Abstract
A 2007 earthquake in the western Solomon Islands resulted in a localised subsidence event in which sea level (relative to the previous coastal settings) rose approximately 30–70 cm, providing insight into impacts of future rapid changes to sea level on coastal ecosystems. Here, we show that increasing sea level by 30–70 cm can have contrasting impacts on mangrove, seagrass and coral reef ecosystems. Coral reef habitats were the clear winners with a steady lateral growth from 2006–2014, yielding a 157% increase in areal coverage over seven years. Mangrove ecosystems, on the other hand, suffered the largest impact through a rapid dieback of 35% (130 ha) of mangrove forest in the study area after subsidence. These forests, however, had partially recovered seven years after the earthquake albeit with a different community structure. The shallow seagrass ecosystems demonstrated the most dynamic response to relative shifts in sea level with both losses and gains in areal extent at small scales of 10–100 m. The results of this study emphasize the importance of considering the impacts of sea-level rise within a complex landscape in which winners and losers may vary over time and space.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence.
Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Predicted increases in global sea level over the 21st century are anticipated to be a critical issue for humanity (Nicholls and Cazenave 2010). Sea-level rise will directly impact people and infrastructure through inundation and erosion of the shoreline, and will indirectly impact people by influencing the distribution and abundance of tropical coastal ecosystems, such as coral reefs, mangroves and seagrass. These ecosystems are important to the well-being of hundreds of millions of coastal people through the provision of ecosystem goods and services. Services provided by coastal tropical ecosystems include habitat for fish, shoreline stabilisation, building materials, and carbon sequestration (Hoegh-Guldberg et al 2014). Developing predictive understanding of how ecosystems will respond to deepening water is crucial to guide adaptation initiatives, and forecasts of where ecosystems will be lost and gained can better inform coastal planning (Bell et al 2014, Mills et al 2016).
The anticipated impacts of increased sea level on human populations (Hinkel et al 2014), shorelines (Albert et al 2016) and ecological communities (Courchamp et al 2014) will, in some cases, be severe and in other cases represent an opportunity for adaptation and gradual movement from areas of highest risk (Cavanaugh et al 2014, Valle et al 2014). In general, the seaward margin of intertidal and shallow subtidal ecosystems is expected to contract, and the landward margin to expand if conditions such as water clarity, hydrodynamics and substratum are suitable (Short et al 2016, Stevens and Lacy 2012, Infantes et al 2009). Mangroves, which are terrestrial plants occurring in intertidal areas between the mean and upper tide levels, are expected to experience dieback on the seaward fringe due to inundation (Lovelock et al 2015) and/or increased water salinity (Cohen et al 2016). However, paleoenvironmental evidence suggests that mangrove response and resilience to sea-level rise is likely to be non-linear and influenced by a range of physical and biological processes and interacting ecological feedback mechanisms (Woodroffe et al 2016). Seagrasses are marine plants which live in shallow coastal waters and have high light requirements. Increased water depth causes the benthic irradiance on the seafloor to decrease, which will lead to seagrass die-off in deeper water (Saunders et al 2013).
Coral reefs are comprised of species of coral that are extending upwards towards the sea surface. In the Indo-Pacific many reef flats may be intertidal and exposed at low water, and therefore lack 'accommodation space' to grow vertically (van Woesik et al 2015). Reef flats are therefore expected to benefit from moderate levels of sea-level rise, because the increased water depth may allow corals to re-establish and provide space into which they can continue to extend (Woodroffe and Webster 2014). The typically clear water on reefs, combined with the shape of reefs (wide and flat on the top, and steeper in deeper water), suggests that the benefits of sea-level rise to reefs may outweigh the overall costs (Perry et al 2015). In theory, all coastal ecosystems could colonise newly inundated areas landward, where substratum and coastal development permits.
