ± 0.1 | 4.3 ± 0.1 | 4.5 ± 0.1 | 4.6 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.1 | * | ns | ns |
| Redox (mV) | 228 ± 6 | 224 ± 12 | 224 ± 6 | 240 ± 2 | 240 ± 4 | 233 ± 5 | ns | ns | ns |
| C (%) | 39.9 ± 1.2 | 44.4 ± 1.6 | 41.4 ± 0.9 | 32.3 ± 1.9 | 38.2 ± 1.1 | 42.0 ± 2.1 | ** | * | ns |
| N (%) | 2.0 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.1 | 1.9 ± 0.1 | 2.1 ± 0.1 | 2.50 ± 0.2 | * | ns | ns |
| C:N | 17.6 ± 1.0 | 18.2 ± 0.9 | 17.4 ± 0.8 | 16.8 ± 0.3 | 18.1 ± 0.6 | 16.9 ± 0.5 | ns | ns | ns |
| Microbial C (µg g−1) | 7258 ± 906 | 6878 ± 1531 | 8096 ± 865 | 4329 ± 475 | 3641 ± 458 | 3867 ± 554 | *** | ns | ns |
| DOC (µg g−1) | 563 ± 33 | 404 ± 93 | 626 ± 65 | 642 ± 62 | 839 ± 81 | 804 ± 143 | ** | ns | ns |
| Microbial N (µg g−1) | 624 ± 57 | 657 ± 289 | 617 ± 45 | 544 ± 61 | 537 ± 97 | 810 ± 121 | ns | ns | ns |
| TDN (µg g−1) | 2153 ± 325 | 1289 ± 352 | 2569 ± 285 | 2186 ± 363 | 2231 ± 422 | 1795 ± 272 | ns | ns | ns |
| Cellobiohydrolase | 0.4 ± 3.2 | 0.6 ± 0.6 | 0.8 ± 0.4 | 0.3 ± 0.5 | 0.2 ± 0.3 | 0.3 ± 0.1 | * | ns | ns |
| TOCRE6 (%) | 29.8 ± 4.8 | 40.6 ± 1.6 | 40.5 ± 0.4 | 37.0 ± 0.8 | 35.2 ± 2.1 | 36.1 ± 1.2 | |||
| HIRE6 (mg HC g−1 TOCRE6) | 327.0 ± 11.8 | 307.2 ± 7.6 | 319.6 ± 12.3 | 294.2 ± 11.2 | 262.8 ± 8.7 | 252.2 ± 10.6 | *** | * | ns |
| OIRE6 (mg O2 g−1 TOCRE6) | 206.6 ± 9.8 | 198.6 ± 7.1 | 205.4 ± 3.9 | 189.2 ± 5.3 | 208.2 ± 5.4 | 230.0 ± 6.5 | ns | * | ** |
| I-index | 0.02 ± 0.004 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.12 ± 0.02 | 0.15 ± 0.01 | 0.13 ± 0.03 | ** | ns | ns |
| R-index | 0.63 ± 0.002 | 0.61 ± 0.01 | 0.61 ± 0.004 | 0.59 ± 0.01 | 0.59 ± 0.01 | 0.59 ± 0.01 | * | ns | ns |
Dissolved oxygen was measured in situ using a combined dissolved oxygen and temperature probe (Jenway 970 DO meter). Measurements were made sequentially in 5 cm increments to a depth of 25 cm following GHG sampling. Measurements were only made from the surface of the water table and below, as peats were not continually inundated during sampling. All measurements were made using the peat surface as a reference point for depth. Only data from April and May was included in the study to account for disturbance effects.
Pore water samples were collected using 10 cm long Rhizon samplers made from hydrophilic porous polymer with a pore diameter of 0.1 µm to exclude peat particles (Rhizosphere Research Products, Wageningen, the Netherlands) at the conclusion of the study in May 2016. Dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) were measured using a TOC-V/TN analyser (Shimadzu Corp, Kyoto, Japan).
Four months after installation, mesocosms were removed and in-growing roots were collected. A 25 cm by 8 cm circular core was excavated under the control collar for comparison. Living fine roots (<2 mm diameter), identified by colour and condition, were washed in deionised water and oven-dried at 65 °C for 3 d before weighing.
Gravimetric moisture was determined as mass loss following oven drying at 105 °C for 24 h. Organic matter content was determined as mass loss after ignition for 7 h at 550 °C. Total peat carbon (C) and total nitrogen (N) were determined using a total element analyser (Thermo Flash EA 1112, CE Instruments, Wigan, UK). Bulk density was measured by collecting 10 cm × 10 cm × 20 cm sections from the peat surface, and oven drying at 105 °C for 24 h. Peat pH and redox potential were measured in a 1:5 suspension of fresh peat to deionized water.
