Abstract
Acellular porcine small intestinal submucosa (SIS) has been successfully used for reconstructing esophagus with half circumferential defects. However, repairing full circumferential esophageal defects with SIS has been restricted due to the latter's poor mechanical properties. In the present study, synthetic polyesters biomaterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) and poly(lactide-co-glycolide) (PLGA) have been used to improve the mechanical properties of SIS. Feasibility of SIS/PHBHHx-PLGA composite material scaffold for esophageal tissue engineering has been assessed through a series of testing. The appropriate mixing ratio of PHBHHx and PLGA polymers has been determined as 5:5 by mechanical testing and in vitro degradation experiment. The morphology of constructed membranous and tubular scaffolds was also characterized. As confirmed by enzyme-linked immunosorbent assay, the contents of VEGF and TGF-β have respectively reached 657 ± 18 ng mL−1 and 130 ± 4 pg mL−1 within the SIS/PHBHHx-PLGA specimens. Biocompatibility of the SIS/PHBHHx-PLGA specimens with rat bone marrow mesenchymal stem cells (MSCs) was also evaluated by scanning electron microscopy and a live–dead cell viability assay. Actin filaments of MSCs on the composite materials were labeled. Biological safety of the extract from SIS/PHBHHx-PLGA specimens, measured as hemolysis rate, was all lower than 5%. Compared with SIS and SIS/PHBHHx-PLGA specimens, inflammatory reaction provoked by the PHBHHx-PLGA specimens in rats was however more severe. Our results have suggested that SIS/PHBHHx-PLGA composite material can offer a new approach for esophageal tissue engineering.
Export citation and abstract BibTeX RIS
1. Introduction
Esophageal cancer has been a difficult-to-treat malignancy due to the poor regenerative capacity of esophageal tissue [1]. Various tissues derived from stomach, jejunum and colon have been used as natural substitutes for repairing surgical defects of the esophagus [2–4]. Unfortunately, such approaches have often been followed by post-operative complications which can negatively impact patient's quality of life [5]. Tissue engineering, which holds great promise for the repair and regeneration of surgical defects or damaged tissues and organs, requires a suitable scaffold upon which regeneration can take place [6–8]. Recently, artificial esophagus scaffold constructed with naturally derived materials, e.g., collagen and alginate, acellular tissue matrices from bladder or small intestinal submucosa (SIS), and synthetic polymers, e.g., polyglycolic acid (PGA), poly(lactide-co-glycolide) (PLGA) and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) have been described [9–15].
As an extracellular matrix (ECM) material, SIS derived from swine has been approved by the US Food and Drug Administration (FDA) for various tissue engineering applications [16]. In common with other ECM materials such as decellularized urinary bladder submucosa, acellular dermal grafts and gastric acellular matrix, SIS itself contains collagen, proteoglycan glycosaminoglycan, glycoprotein and a variety of growth factors including vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) [17–19]. Natural collagen enriched SIS–ECM has been used as a scaffold in tissue engineering for repairing anterior urethral stricture [20], abdominal body wall [21], partial and full thickness skin defects [22] and lower urinary tract defects [23]. SIS is more advantageous than synthetic materials in promoting angiogenesis, cell growth and differentiation, and tissue regeneration without prior cell seeding during the remodeling process due to its innate growth factors that may be released during scaffold degradation. Acellular SIS has been successfully used for remodeling partial esophagus with appearance and histology resembling those of the natural organ [24, 25]. In our previous study, we have obtained promising results where SIS was used for replacement of half circumferentially resected esophagus in a dog model [26, 27]. However, to repair whole circumferential defects with SIS has been restricted because of its inconsistency in supporting tissue regeneration for larger areas [28].
As a synthetic polyester biomaterial, PLGA has been widely used as a scaffold to support tissue regeneration for its sound biocompatibility, biodegradability and approval by the FDA [29, 30]. Of note, this polymer can be degraded into lactic acid and glycolic acid by nonspecific hydrolysis, and easily metabolized by the body via the Krebs cycle and excreted through respiration and urination as carbon dioxide and water without residual metabolites [31]. PLGA is commercially available and its degradation time can vary from several months to several years depending on molecular weight and copolymer ratio [32]. For esophageal regeneration, we have selected PLGA with a 50:50 monomer ratio of lactide to glycolide, which requires about three months to degrade. However, the brittleness and poor mechanical properties of this polymer have hampered its suitability for the engineering of esophageal scaffold. Therefore, we have tried poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx), a member of polyhydroxyalkanoates (PHA) family, which may be produced by microorganisms under unbalanced growth conditions with desirable mechanical and elastic properties [33, 34]. In vitro testing has demonstrated that PHBHHx has sound biocompatibility for several types of cells [35–37] and tissues [38]. Despite the poor degradation rate noted for clinical applications [39], this polymer can also be degraded into small molecules which can be absorbed by the body without any toxic effect [40].
In this study, we have tried to improve the regenerative properties of SIS using PHBHHx and PLGA polymers with suitable mechanical properties and degradation time. Important properties of the SIS/PHBHHx-PLGA scaffold were investigated, which included mechanical strength, degradation in vitro, morphology, release of growth factors and hemolysis testing. Furthermore, the cytocompatibility and histocompatibility of SIS/PHBHHx-PLGA scaffolds were also evaluated.