A range of modelling tools have been utilized to predict both species and habitat level changes in distribution under various sea-level rise scenarios (Davis et al 2016, Saunders et al 2014, Lovelock et al 2015, Saunders et al 2013, Storlazzi et al 2011, Hamylton et al 2014). Insights into how ecosystems will respond to sea-level rise can be obtained from modelling, but real world examples are important for the validation of theoretical models. Local factors such as bathymetry, topography, sediment supply, water quality, substrate, currents and wave exposure can have a significant influence on the response of ecological systems to rising seas. As such, local scale (<100 km coastline) case studies of responses to relative changes in sea level can provide critical insight into larger ecosystem scale dynamics over 1000's km of coastline. Further, there are interdependencies among coastal marine habitats (Gillis et al 2014) which affect their response to climate change (Saunders et al 2014). Therefore, we need to assess the response of interconnected habitats at landscape scales.
Few studies have examined the impacts of increases in relative water depth on modern tropical marine ecosystems in situ. Impacts due to the increase in relative water depth have been assessed for coral reef flats using: (1) climatic variability in water level in the Andaman sea (Brown et al 2011) (2) engineering works on a reef flat at Heron Island on the Great Barrier Reef (Scopélitis et al 2011), and (3) subsidence caused by a subduction earthquake in Solomon Islands (Saunders et al 2016). There are no studies that have assessed modern in situ impacts of water level increase on seagrass, although a retraction of the shallow edge of seagrass meadows in Corsica was hypothesised to be the result of recent sea-level rise (Pergent et al 2015). Mangroves elicit complex responses to sea-level rise and shallow sub-surface processes, sediment accretion rates, coastal development and topography all play a critical role (Sasmito et al 2016, Krauss et al 2014, Lovelock et al 2011, Duke et al 2017). Many studies have used coastal ecosystems to infer water levels over geologic history (e.g. Grigg et al 2002, Rajendran et al 2007, Dura et al 2011, Blanchon and Shaw 1995, Woodroffe and Webster 2014, Harvey et al 1999). This present study demonstrates the diverse responses of adjacent seagrass, mangroves and coral ecosystems to an earthquake with associated sudden increase in relative sea level.
We tested the theoretical model of tropical coastal ecosystem response to sea-level rise using field data and remote sensing imagery from a unique in situ 'proxy' of sea-level rise caused by tectonic subsidence. The study was conducted in Roviana Lagoon (8.27 °S, E 157.5 °E), Western Province, Solomon Islands, where a magnitude 8.1 megathrust subduction earthquake caused 30–70 cm subsidence of the marine environment in April 2007 (Chen et al 2009, Taylor et al 2008, Saunders et al 2016). We quantified the response of coral reef, seagrass and mangrove ecosystems in a shallow lagoon to rapid relative sea-level rise. The area of each of the three ecosystems was mapped using remote sensing imagery in 2006, 2009, 2012, and 2014. Field measurements that were used to validate the satellite image based mapping were obtained in May 2013 (Saunders et al 2016).
Figure 1. Map of field sites in Roviana Lagoon, Solomon islands (a) with Mangrove, Seagrass and Coral study areas highlighted (b). Magntiude of earthquake induced subsidence measured by Taylor et al (2008) and Saunders et al (2016) indicated.
Download figure:
Standard image High-resolution imageMethods
Study site
The M8.1 megathrust earthquake on April 2 2007 (Taylor et al 2008) resulted in a significant rupturing that caused the islands of Ranongga in the west to be uplifted by up to 2 m, and the areas of Roviana and Vonavona lagoons in the east to subside. Our study assessed areas of mangrove, seagrass and coral in an area of Roviana Lagoon that subsided by 30–70 cm (Saunders et al 2016, Taylor et al 2008). We studied the largest expanses of mangrove (8 km2), seagrass (5 km2) and shallow coral (2 km2) within this region (figure 1). These coastal ecosystems provide critical ecosystem services for subsistence communities in Solomon Islands (Albert et al 2015, Warren-Rhodes et al 2011). Thus changes in these ecosystems under sea-level rise scenarios can have significant impacts on these rural communities that depend on them for survival.