Microbial biomass nutrients were determined by chloroform fumigation. Microbial carbon and nitrogen were measured in 0.5 M K2SO4 extracts from peat that had been fumigated with chloroform for 24 h. Microbial nutrients were calculated as the difference between fumigated and non-fumigated samples. A correction factor of 2.64 was used to account for unrecovered biomass carbon and 1.85 for unrecovered nitrogen [21].
The activity of six hydrolytic enzymes involved in phosphorus and carbon release from organic compounds were measured using fluorimetric assays using methyumbelliferone (MU) linked substrates [22]. Enzymes assessed were (i) phosphomonoesterase (degrades monoester-linked organic phosphates); (ii) phosphodiesterase, (iii) β-glucosidase (degrades ß-bonds in simple sugars); (iv) N-acetyl-β-glucosaminidase (degrades N-glycosidic bonds); (v) xylanase (degrades hemicellulose); (vi) cellobiohydrolase (degrades cellulose). Fluorescence was measured on a FLUOstar Optima microplate reader (BMG Labtech, Offenburg, Germany), and calculated in nmol MU g−1 min−1 [23]. Detailed methods are provided in supplementary information.
Phospholipid fatty acids (PLFAs) were extracted from peat samples following the Bligh and Dyer protocol (1959) and quantified via GC analysis. Detailed methods and PLFA designations are provided in supplementary information [24].
Peat organic matter properties were assessed by Rock-Eval 6 pyrolysis configured in standard mode [25]. Organic matter properties were assessed using the following selected parameters: (i) total organic carbon (TOCRE6); (ii) Hydrogen Index (HI), a measure of released hydrocarbons relative to TOCRE6; (iii) Oxygen Index (OI), corresponding to the oxygen released as CO and CO2 relative to TOCRE6; (iv) the I index, describing thermally labile organic matter; (v) the R-index describing highly thermostable mature organic matter [26]. Detailed methods are provided in supplementary information.
2.5. Statistical analysis
Statistical analyses were carried out in Genstat v17.0. Differences between peat properties, enzyme activities, microbial community abundances and CH4 fluxes were assessed using a linear mixed effects model fitted using Residual Maximum Likelihood (REML) to account for variable dependence between mesocosms. Peat biochemical properties and enzyme activities included treatment and peat type as fixed effects and plot number as a random effect. CH4 fluxes and enzyme activities were log-transformed to meet assumptions of normality. Models of CH4 fluxes also included sampling day as a fixed effect, and models of dissolved oxygen included sampling day and depth as fixed effects. The percentage of oxidized CH4 was calculated by comparing CH4 effluxes from paired treatment (root-accessible versus closed) mesocosms. Comparisons were made between treatment mesocosms rather than to the control to account for the increase in fluxes derived from root necromass. Regression models were used to assess the correlation between transformed CH4 and root biomass. Relationships between peat biochemistry and CH4 fluxes were assessed by Principal Component Analysis (PCA) based on correlation matrices.
3. Results and discussion
3.1. Dissolved oxygen in peat profiles
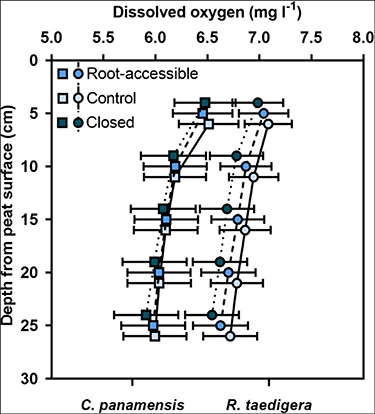
Supporting our first hypothesis, root exclusion significantly reduced dissolved oxygen concentrations under both species and across all depths (p < 0.01), with lowest dissolved oxygen concentrations found in closed mesocosms (figure 1). Reductions ranged from 0.2–3.1% for C. panamensis, and 0.4%–3.3% for R. taedigera compared to the root-accessible mesocosm and up to 2.9% and 5.4% compared to the control. Dissolved oxygen concentrations were significantly higher under R. taedigera, indicating greater root inputs of oxygen (p < 0.001), supporting our second hypothesis. Concentrations also varied by depth (p < 0.001), with decreases most pronounced between 5–10 cm depth. The most substantial reductions in mean dissolved oxygen following root exclusion were found at 25 cm depth for C. panamensis (1.2%), and 15 cm depth for R. taedigera (1.5%).