2. Materials and methods
2.1. Materials
PLGA with an average molecular weight of 3000 g mole−1 was purchased from Polymer Ruitong Medical Instrument (Shandong, China) with a 5:5 monomer ratio of lactide to glycolide. PHBHHx containing 15 mol% HHx with a molecular weight of 500 000 g mole−1 was kindly given by Professor Qiong Wu from Tsinghua University (Beijing, China).
2.2. Preparation of SIS
The preparation of SIS followed a previously described procedure [41]. A segment of fresh porcine jejunum was harvested from market-sold pigs (weighing about 100 kg at six months) within 4 h of sacrifice. Following careful washing in water, porcine small intestine was split longitudinally and sectioned into approximately 10 cm lengths. Tunica mucosa, serosa and tunica muscularis were mechanically cleaned and washed in a saline solution, rinsed with a solution containing methanol and chloroform (1:1, V/V) in a fume cupboard for 12 h and then with deionized water. Subsequently, the membrane was incubated with 0.05% trypsin/0.05% ethylenediamine tetraacetic acid at 37 °C for 12 h to remove resident cells and washed with a saline solution. Then the membrane was rinsed with 0.5% sodium dodecylsulphate (SDS) in 0.9% sodium chloride for 4 h. Detergent was thoroughly removed with saline solution. Finally, the submucous membrane was soaked in 0.1% peroxyacetic acid and 20% ethanol for 30 min and rinsed with saline solution. The derived SIS was freeze-dried in a freeze dryer (Christ, Germany) under −70 °C for 48 h.
2.3. Fabrication of PHBHHx-PLGA membranes, SIS/PHBHHx-PLGA membranes and tubular scaffolds
PHBHHx and PLGA polymers (2 g each) were dissolved in 100 mL of methylene chloride. The mixture was placed on a magnetic stirrer overnight to completely dissolve the polymers. A 15 mL microcentrifuge tube was cut at the 5 mL mark. A piece of SIS was placed between the lid and the tube. The lid was lightly covered. Five milliliter of prepared PHBHHx-PLGA solution was added to the mucosal side of the SIS via the hole in the tube. The SIS/PHBHHx-PLGA membranes were then taken out and air-dried for 24 h, with irregular edges trimmed. The same volume of PHBHHx-PLGA solution was poured into dishes and maintained for 24 h. Vacuum drying was applied for another 24 h to remove the remaining solvent from PHBHHx-PLGA and SIS/PHBHHx-PLGA membranes. They were then sterilized with ethylene oxide and kept desiccated till further use. Procedures for the construction of SIS/PHBHHx-PLGA membranes are shown in supplementary figure 1, available from stacks.iop.org/BMM/9/015012/mmedia.
To fit with the anatomy of rat cervical esophagus, tubular scaffolds with an inner diameter of 2 mm were prepared with the SIS and PHBHHx-PLGA solution. Briefly, multilayer tubes were created by wrapping hydrated double sheets of SIS around a mandrel with a diameter of approximately 2 mm and a length of 10 cm. Five milliliter of PHBHHx-PLGA solution was added to the mucosal surface of SIS. After air-drying for 24 h and further drying in a vacuum for 24 h, the mandrel was withdrawn, and the tubular scaffolds were sterilized with ethylene oxide.
2.4. Determination of appropriate mixture ratio of PHBHHx and PLGA polymers
2.4.1. Mechanical testing
PHBHHx and PLGA polymers (4 g in total) with various ratios, e.g., 3:7, 5:5 and 7:3 (by weight), were dissolved in 100 mL of methylene chloride. SIS/PHBHHx-PLGA specimens were prepared with a method detailed in section 2.3. A segment of fresh cervical esophagus of rats (150–160 g in body weight) was harvested and split longitudinally. Tensile strain on split films of esophagus, SIS and SIS/PHBHHx-PLGA specimens, each spanning approximately 6 mm in width and 3 cm in length, was measured with a tensile tester (Shimazdu Corp., Japan) and a crosshead speed of 3 mm min−1, respectively. Mechanical tensile value was calculated based on the average of six samples.
2.4.2. In vitro degradation
To observe hydrolytic degradation, SIS/PHBHHx-PLGA (5:5) and SIS/PHBHHx-PLGA (7:3) specimens, weighing approximately 25 mg each and measuring 1 cm in diameter, were individually placed into test tubes containing 10 mL of simulated body fluid (SBF) at 37 °C (n = 3). The degradation process was evaluated by weight loss and shape change. Prior to analysis, samples were periodically removed at 1, 2, 4, 6, 8, 10 and 12 weeks, gently washed in deionized water and dried in a vacuum by lyophilization. Weight loss after incubation was calculated based on the average of three samples.
2.5. Morphological examination of SIS/PHBHHx-PLGA membranes and tubular scaffolds
To analyze the structure and pore size of SIS, the surface and cross-section morphology of the SIS/PHBHHx-PLGA specimens were observed under a scanning electron microscope (SEM) (Hitachi, Japan). Prior to the microscopy, the specimens were mounted on aluminum stumps, followed by coating with gold in a sputtering device.