A suitable 'control' site where subsidence did not occur was not available in the same geographic and ecological region due to the widespread and varied nature of the subsidence and uplift. Using a control site from a different region would introduce a range of confounding ecological, anthropogenic and oceanographic variables. However, time series satellite imagery from 2003–2006 of the coral reefs in Roviana Lagoon provided a 'temporal' control, and showed no changed in coral extent prior to subsidence, and a pronounced change in coral cover and canopy height after subsidence (Saunders et al 2016). Suitable satellite imagery from pre-2006 was not available for the mangrove and seagrass areas.
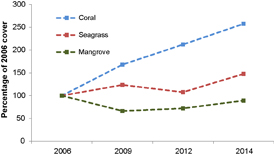
Figure 2. Area (relative to 2006) of live cover of mangrove, seagrass and coral between 2006 and 2014.
Download figure:
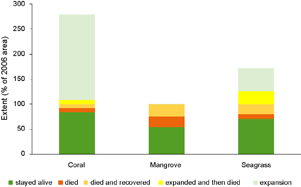
Standard image High-resolution imageFigure 3. Dynamics of coral, mangrove and seagrass between 2006 and 2014. Note total extent in 2006- Coral 5.8 ha, Mangrove 380.7 ha, Seagrass 174.3 ha.
Download figure:
Standard image High-resolution imageMapping
The extent and composition of mangrove, seagrass and shallow coral areas in Roviana Lagoon were mapped at four time periods (2006, 2009, 2012, 2014) through either manual digitization or object-based image analysis of high spatial resolution, pan-sharpened satellite imagery. Archived high spatial resolution multispectral satellite imagery (cloud free) from 2006 and 2009 (Quickbird-2), 2012 and 2014 (WorldView-2; table S1 available at stacks.iop.org/ERL/12/094009/mmedia) was acquired. All image data were atmospherically corrected to represent reflected sunlight from the water surface (ENVI 4.8 FLAASH ® module). The panchromatic band was used to pan-sharpen the blue, green and red bands (visible wavelengths), resulting in colour image data with a 0.64 m (Quickbird-2) or 0.5 m (WorldView-2) pixel resolution. The 2012 image was used as the base image, to which all other years were georeferenced.
Due to the specific characteristics of the individual habitat types, one of two mapping approaches was used. For the larger mangrove and seagrass areas, Object Based Image Analysis (OBIA) was applied, whereas manual delineation, which is more efficient for small areas, was used to map the extent of dense hard coral cover. OBIA is a multistep approach that first divides the image into objects of like pixels based upon their shape, colour and/or texture (Blaschke 2010). Subsequently classes are assigned to the objects using regional-specific rule sets that further differentiate objects from each other based on their neighbourhood relationship in addition to shape, colour, texture (Kamal et al 2015, Roelfsema et al 2013). Contextual editing was utilised in instances where automatic class assignment was deemed incorrect following visual interpretation of the OBIA classification. Field ground-truthing was conducted at the coral and seagrass sites in May 2013 and in October 2014 for the mangrove site, a detailed description of coral mapping methodology is provided in (Saunders et al 2016).
Qualitative validation was based on visual assessment by the authors of the final time series of maps for each habitat type. No quantitative accuracy assessment could be conducted as limited field validation data was available (only available for 2013 for coral and seagrass habitats, for 2014 for the mangrove area, with no validation data collected for any habitat, in any of the other years). From the time series (2006, 2009, 2012, 2014) of mangrove, seagrass and coral habitat maps, the expansion/retraction of each habitat was assessed and difference maps were created using ArcGIS software. The limited field data, only four different times and reliance on satellite based mapping, limits the interpretation of the results to landscape scale changes rather than fine scale processes.