Figure 1. Dissolved oxygen at 5–25 cm depth for C. panamensis (▪) and R. taedigera (•) mesocosms. Means ± 1 SE (n = 5).
Download figure:
Standard image High-resolution imageSignificant differences in dissolved oxygen concentrations between peat types at depth are likely associated with contrasts in root structures between plant species [15], including the anatomical features of the root cortex, epidermis and hypodermis [27]. Differences in the extent of CH4 oxidation between species supports our second hypothesis, that plants with different root structures would be associated with contrasting oxygen inputs and resultant GHG emissions.
3.2. Peat surface methane fluxes
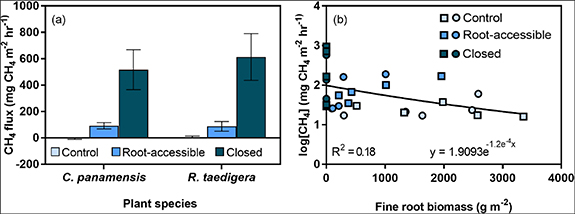
Supporting our third hypothesis, root exclusion increased CH4 fluxes in both treatment mesocosms (figure 2(a)). Control collar measurements indicated that C. panamensis peat was a small sink for CH4, and R. taedigera peat a small source. However, root-accessible and closed mesocosms were significant sources of CH4 for both peat types (p < 0.05). CH4 fluxes from the root-accessible mesocosm were 92.3 mg CH4 m−2 h−1 and 87.8 mg CH4 m−2 h−1 under C. panamensis and R. taedigera respectively, with maximum fluxes measured in the closed mesocosm under both species (517.8 mg CH4 m−2 hr−1 and 613.8 mg CH4 m−2 hr−1). CH4 fluxes increased in the closed mesocosm compared to the root-accessible mesocosm by 92 ± 4% under C. panamensis, and 85 ± 6% under R. taedigera, indicating full root exclusion drove substantial emissions. The strength of this effect is underlined by the incomplete regrowth of roots in the root-accessible mesocosm compared to the control indicating that relatively low root biomass (table 1) can result in substantial peat surface CH4 flux mitigation. There were no significant differences in CH4 fluxes between species. There was a significant correlation (p < 0.05, R2 = 0.18) between fine root biomass and log-transformed CH4 fluxes, with increased root biomass associated with reduced CH4 fluxes (figure 2(b)).
Figure 2. (a) CH4 fluxes from C. panamensis and R. taedigera from experimental mesocosms and (b) CH4 fluxes and mesocosm fine root biomass for C. panamensis (▪) and R. taedigera (•) control, root-accessible and closed mesocosms. Means ± 1 SE (n = 5).
Download figure:
Standard image High-resolution imageShallow subsurface peat has previously been identified as a dominant source of CH4 in the profile [18], and our results suggest that substantial oxidation is driven by root oxygen inputs at these depths, because the most substantial reductions in dissolved oxygen following root exclusion were found at 10–15 cm depth (1.6%–2.9%). An ex situ study of R. taedigera seedlings demonstrated that root oxygen can suppress CH4 fluxes by 40% up to 2 cm from root surfaces and was more pronounced in later growth stages of seedlings, most likely due to the increased spread of root aerenchymateous tissue enhancing the release of oxygen into peat [28]. Similar effects occur during rice development, whereby CH4 emissions decline in more advanced growth stages due to increased CH4 oxidation and the inhibition of methanogenesis [29, 30]. Estimates of CH4 oxidation by rice root oxygen are consequently numerous and varied, ranging from 7%–90% [31, 32].
Previously reported proportions of CH4 oxidation driven by combined root oxygen inputs and the surface oxic layer in temperate and boreal systems are highly variable, including 22% toxidation in a Sphagnum dominated peatland in northern Scotland [13], 11%–100% in a peatland in the Appalachian Mountains [12], and 22.9%–79% in the rhizosphere of some wetland plants [33, 34]. The roots present in the root-accessible mesocosm may also be acting as a transport pathway for CH4 efflux, as may dead roots in the closed mesocosm if they connect to the surface. However, this proportion is likely to be relatively low because fully grown plants only accounted for approximately 30% of CH4 transport at the site, and root-accessible mesocosms had reduced fine root biomass compared to the controls [35]. The extent of root transport is also species dependent, differing between broadleaved evergreen trees and palms [35], and likely accounts for low peat surface fluxes from control mesocosms, alongside differences in production rates due to peat chemistry and microbial community structure and function.