Acridine orange (AO) (Sigma, USA) fluorescence staining was used to assess the bilayer structure of SIS/PHBHHx-PLGA membranes and tubular scaffolds. Thin cross-sectional slices were incubated in PBS containing 1 µM of AO for 2 h and thoroughly washed with deionized water. Fluorescence images were taken under a confocal laser scanning microscope (CLSM) (Nikon, Japan) under wavelengths of 488 nm (green) and 647 nm (blue).
2.6. Measurement of released growth factors in SIS and SIS/PHBHHx-PLGA specimens
Growth factors including VEGF and TGF-β released from SIS and SIS/PHBHHx-PLGA specimens were quantified with an enzyme-linked immunosorbent assay (ELISA). Briefly, 0.1 g SIS and SIS/PHBHHx-PLGA (containing 0.1 g SIS) were respectively placed in a centrifuge tube containing 3 mL of PBS. Each sample was triplicated. The samples were incubated at 37 °C up to 30 d in a constant-temperature shaking incubator (Hasuc, China) with a speed of 50 rev/min. For ELISA analysis, 1 mL of PBS solution was collected every two days and stored at −20 °C. The incubation was replenished with an equal volume of fresh PBS. By following the instructions of the manufacturer (Cusabio Biotech, USA), each sample was run twice, and the signal was measured with an automated spectrophotometric plate reader (Hach, USA) at a wavelength of 450 nm. The data were plotted as background-corrected absorbance versus concentration.
2.7. Isolation and culture of rat bone marrow mesenchymal stem cells and co-culture with SIS/PHBHHx-PLGA specimens
Bone marrow mesenchymal stem cells (MSCs) were flushed by L-DMEM (Gibco, USA) from femora and tibia harvested from 1 to 3 day old Sprague Dawley rats. The cells were cultured in L-DMEM supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) in a humidified atmosphere containing 5% CO2 at 37 °C. Culture medium was replaced after 24 h to remove non-adherent cells and then every two days. Adherent spindle-shaped MSC population was further expanded. When they had grown to 90% confluence, the MSCs were digested with 0.25% trypsin-EDTA (Gibco, USA) and resuspended in complete medium. Cells from the third passage were harvested for subsequent experiments.
Sterilized SIS/PHBHHx-PLGA membranes of suitable sizes were spread on 12-well plates and individually hydrated in culture medium for 24 h. After removing the medium, MSCs from the third passage were seeded with a concentration of 5 × 104 cells/well on the SIS-surface of membranes. Culture medium was replaced every two days. L-DMEM containing 10% FBS was added to completely immerse the specimens, which were then cultured for two and four days in a humidified atmosphere at 37 °C containing 5% CO2.
2.8. Examination of co-culture specimens
2.8.1. Scanning electron microscopy
To study their general morphology, MSCs combined with SIS/PHBHHx-PLGA membranes in 12-well plates were washed twice with PBS and fixed with 2.5% glutaraldehyde for 4 h at room temperature. Thereafter, the co-culture was dehydrated through an ethanol gradient with increasing concentrations from 30% to 100% for 15 min each. After critical point drying and gold-coating, the specimens were examined under a SEM.
2.8.2. Live–dead cell viability assay
Third passage MSCs and SIS/PHBHHx-PLGA membranes were co-cultured for two and four days. Viability of the cells was determined by live–dead cell staining using Calcein-AM and propidium iodide (PI). Briefly, co-cultured specimens were washed with PBS several times and incubated in PBS containing 2 µM of Calcein-AM (Sigma, USA) and 4 µM of PI (Sigma, USA) for 30 min at room temperature in darkness. Viable and dead cells were then detected under a fluorescence microscope (Olympus, Japan).
2.8.3. Confocal laser scanning microscopy of co-cultured specimens
Pieces of SIS/PHBHHx-PLGA membrane were spread on 12-well plates incubated 4 h in PBS containing 1 µM AO under sterile conditions. After washing with sterilized PBS, MSCs from the third passage were seeded with a concentration of 5 × 104 cells/well and cultured as described before. On the second and fourth days, the cells were washed with PBS to remove the medium, fixed with 3.7% formaldehyde solution for 5 min and extensively washed in PBS, permeabilized with 0.1% Triton X 100 (Sigma, USA), washed again with PBS, and incubated for 40 min at room temperature with a 50 mg mL−1 fluorescent phalloidin conjugate solution tetramethyl-rhodamine B isothiocyanate (TRITC) (Sigma, USA) to label the actin filaments. Finally, the cells were washed several times with PBS to remove unbound phalloidin conjugate and immediately observed under a CLSM with wavelengths of 488 nm (green), 568 nm (red) and 647 nm (blue).