Results
Overview
The response of mangroves, seagrass and corals differed between ecosystem type according to the conceptual model that we outlined in the introduction. However, the trajectories of change differed among ecosystems, highlighting key differences in the vulnerability and responsiveness of different ecosystems to environmental changes. There was an initial 30% decline in mangrove areal extent followed by a steady recovery to 90% of the pre-disturbance area (figure 2). Seagrass initially increased in area, followed by a decline to the pre-disturbance extent and, finally, another increase (figure 2). Corals showed a steady increase in area through all time points with a final 257% increase on pre-disturbance extent (figure 2).
By assessing the changes in each specific 1 m2 grid over the four satellite images (2006, 2009, 2012, 2014) we can gain an understanding of how stable or resilient each ecosystem is (figure 3). Over 80% of the coral present in 2006 remained alive in all years through to 2014. With only small areas of coral dying, dying and recovering or expanding and then dying (8%, 7.5% and 8% respectively). Only 54% of the mangrove that was present in 2006 persisted through all years to still be alive in 2014. With 21% of 2006 mangroves dying and remaining dead through all years and 24% dying after 2006 but recovering by 2014. The seagrass ecosystem proved to be the most dynamic with 70% staying alive through all years, 10% dying, 20% dying and then recovering, 26% expanding and then dying and 46% expanding and remaining alive in 2014 (figure 3).
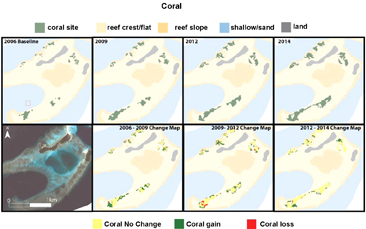
Figure 4. Extent (top) and change (below) of coral between 2006 and 2014.
Download figure:
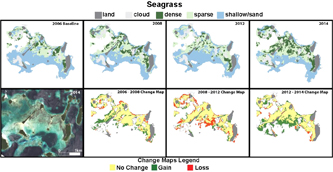
Standard image High-resolution imageFigure 5. Extent (top) and change (below) of seagrass between 2006 and 2014.
Download figure:
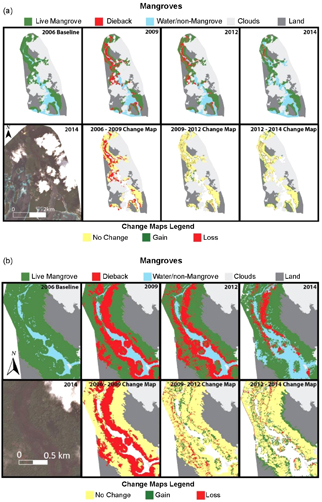
Standard image High-resolution imageFigure 6. (a) Extent (top) and change (below) of mangroves between 2006 and 2014 showing loss after 2007 subsidence and recovery between 2012 and 2014, (b) zoom of extent (top) and change (below) of mangroves between 2006 and 2014 showing fine scale patterns in dieback and recovery.
Download figure:
Standard image High-resolution imageFigure 7. (a) Dieback of mangroves In Lulu Channel, NW of Munda during April 2008 (Photo: Bruno Manele), (b) recovery of Rhizophora in November 2014 adjacent to dead mature Bruguiera of the mid-intertidal zone.
Download figure:
Standard image High-resolution imageCoral
In 2006, the live coral in the Kundukundu area covered an area of 5.8 ha. Between 2006 and 2009, 4.3 ha of live coral colonised in areas from which coral was previously absent, while 0.4 ha of coral present in 2006 died, resulting in a total area of live coral of 9.8 ha by 2009. This rate of coral expansion into new areas with minimal loss of coral continued between 2009–2012 and 2012–2014 to result in 15 ha of live coral occurring in 2014, representing a 157% increase in coral area from 2006 (figure 4). Further information on the response of the coral reefs to relative sea-level rise in the region is available in Saunders et al 2016.