Changes in oxygen concentrations in the peat profile may also have affected the balance between ebullition and diffusion transport pathways. High oxygen concentrations may reduce the accumulation of CH4 in bubbles, resulting in lower transport of CH4 via ebullition [36]. However, as the exclusion of roots decreased oxygen concentrations, this indicates that the suppression of ebullition is partially driven by root inputs of oxygen. The exclusion of probable ebullition events from the calculation of CH4 oxidation events may have slightly underestimated the role of root inputs of oxygen in suppressing peat surface fluxes. The majority of models of CH4 dynamics in peatlands and wetlands assume negligible rates of oxidation for CH4 ebullition [37]. Longer-term studies of ebullition in tropical peatlands are therefore needed to fully elucidate the regulation of this CH4 transport pathway.
Taken together, our estimates of CH4 oxidation by roots are amongst the highest estimates reported for peatland CH4 oxidation but may be more uncertain than our results suggest due to the possibility of overestimation from the unquantified role of root CH4 transport, and underestimation from the exclusion of ebullition events. Our estimates are the first for tropical peatlands, and therefore demonstrate the critical role of vegetation in mitigating peat surface CH4 emissions.
3.3. Peat microbial community structure
Microbial communities for both peat types were bacteria dominated, accounting for two-thirds of total PLFA biomarkers in control peats (table 2). Fungi accounted for only 7% of identified biomarkers. Only Gram negative bacteria abundance was significantly affected by changes in root inputs, with a significant decrease in closed mesocosms for both plant species (p < 0.05). This change was predominantly driven by a significant (p < 0.05) decrease in C16:1ω5 biomarker abundance, one of two methanotroph biomarkers (the other being C18:1ω7), which accounted for 37% of total Gram negative biomarker abundance in control treatments. Changes in Gram negative abundance did result in a slight but non-significant (p > 0.05) increase fungi:bacteria, but did not alter Gram positive:Gram negative. We propose that the significant decline in C16:1ω5 abundance, indicates that decreased methanotrophy was a likely cause of the substantial CH4 fluxes observed, supporting our fourth hypothesis. However, PLFA biomarkers are restricted to fungi and bacteria and are not found in methanogenic Archaea. Consequently, decreased methanogen abundance, possibly combined with increased methanotroph abundance, may also account for the observed pattern of fluxes [38].
Table 2. PLFA biomarker abundance for C. panamensis and R. taedigera in situ mesocosms. Means ± 1 SE (n = 5). * p < 0.05, ** p < 0.01, *** p < 0.001, ns = not significant.
| C. panamensis | R. taedigera | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Root-accessible | Closed | Control | Root-accessible | Closed | Peat type | Treatment | Peat type × treatment |
| Total PLFAs (µg g−1) | 82.3 ± 11.5 | 64.5 ± 1.6 | 54.5 ± 11.1 | 64.2 ± 7.5 | 54.8 ± 14.3 | 47.2 ± 6.4 | ns | ns | ns |
| Fungi (µg g−1) | 5.9 ± 1.7 | 3.7 ± 5.7 | 3.9 ± 0.5 | 4.3 ± 1.8 | 3.7 ± 1.0 | 4.7 ± 0.5 | ns | ns | ns |
| Gram positive (µg g−1) | 21.3 ± 3.7 | 17.4 ± 10.8 | 17.8 ± 2.9 | 19.1 ± 2.6 | 14.3 ± 4.4 | 11.4 ± 1.3 | ns | ns | ns |
| Gram negative (µg g−1) | 35.0 ± 8.9 | 28.1 ± 0.03 | 15 ± 5.4 | 24.7 ± 3.1 | 22.9 ± 6.1 | 18 ± 3.4 | ns | * | ns |
| C16:1ω5 (µg g−1) | 13.2 ± 3.2 | 10.3 ± 1.5 | 5.4 ± 2.0 | 8.9 ± 1.2 | 8.5 ± 2.3 | 5.9 ± 1.2 | ns | * | ns |
| C18:1ω7 (µg g−1) | 5.4 ± 1.9 | 5.1 ± 0.0 | 3.1 ± 0.9 | 3.7 ± 0.7 | 3.8 ± 1.1 | 3.3 ± 0.4 | ns | ns | ns |
| Fungi:bacteria | 0.11 ± 0.01 | 0.08 ± 0.03 | 0.22 ± 0.01 | 0.10 ± 0.05 | 0.10 ± 0.01 | 0.15 ± 0.0 | ns | ns | ns |
| Gram positive:gram negative | 0.61 ± 0.0 | 0.6 ± 3.9 | 0.62 ± 0.01 | 0.81 ± 0.08 | 0.6 ± 0.06 | 0.62 ± 0.07 | ns | ns | ns |
3.4. Peat properties, root oxygen and methane flux regulation
Mesocosm installation significantly reduced live root biomass (p < 0.001, table 1). In root-accessible mesocosms, there were 798 g m−2 and 343 g m−2 under C. panamensis and R. taedigera, respectively. No live roots were found in the closed mesocosm. At the conclusion of the experiment only total carbon was found to be significantly higher in treatment mesocosms (p < 0.01), providing further evidence of overall recovery from disturbance following installation.