2.9. Hemolysis test
Hemolysis testing was carried out as a standard biological safety test of the materials. According to the ISO standard [42], 1 g of SIS/PHBHHx-PLGA samples were soaked in 10 mL normal saline at 37 °C for 24 h to obtain a material extract. Fresh arterial blood of New Zealand white rabbit was collected in a vacuum tube containing EDTA and diluted with sodium chloride repeatedly to a density of 2%. A set of assay tubes were prepared in triplicate for each group. The volume of diluted blood, physiological saline and material extract were as listed table 1, with tubes 1–5 as the test groups and 6–7 as the negative and positive control groups, respectively. To each tube was added 2.5 mL normal saline and 2.5 mL deionized water. All assay tubes were gently mixed, equilibrated at 37 °C for 4 h, and centrifuged for 10 min at 1500 rev/min. Supernatant was removed and average optical density (OD) for each tube were measured based on the average of five samples at 545 nm. Hemolysis rate was estimated using the average OD values with the following formula. The test material was considered hemolytic should the hemolysis rate be greater than 5%:
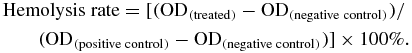
Table 1. Results of hemolysis test.
| Tubes | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Diluted blood (2%) (mL) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Physiological saline (mL) | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | 0 |
| Deionized water (mL) | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 |
| Material extract (mL) | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | 0 | 0 |
| Hemolysis rate (%) | 1.45± | 1.16± | 0.70± | 0.51± | 0.29± | 0 | 100 |
| 0.13 | 0.15 | 0.08 | 0.07 | 0.01 |
2.10. In vivo implantation
Five-week-old adult Sprague Dawley male rats weighing 150–160 g each were purchased from the Laboratory Animal Academy of Sichuan Medical Sciences Institute (License Number SCXK2004-15). The rats were housed in cages under standard laboratory conditions and given rat chow and free access to water. All animal experimental procedures have been approved by Sichuan University Animal Care and Use Committee in concordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research. To evaluate the inflammatory reaction, the rats were divided into three groups (n = 6 each) and anaesthetized with a muscular injection of sumianxin (0.3–0.4 mL/100 g). Thereafter, SIS, PHBHHx-PLGA and SIS/PHBHHx-PLGA specimens, of size approximately 1 cm2 each and sterilized using ethylene oxide, were implanted subcutaneously. After 14 and 28 days, three rats from each group were randomly selected and sacrificed. Implant areas containing tissues from the subcutaneous dorsum were dissected. The latter was immediately fixed with 10% buffered formalin, embedded in paraffin and sectioned along the longitudinal axis. The sections were stained with hematoxylin and eosin (H&E) for histological examination. In addition, inflammatory cells surrounding each sample were quantified with a computer-based image analysis system (Image-Pro Plus, USA) under a 400 × microscopic field.
2.11. Statistical analysis
All data were presented as mean ± standard deviation (SD). Differences between continuous variables were analyzed with unpaired Student's t-test. The data of multiple comparisons were first analyzed with one-way ANOVA. The Student–Newman–Keuls (S–N–K) test was chosen as a post-hoc test to show individual differences. All statistical analysis was performed with an SPSS 13.0 statistical package (SPSS Inc., USA). P < 0.05 was indicative of a statistically significant difference.
3. Results
3.1. Mechanical testing and in vitro degradation
Mechanical properties of the SIS/PHBHHx-PLGA specimens with various ratios of PHBHHx and PLGA polymers (3:7, 5:5 and 7:3) were compared with those of SIS and normal cervical esophageal tissue of rats. Peak load (N) (figure 1(a)) and maximum strain (figure 1(b)) of all specimens were determined. As shown, the peak load of SIS specimens can be significantly enhanced by simple blending of PHBHHx with PLGA polymers (5:5 and 7:3). In particular, SIS/PHBHHx-PLGA (5:5) specimens have shown a peak load of 11.29 ± 1.04 N, which was much higher than those of 3:7 or 7:3 in ratio, i.e. 6.91 ± 1.14 N and 8.63 ± 1.21 N, respectively, but was close to that of esophageal tissue (9.61 ± 0.40 N). The maximum strain testing, however, suggested superiority of SIS over SIS/PHBHHx-PLGA specimen. This is because both SIS and normal cervical esophagus are derived from natural tissue. The stretching process may therefore induce similar deformation behavior in both tissues. Furthermore, SIS/PHBHHx-PLGA (5:5) and SIS/PHBHHx-PLGA (7:3) specimens, both with mechanical properties close to esophageal tissue, showed a weight loss in SBF at 37 °C after 12 weeks of hydrolytic biodegradation. Results were presented by a percentage ratio of residual and original weights. As shown in figure 2(c), whilst the residual weight of SIS/PHBHHx-PLGA (7:3) specimens measured 49.6 ± 5.9%, SIS/PHBHHx-PLGA (5:5) specimens had only 34.8 ± 2.8% of residual weight by week 12. Likewise, morphological changes of the specimens were observed during the degradation. The diameter of SIS/PHBHHx-PLGA (5:5) specimens (figure 2(a)) appeared to have diminished more than SIS/PHBHHx-PLGA (7:3) specimens (figure 2(b)) by week 12. Therefore, we have selected SIS/PHBHHx-PLGA (5:5) specimens for subsequent study considering it has optimum mechanical properties, especially peak load and proper biodegradation time, compared with the others.