Seagrass
In 2006 174.3 ha of seagrass meadow (Cymodocea sp., Syringodium isoetifolium, Halophila sp.) extended across the shallow sandy region studied in Roviana lagoon. By 2009, the total area of seagrass had increased to 215.4 ha, driven largely by colonisation of sparse seagrass onto areas previously defined as 'shallow sand' in 2006. Between 2009 and 2012 the total seagrass area declined to 187.5 ha driven largely by a transition of sparse seagrass to shallow sand on areas on the edge of the meadow. From 2012 to 2014 the seagrass meadow expanded from 187.5 ha to 257.5 ha, driven by 66.9 ha of previously shallow sand being colonised by sparse seagrass along the southern edge of the meadow (figure 5).
Mangroves
There was 381 ha of dense mangrove forests within the study area in 2006. This forest consisted of a diverse assemblage of species with Rhizophora stylosa dominating the seaward fringe, Ceriops tagal, Rhizophora apiculata and Bruguiera gynmorhiza in the mid forest and Xylocarpus granatum, Heritiera littoralis and Lumnitzera littorea in the landward forest. Within 3–6 months after the 2007 earthquake, widespread dieback of the mangroves began to occur and by early 2008 had become widespread (figure 6(a)). By 2009, as shown in the conceptual profile diagram (figure 8(b)), 35% (130 ha) of the mangrove forest had died with the majority of dieback occurring along the seaward fringe and mid-intertidal zones. The rate of mangrove forest loss over this period is orders of magnitude above the background levels of mangrove forest loss in Solomon Islands of 0.05% per annum (Hamilton and Casey 2016). By 2012, 38% (50 ha) of the dieback areas had recovered with new seedling growth (figure 6, figure 7(b) and figure 8), however a further 24 ha of mangrove forest had died. By 2014, the rate of new dieback was negligible with only 3.2 ha of mangroves lost between 2012–2014 and 51 ha of new regrowth over this period. By 2014, the live mangrove forest, including dense regrowth, occupied 339 ha or 89% of the original 2006 area. The mangroves along the seaward fringe suffered the most dieback with over 80% loss between 2006 and 2009 (figure 9). By 2014 these fringe forests experienced rapid recovery to 75% of the 2006 area. However, recovery was not spatially uniform. Figure 6 shows some large areas of mangrove, particularly in the upstream channel, which did not have observable recovery by 2014. Areas not mapped as recovered appear to be within the lower mid-intertidal zone as shown in figure 6(b).
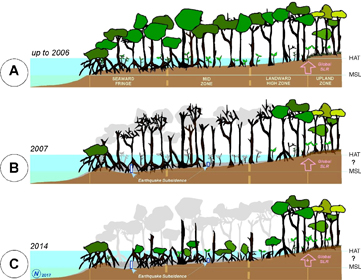
Figure 8. Mangrove zones across the intertidal profile before the Solomon Islands earthquake in 2007 (a), around 6 months afterwards (b), and 7 years afterwards (c). Note original presence of stilt-rooted Rhizophora species at the Seaward Fringe zone, buttressed Bruguiera species in the Mid Zone, and buttressed Heritiera and Xylocarpus at the High Zone. Mangrove dieback had occurred through the tidal zone from Seaward to Mid zones, leaving remnant Rhizophora trees at the seaward edge as well as along the high intertidal landward fringing zones.
Download figure:
Standard image High-resolution imageFigure 9. Area (relative to 2006) of live mangroves in seaward fringe, midzone and landward highzone between 2006 and 2014.
Download figure:
Standard image High-resolution imageDiscussion
The M8.1 earthquake and subsequent relative sea-level rise of 30–70 cm in Solomon Islands in 2007 induced substantially different responses from the three ecosystems assessed over local areas < 10 km2. Mangroves were negatively impacted initially followed by a recovery phase. Seagrass were relatively unaffected by the relative sea-level rise, and showed a small relative increase in area through time. Coral communities appeared to benefit through increased live cover extent. This is notable, given declines in coral coverage regionally (Selig and Bruno 2010) and elsewhere globally (Gardner et al 2003).