Selected indices from Rock-Eval 6 pyrolysis were used to assess differences in organic matter decomposition between peat types and following root exclusion (table 1). The hydrogen index (HI), a measure of hydrocarbons released relative to TOCRE6, varied significantly between treatments (p < 0.05), decreasing in root-accessible and closed mesocosms. The oxygen index (OI), corresponding to the amount of oxygen released as CO2 relative to TOCRE, varied significantly between treatments (p < 0.05) and in the interaction between treatment and species (p < 0.01). In C. panamensis peats, OI decreased somewhat in both treatment mesocosms, but showed a pronounced increase in R. taedigera mesocosms, with greatest OI found in the closed mesocosm (230 mg O2 g−1 TOCRE6). The hydrogen index (HI), a measure of released hydrocarbons relative to TOCRE6, also decreased in both treatment mesocosms. Changes in HI and OI indicate the extent of organic matter decomposition and have previously been applied to tropical peats [20, 39]. There were, however, significant differences in I (p < 0.01) and R (p < 0.05) indices between peat types, which describe the preservation of thermally labile and highly thermostable organic matter respectively.
Several differences in peat properties were found between peat types. Organic matter content was greater in peat under C. panamensis (92%) than R. taedigera (88%), as were total carbon and nitrogen, and microbial biomass carbon (MBC) (p < 0.01, table 1). In contrast, dissolved organic carbon (DOC) was higher in R. taedigera peats (p < 0.01). Most enzyme activities were greater in C. panamensis peat (supplementary table 1), but this difference was only significant for cellobiohydrolase activity (p < 0.05, table 1). HI was significantly higher under C. panamensis compared to R. taedigera across all mesocosms (p < 0.001), although OI did not differ (p > 0.05). The I-index was higher under R. taedigera (p < 0.05), and the R-index higher under C. panamensis (p < 0.01).
Taken together, these results indicate broad scale differences in organic chemistry between peat types, reflecting previously identified differences between dominant vegetation types [7, 20]. Moreover, the relatively limited differences in organic chemistry between treatments supports recovery following mesocosm installation, with the exception of increased root necromass in the root-accessible and closed mesocosms (table 1).
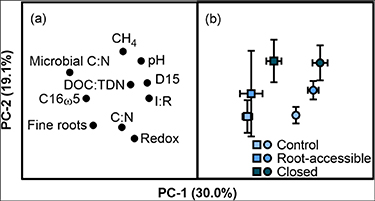
Principal component analyses (PCA) was run to test the relationship between CH4 fluxes, dissolved oxygen, and peat properties. The first principal component (PC-1) separated scores by peat type, with distribution driven primarily by dissolved oxygen concentrations at 15 cm depth (D15), the extent of decomposition (I:R), and microbial C:N (figures 3(a) and (b)). The second principal component (PC-2) separated scores by treatment and is driven by differences in fine roots, total C:N, redox potential, and pH. Collectively, these variables accounted for 49.1% of variance. Taken together, these results support our third hypothesis, as combined changes in organic matter chemistry and microbial community structure, associated with reduced root inputs of oxygen, resulted in increased CH4 fluxes.
Figure 3. (a) Loadings and (b) scores for CH4 fluxes and peat biochemical properties for C. panamensis (▪) and R. taedigera (•) control, root-accessible and closed mesocosms.