Figure 1. Mechanical properties of (a) peak load (N) and (b) maximum strain of the SIS/PHBHHx-PLGA specimens (with various ratios of PHBHHx and PLGA polymers, e.g., 3:7, 5:5, 7:3), SIS and normal rat cervical esophageal tissue. #: P < 0.01 indicates a significant difference compared with the esophagus group. *: P < 0.01 indicates a significant difference compared with the SIS group.
Download figure:
Standard image High-resolution imageFigure 2. Degradation study of SIS/PHBHHx-PLGA (5:5) and SIS/PHBHHx-PLGA (7:3) specimens submerged in SBF for 12 weeks at 37 °C.The specimens (n = 3) were periodically removed and dried under lyophilization prior to analysis. Photographs show changes in the shape of (a) SIS/PHBHHx-PLGA (5:5) and (b) SIS/PHBHHx-PLGA (7:3) specimens. (c) Plot of percent residual weight of specimens versus degradation time. Numbers 1–8 indicate the changes of specimens in weeks 0, 1, 2, 4, 6, 8, 10 and 12, respectively. *: a significant difference (P < 0.05) has been found between the two groups in weeks 8, 10 and 12.
Download figure:
Standard image High-resolution image3.2. Morphology of the SIS/PHBHHx-PLGA membranes and tubular scaffolds
As revealed by SEM, the surface of SIS was rough and porous with pore sizes ranging from 30 to 50 µm (figure 3(a)). By contrast, the surface of PHBHHx-PLGA polymer was smooth and less porous (figure 3(b)). SIS in the upper layer has become tightly adhered to PHBHHx-PLGA polymer in the lower layer (figure 3(c)). The variable ranges of thicknesses of SIS layer and PHBHHx-PLGA polymer layer were controlled at around 100 ± 20 µm due to operational differences (figures 3(c) and (d)). SIS/PHBHHx-PLGA tubular scaffolds (figure 3(e)) with an inner diameter of 2 mm were produced with double sheets of SIS and PHBHHx-PLGA polymer. By CLSM, AO staining has shown green and blue fluorescence respectively for the SIS and PHBHHx-PLGA polymer within the membranes (figure 3(d)) and tubular scaffolds (figure 3(f)). Figure 3(f) also revealed a small gap between the sheets of SIS, which may cause a problem for cell migration, but on the other hand may be in favor of release of growth factors as well as provide space for epithelial cells coverage.
Figure 3. Morphology of SIS/PHBHHx-PLGA membrane and tubular scaffold. (a) SIS-surface of membrane. Bar = 100 µm; (b) PHBHHx-PLGA polymer-surface of membrane. Bar = 100 µm; (c) SEM images of cross-section of the membrane. Bar = 200 µm; (d) CLSM images of cross-section of membrane stained with AO. Green and blue fluorescence represents SIS and PHBHHx-PLGA polymer, respectively. Bar = 100 µm; (e) Fabricated SIS/PHBHHx-PLGA tubular scaffold with an inner diameter of 2 mm; (f) CLSM images of cross-section of tubular scaffold stained with AO. Green and blue fluorescence represents SIS and PHBHHx-PLGA polymer, respectively. Bar = 600 µm.
Download figure:
Standard image High-resolution image3.3. Released growth factors within SIS and SIS/PHBHHx-PLGA specimens
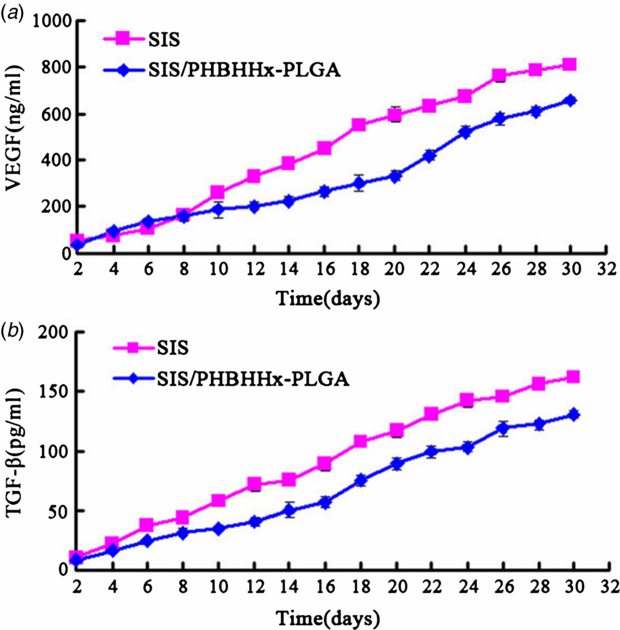
An ELISA method was used for assaying the release of growth factors by SIS and SIS/PHBHHx-PLGA specimens over 30 days. As shown in figures 4(a) and (b), the release of VEGF and TGF-β gradually increased with time. The speeds of release were similar. The content for VEGF and TGF-β in SIS/PHBHHx-PLGA specimens were measured as 657 ± 18 ng mL−1 and 130 ± 4 pg mL−1, respectively, and those in SIS specimens measured 811 ± 12 ng mL−1 and 161 ± 3 pg mL−1, respectively.