The study site offered a unique opportunity to study the impacts of rapid relative sea-level rise on three interconnected tropical marine ecosystems. Despite the lack of a suitable control site to compare these responses to, we offer several lines of evidence to suggest that responses are related to the change in relative sea level. Firstly, the magnitude of change in extent for both coral and mangrove ecosystems are orders of magnitude above background levels of change observed elsewhere in the region (Selig and Bruno 2010, Hamilton and Casey 2016). Secondly, the changes in both coral and mangrove ecosystems are in agreement with previous assessments and analysis of past, modern and future sea-level rise influences on these ecosystems (Saunders et al 2016, Lovelock et al 2015, Scopélitis et al 2011, Brown et al 2011). Thirdly, the timing and consistency in these responses in the three time points assessed after the subsidence indicate the results are not the outcome of natural variability or other variables unrelated to the subsidence event. Fourth, time series satellite imagery from 2003–2006 of the coral reefs in Roviana Lagoon showed no change in coral extent prior to subsidence, in contrast to the pronounced increase in coral cover and canopy height observed after subsidence (Saunders et al 2016).
Mangroves have been identified as a particularly vulnerable ecosystem to shifts in sea level due to their strong dependence along the tidal profile. Paleo-environmental evidence suggests mangroves may have been able to keep up with sea-level rise rates up to 8–10 mm yr−1 when sedimentation has been sufficient (Woodroffe 1990). Furthermore, accumulation of organic matter from biological production has also been demonstrated to enable mangroves to be stable in the face of relative sea-level rise (Morris et al 2002). However, depending on; sedimentation rates, tidal range, salinity and wave exposure, mangroves have exhibited a large diversity of responses to shifts in sea level over the past 10 000 years (Woodroffe 1999, Cohen et al 2016). In particular, biological accretion from root material has been identified as an important process for mangroves to maintain elevation in the face of sea-level rise (McKee et al 2007). The rapid rise in relative sea levels in Roviana resulted in a dramatic decline in mangroves followed by a rapid recovery phase with forest cover partially re-established by 2014. In this, it is useful to consider that mangrove trees lost were of mixed age classes up to around 200 years old. So, whilst tree maturity may be attained in a decade (Duke 2001), recovery here is measured in terms of seedling re-establishment observed after seven years.
The early recovery of mangrove forests following the earthquake is encouraging. Field observations indicate the mangrove re-establishment is primarily driven by colonisation of Rhizophora replacing Bruguiera within the mid intertidal range, which may be indicative of up-profile zonal shift. Rhizophora are a fast growing, well dispersed colonising species and can be expected to expand in area following disturbances. Landward migration of wetlands can also occur as a result of sea-level rise if rates and topography allow (Doyle et al 2010). In this instance minimal landward migration was observed. Coastal topography and limited impact to high-intertidal mangroves and adjacent terrestrial forest is likely to have restricted mangrove landward migration. The shift in species from Bruguiera to Rhizophora will result in reduced ecosystem services from this forest as Bruguiera are commonly used as food, firewood and building materials for subsistence communities in Solomon Islands (Warren-Rhodes et al 2011).
This short-term recovery of Rhizophora will have an important role in stabilisation of sediments (Lovelock et al 2015) and preventing sediment loss that can compound sea-level rise impacts (Hayden and Granek 2015). The rapid recovery can also maintain sediment levels through biological accretion, with rates of accretion in fringe mangroves exceeding that of landward mangroves thus increasing sea-level rise resilience (McKee et al 2007). In sum, the roles that functional mangrove ecosystems play can create a series of feedback mechanisms that can mitigate the influence of sea-level rise (Kirwan and Megonigal 2013). Thus, despite the widespread dieback that initially occurred in Roviana as a result of the earthquake and associated sudden change in sea level, the rapid recovery observed indicates this mangrove ecosystem will likely continue to function under future sea-level rise scenarios, albeit with a different distribution of species.
Mangroves are nonlinear, non-equilibrium, and highly dynamic systems (Schmitt and Duke 2015). The lack of observable recovery in some areas provides practical evidence that mangrove response to disturbance, in particular sea level rise, is likely to be non-linear and influenced by a range of physical and biological processes and complex ecological feedback mechanisms (Woodroffe et al 2016).