Download figure:
Standard image High-resolution imageDespite a significant difference in dissolved oxygen concentrations between species, differences in peat surface CH4 fluxes were not significant. First, differences in root oxygen input between species and the interaction with contrasting organic matter properties are likely to be strong drivers of peat decomposition. This interaction is also likely to explain part of the previously reported small scale variation in organic matter properties [20]. While some previous studies at Changuinola have reported significant differences in CH4 flux from peats under contrasting plant communities [15], fluxes display a high variability and differences have not been consistently reported [8, 20]. Second, plant-mediated CH4 transport at the site is species dependent and is generally lower in palms than for broadleaved evergreen trees [35], although there were no significant differences in fine root biomass between species (table 1). However, substantial CH4 transport can occur through palm pneumatophores [40]. Consequently, the similar peat surface CH4 fluxes between species, despite different dissolved oxygen inputs, is likely differing contributions of root transport of CH4, contrasting peat organic chemistry and rates of decomposition under alternating aerobic or anaerobic conditions [17], and variation in methanogenic and methanotrophic community structure and activity [24, 41, 42].
In general, litter from R. taedigera decomposes more rapidly than C. panamensis litter in the presence of oxygen, most likely because of higher nitrogen and lignin content [17], and because ligninolytic microbes are obligate aerobes [43]. In the root-accessible mesocosms, the decrease in total carbon for R. taedigera relative to the closed mesocosms is likely driven by the higher oxygen inputs. Compared to R. taedigera, C. panamensis litter has lower lignin content and therefore anaerobic decomposition occurs more quickly [17]. This is supported by higher total carbon in the root-accessible relative to the closed mesocosm. In addition, peats were not consistently waterlogged throughout the experiment due to fluctuating water tables, potentially further enhancing aerobic decomposition for both peat types [16]. In addition, diffusion of oxygen in surface layers will have resulted in continued CH4 oxidation where CH4 and oxygen profiles overlap [44]. Surface fluxes relative to subsurface production are substantially lower due to CH4 oxidation to CO2 by a highly efficient methanotropic community [18]. Taken together, these results demonstrate that the presence of relatively small masses of fine roots can significantly reduce CH4 fluxes in situ, through a combination of transport (<30%) [35] and root oxygen inputs (up to 92%) (figure 2(b)).
4. Conclusion and discussion
Root exclusion reduced dissolved oxygen and the abundance of a PLFA biomarker previously identified in methanotrophs. Matching these declines was a considerable increase in CH4 emissions. This demonstrates the critical role of roots as regulators of tropical peatland GHG fluxes through species-specific inputs of oxygen, which regulates microbial community abundance and therefore the degree to which CH4 is consumed in the peat profile.
Our results demonstrate that in tropical peatlands the presence of living roots can strongly influence oxygen concentrations and CH4 fluxes. Root exclusion resulted in a 92% reduction of CH4 fluxes, most likely driven by the 0.2%–3.3% reduction in root inputs of oxygen between root-accessible and closed mesocosms, but also through changes in the balance of ebullition, plant-mediated and diffusion pathways. This maximum rate of oxidation is broadly comparable to previous estimates for wetland plants [33, 34], and the first for tropical peatland ecosystems. Declines in PLFA biomarkers previously linked to methanotrophs suggests that root oxygen inputs play a key role in their abundance and that their presence mitigates otherwise substantial CH4 emissions. Root inputs of oxygen varied significantly between species which, combined with different litter chemistry, exerts a key limitation on rates of decomposition. Differences between species are significant in the context of global land use and climate change, as shifts in peatland plant community composition may alter regional patterns of GHG fluxes through changes in root oxygen inputs, and elevated temperatures can drive substantial increases in CH4 production [45, 46]. As a consequence, we propose that plants have an important role in reducing peat CH4 fluxes through root inputs of oxygen, and should be included in future models of GHG emissions from tropical peatlands.
Acknowledgments
This work was supported by the Natural Environment Research Council (Grant No. NE/L002604/1), and a Smithsonian Tropical Research Institute short-term fellowship. We thank Eric Brown for his support in the field, the staff at the Smithsonian Tropical Research Institute in Panama City and Bocas Del Toro for their logistical support, James Verran and Dr Saul Vasquez Reina at the University of Nottingham, Dr Annette Ryan at Lancaster University, and Vicky Moss-Hayes at the British Geological Survey for analytical support. We thank Professor Neil Crout for his feedback on early drafts. CHV publishes with the permission of the Executive Director of the British Geological Survey (NERC).
Data availability statement
The data that support the findings of this study are openly available. Please cite this paper and the data. Data to be cited as Girkin, N T, Vane, C H, Turner, B L, Ostle, N J, Sjögersten, S (2020). Root oxygen mitigates methane fluxes in tropical peatlands: data. DOI: https://doi.org/10.17862/cranfield.rd.12032145.