Figure 4. Growth factors released by SIS and SIS/PHBHHx-PLGA specimens quantified by ELISA. (a) For VEGF, P < 0.05 for day 10–30 indicates a significant difference between the two groups. (b) For TGF-β, P < 0.05 for day 4–30 indicates a significant difference between the two groups.
Download figure:
Standard image High-resolution image3.4. Examination of cytocompatibility with MSCs
3.4.1. Culture of MSCs
After 24 h of culture, primary MSCs from rat bone marrow adhered to the bottom of culture plates and formed visible colonies 2–3 days later. After five days of culture, primary cells developed a satellite or spindle shape and became closely connected. Cells were passaged when they reached 80–90% confluence. Non-adherent cells were periodically removed by replacing the culture media and passaging. MSCs from the third passage were cultured for five days and used for subsequent experiments when they displayed a uniform fibroblast-like shape and formed clusters.
3.4.2. Examination of scanning electron microscopy
MSCs from the third passage were seeded on SIS/PHBHHx-PLGA specimens and cultured in vitro. After two days, SEM examination showed that a small number of MSCs had attached and spread on the SIS-surface of specimens (figure 5(a)). By day 4, the cells were in contact with each other and reached confluence (figure 5(b)).
Figure 5. SEM images of MSCs cultured on SIS/PHBHHx-PLGA specimens on day 2 (a) and day 4 (b). Fluorescence microscopy images for the live–dead cell viability assay of MSCs cultured on SIS/PHBHHx-PLGA specimens on day 2 (c) and day 4 (d). CLSM images of the MSCs cultured on SIS/PHBHHx-PLGA specimens on day 2 (e) and day 4 (f). Green fluorescence represents the SIS-surface of SIS/PHBHHx-PLGA specimens stained with AO. Red fluorescence represents actin filaments of MSCs stained with phalloidin. Bar = 100 µm.
Download figure:
Standard image High-resolution image3.4.3. Live–dead cell viability assay
After two and four days of proliferation on SIS/PHBHHx-PLGA specimens, viability of MSCs was assessed by live–dead cell staining. Respectively, calcein-AM and PI solutions stained viable and dead cells with green and red fluorescence. As shown in figures 5(c) and 6(d), nearly all MSCs on the surface and inside the specimens were stained green, whilst a very few disrupted cells had a red nucleus. Compared with day 2 (figure 5(c)), MSCs cultured on specimens after four days (figure 5(d)) of culture had grown more densely and had more extensive connections in between.
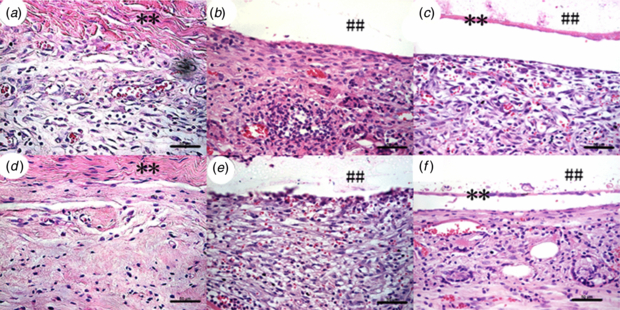
Figure 6. H&E staining of sections (400 × magnification) from retrieved SIS (a), PHBHHx-PLGA (b) and SIS/PHBHHx-PLGA (c) specimens implanted subcutaneously in rats at week 2, and those of SIS (d), PHBHHx-PLGA (e) and SIS/PHBHHx-PLGA (f) specimens retrieved at week 4. Bar = 50 µm. ** and ## signs represents SIS and PHBHHx-PLGA, respectively.
Download figure:
Standard image High-resolution image3.4.4. Confocal laser scanning microscopy for co-cultured specimens
Co-cultured MSCs and specimens were stained with AO and phalloidin. The SIS surface of the SIS/PHBHHx-PLGA specimens and actin filaments of MSCs were respectively stained with red and green fluorescence. Under a CLSM, extensive formation of microfilament bundles spanning the length of widely spread cells on the specimen was noticed. By day 4 (figure 5(f)), the lengths of cytoskeletons (actin) became even longer compared with day 2 (figure 5(e)).
3.5. Hemolysis test
The hemolysis rates of material extracts with volumes ranging from 0.5 to 0.1 mL (tubes 1–5), negative control (tube 6) and positive control (tube 7) were measured as 1.45 ± 0.13%, 1.16 ± 0.15%, 0.70 ± 0.08%, 0.51 ± 0.07%, 0.29 ± 0.01%, 0% and 100%, respectively, which were all below 5% (table 1). This suggested that the SIS/PHBHHx-PLGA materials have excellent in vitro hemocompatibility and will not lead to severe hemolysis according to the ISO standard [42]. Supplementary figure 2 (available from stacks.iop.org/BMM/9/015012/mmedia) shows the general appearance of hemoglobin. Similar to that of the negative control, supernatants from the test groups were all clear and transparent with erythrocytes sedimented to the bottom of tubes, indicating that erythrocytes have not ruptured. By contrast, hemoglobin release of the positive control yields a muddy supernatant, suggesting full-scale hemolysis.