Although seagrass occupy a broader extent of the tidal profile than mangroves, being predominantly sub-tidal, their distribution is tightly coupled to light availability (a function of depth and water clarity) (Abal and Dennison 1996) and also regulated by substrate and wave exposure (Callaghan et al 2015). Given the sensitivity of seagrass to changes in water quality, they are often considered a biological sentinel or 'coastal canary' (Orth et al 2006). Several studies have indicated seagrass habitats would contract under sea-level rise scenarios due to reduced light penetration (Davis et al 2016) or coastal squeeze (Mills et al 2016) unless water quality is improved (Saunders et al 2013). However, water clarity in our shallow study area is relatively high and hence seagrass distribution is unlikely to be light limited. In terms of substrate, the shallow seagrass meadows studied occur in soft sediments which are dynamic in response to water level and hydrodynamic changes (Tecchiato et al 2015). Numerical models have shown that a 0.5–1 m rise in sea level can result in increased wave-induced stresses, including resuspension and erosion of sediments (Storlazzi et al 2011), capable of negatively impacting ecological processes (Storlazzi et al 2011) (Saunders et al 2014). The fast growing nature of tropical seagrass has likely contributed to the rapid adaptation and colonization of emergent suitable habitat. Thus, vegetated ecosystems such as mangrove and seagrass are more likely to rapidly adapt to changes in substrate, sea level and hydrodynamics than those that are founded on fixed, hard substrates such as corals.
The positive impact of local sea-level rise on coral ecosystems observed in this study was related to the presence of fast growing Acropora corals (Saunders et al 2016). In some areas where accommodation space is the limiting factor and healthy areas of fast-growing species exist, we can expect sea-level rise to provide some positive outcomes for shallow reefs. However, it is important to consider other factors when using these findings to understand future coral community responses to sea-level rise. First, increases in the availability of habitat in shallow water environments will necessarily be accompanied by a loss of habitat in the deeper limits to coral growth. As well, the expected warmer and less alkaline oceans will slow coral growth (Hoegh-Guldberg et al 2007, Albright et al 2016) and may lead to reefs that are no longer grow fast enough to keep up with sea-level rise (Dove et al 2013). Furthermore, direct anthropogenic impacts of overfishing, coastal development and degraded water quality will likely exceed positive effects from sea-level rise in many instances.
While assessments of how single species and ecosystem scale responses to sea-level rise are important, consideration is also needed of landscape scale responses. Landscape scale connectivity between seagrass, coral and mangroves is critical to support fisheries in Roviana (Olds et al 2013). As the extent and distribution of these ecosystems changes with sea-level rise and other climate change related pressures, we can expect species that rely on connectivity between the ecosystems to also be impacted (Leonard et al 2016, Iwamura et al 2013). The response of coral reef ecosystems to sea-level rise will affect the persistence of adjacent ecosystems in lagoonal environments due to changes in the hydrodynamic environment caused by deepening water (Saunders et al 2014). Improving our ability to predict these landscape scale changes as a result of sea-level rise can help guide pre-emptive adaptive conservation of these landscapes by including predicted future habitats in conservation prioritisation processes (Rogers et al 2015).
This present study has demonstrated that changes to ecosystems as a result of sea-level rise will be complex and varied. Whilst there are limitations due to this study assessing a rapid 30–70 cm rise in sea levels which does not represent the incremental eustatic sea-level rise expected in the order of 5–10 mm yr−1 over the coming century (Australian Bureau of Meteorology and CSIRO 2014, Jevrejeva et al 2012). Regardless of the rates of rise, this study illustrates the differential responses of ecosystems, with seagrass being dynamic in the face of change, corals winning out through lateral expansion and mangroves initially losing substantial area prior to recovery to an altered state. Through more detailed studies of the processes involved in these varied responses, we can begin to identify the features that provide ecosystem level resilience to sea-level rise.
Supplementary data. (250 KB, PDF)