3.6. In vivo implantation and inflammatory response
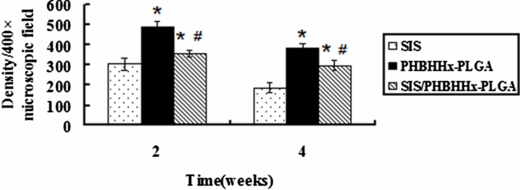
To assess the inflammatory reaction, we have respectively implanted SIS, PHBHHx-PLGA and SIS/PHBHHx-PLGA specimens subcutaneously into experimental rats. As shown by H&E staining of histological sections of specimens harvested two and four weeks after the implantation, the PHBHHx-PLGA specimens showed a dense accumulation of inflammatory cells including monocytes, macrophages, eosinophils and others around the implants (figures 6(b) and (e)). By contrast, the SIS specimens had significantly milder accumulation of inflammatory cells (figures 6(a) and (d)). Probably due to the soft nature of SIS, histological sections of SIS have shown a pellet instead of linear form. The SIS/PHBHHx-PLGA specimens have shown intermediate accumulation of inflammatory cells in the surrounding tissues (figures 6(c) and (f)). Moreover, inflammatory reaction of various specimens at week 4 (figures 6(d)–(f)) was milder than at week 2 (figures 6(a)–(c)). Quantification of inflammatory cells with an image analysis system under a 400 × microscopic field (figure 7) has indicated the mean density of 304 ± 29, 488 ± 26 and 354 ± 19 at week 2 for SIS, PHBHHx-PLGA and SIS/PHBHHx-PLGA specimens, respectively. However, by week 4, the presence of macrophages and other inflammatory cells was significantly reduced and the mean density of inflammatory cells for the three specimens became 184 ± 25, 380 ± 25 and 294 ± 24, respectively.
Figure 7. Density of inflammatory cells (under 400 × microscopic field) in the reaction zone of SIS, PHBHHx-PLGA and SIS/PHBHHx-PLGA specimens implanted subcutaneously into rats. *: a significant difference (P < 0.05) was detected when compared with the SIS group. #: a significant difference (P < 0.05) was detected when compared with the PHBHHx-PLGA group.
Download figure:
Standard image High-resolution image4. Discussion
An excellent scaffold should offer not only a three-dimensional configuration but also controllable biodegradability, biocompatibility and desirable mechanical properties. For esophageal tissue engineering, various materials ranging from resorbable substances, acellular matrices to cellularized patches have been used as scaffolds. Miki et al [9] have constructed a tube using PGA as the frame, upon which a collagen layer with esophageal fibroblasts and an inner layer of esophageal epithelial cells were combined. Histology of the rat epithelium revealed 20 layers of stratification at 14 days. No stenosis was observed. Bhrany et al [10] have developed an acellular matrix tissue scaffold using preserved ECM proteins to serve as the foundation for a tissue-engineered esophagus. Beckstead et al [11] have studied the interaction between esophageal epithelial cells and natural and synthetic scaffolds, and concluded that both decellularized human skin and degradable polyesters can support esophageal epithelial cell adhesion and proliferation.
Researchers have also tried to use porcine-derived SIS to create a tissue-engineered esophagus scaffold. Badylak et al [24] have used acellular SIS for remodeling of half circumferential resected esophagus in a dog model. Thirty-five days after the surgery, this has achieved esophagoplasty, along with tissue-specific construction and remodeling with resemblance to the natural organ in both appearance and histology. However, only partial epithelialization was achieved. Similar results were obtained with a rat model by Lopes et al [25], who also discovered the presence of nerve growth suggestive of neo-innervation. SIS was used as a scaffold by Wei et al [26], where autologous oral mucosal epithelial cells (OMECs) were pre-seeded before transplantation into a canine model. Tan et al [27] have seeded MSCs onto an SIS scaffold for half circumferential esophageal reconstruction, which obtained promising results in a canine model by the 12th week after the surgery. In addition to its feasibility and effectiveness for esophageal repair, complete re-epithelialization and long bundles of skeletal muscles were also observed. To repair whole circumferential defects, Doede et al [28] have stitched tubular SIS prosthesis with cervical esophagus of 14 piglets. Only 1 of the 14 animals survived the 4th week after the surgery. Because of severe esophageal stenosis, the prosthesis could not be found either macroscopically or histologically in other piglets.
In the present study, we have tried to modify SIS by combination with PHBHHx and PLGA polymers in order to construct a tissue-engineered scaffold suitable for repairing whole circumferential esophageal defects. The three-dimensional microarchitecture of SIS surface seems to be in favor of cell adherence, growth and migration. In addition, the mechanical support offered by the PHBHHx-PLGA polymer and excellent adhesiveness of the bilayer structure have made the SIS/PHBHHx-PLGA composite materials more suitable for in vivo applications.
4.1. Mechanical testing and in vitro degradation
Mechanical properties and degradation time of scaffolds for replacing or supporting native tissues are crucial for their in vivo functions. To test such properties is important for choosing an appropriate mixture ratio. For mechanical properties study, we have tested the peak load and maximum strain. As shown by the results, SIS specimens can be remarkably improved with supplemental synthetic polymers and the mechanical properties of SIS/PHBHHx-PLGA (5:5) specimens can probably meet the requirement for engineering an esophageal scaffold. The progress of reconstructing esophagus with half defects by SIS takes about 2–3 months [27]. However, regeneration of full circumferential defects will take longer, due to missing native tissue's mechanical support and natural integration with surrounding tissue. Therefore, SIS/PHBHHx-PLGA (5:5) specimens, which had 34.8 ± 2.8% of residual weight by 12th week, showed a suitable degradation rate in vitro for regeneration of full circumferential defects. Notably, the weight of both types of specimens has decreased slightly during the last week of incubation. This may be attributed to the residual unbiodegradation of PHBHHx polymer which has a long degradation time [39]. Following the degradation of SIS and PLGA, many large pores can be seen on the residual specimens, into which regenerated cells and tissue may grow. A major limitation of the present study has been that the viscoelastic properties were not measured, and this should be taken as an immediate next step.
4.2. Release contents of growth factors within SIS and SIS/PHBHHx-PLGA specimens
Bioinductive properties of SIS play an important role in tissue regeneration. In addition to other components, growth factors can promote angiogenesis and host cell infiltration and mitogenesis during scaffold degradation of the remodeling process [18, 19]. Among these, VEGF is well known for its capacity to promote angiogenesis, vascular permeability for endothelial cells and stimulate mitogenesis and differentiation of endothelial cells [17]. Transforming growth factor-beta (TGF-β), which may be retained through sterilization and lyophilization [43], also plays an important role in tissue regeneration. In the present study, the composition of PHBHHx-PLGA polymer with SIS only slightly reduced the release of VEGF and TGF-β in 30 days compared with SIS (P < 0.05). We expect that, with time, adequate amount of growth factors can be released by the SIS/PHBHHx-PLGA specimens.
4.3. Evaluation of cytocompatibility with MSCs
Isolated MSCs have started to adhere 24 h after being seeded in primary cultures, and begun to expand, aggregate and grow in colonies. MSCs from the third passage were seeded onto SIS/PHBHHx-PLGA specimens. After 24 h of culture, the cells have adhered and begun to spread. The rough topography of SIS/PHBHHx-PLGA specimens, consisting of pores on the surface, seemed to have provided an anchor for cell attachment, proliferation, migration and growth into the scaffold. We have further evaluated the biocompatibility of co-cultivation construction of SIS/PHBHHx-PLGA specimens with the MSCs. SEM examination and live–dead cell assay both found that MSCs have stretched out and grown a spindle shape on the scaffolds. On day 4, the MSCs have aggregated and formed clusters on the scaffolds. Their spreading areas have become greater compared with day 2. Also shown by a live–dead cell assay, MSCs grown on the SIS/PHBHHx-PLGA specimens have remarkable viability. Little cytotoxicity was observed, which indicates excellent compatibility for SIS/PHBHHx-PLGA specimens and MSCs. F-actin fiber bundles in MSCs labeled with fluorescent conjugates of phalloidin were observed on the scaffolds, which could be stained with AO. Several cytoskeleton proteins connected with F-actin fiber bundles could induce cell shape change and promote cell spreading on the scaffolds. These have indicated that, in addition to sound cytocompatibility, SIS/PHBHHx-PLGA specimens can provide ideal sites for cell attachment and proliferation.
4.4. Evaluation of hemocompatibility and histocompatibility
In the present study, SIS/PHBHHx-PLGA has proven to be a degradable biomaterial. In vitro toxicity of this material was assessed with a hemolysis test, which measures the degree of hemoglobin dissociation and erythrolysis when extract of the material comes into contact with erythrocytes [42]. During the experiment, fresh anticoagulant-treated rabbit blood was diluted and added to the test samples as well as the negative and positive controls. The results showed the hemolysis rate of different volume extracts of materials to be lower than 5%. A photograph of the general appearance was consistent with the above findings. This suggested that SIS/PHBHHx-PLGA specimens have no significant destructive effect on erythrocytes but sound in vitro hemocompatibility.
For in vivo experiment, SIS, PHBHHx-PLGA and SIS/PHBHHx-PLGA specimens were transplanted subcutaneously into SD rats. The surrounding tissues were examined at two and four weeks. As revealed by H&E staining, implantation of the PHBHHx-PLGA specimens provoked more severe inflammation compared with SIS. On the other hand, SIS/PHBHHx-PLGA specimens only induced moderate immune response, suggesting that the addition of SIS to PHBHHx-PLGA may decrease inflammatory properties of PHBHHx-PLGA specimens. Furthermore, a gap between the PHBHHx-PLGA and SIS/PHBHHx-PLGA scaffold and host tissue has been observed, which may only be an artifact of sectioning rather than insufficient infiltration of host cells. In subsequent animal experiments, we shall continue to assess the infiltration of host cells into the SIS/PHBHHx-PLGA scaffolds.
5. Conclusion
As an initial step towards esophageal regeneration, a porous 3D scaffold has been constructed with SIS and PHBHHx-PLGA polymer. The scaffold has shown desirable mechanical properties and capability for in vitro degradation, which may meet the requirement for esophageal regeneration. The scaffold has also exhibited a porous nature and three-dimensional microarchitecture on its surface, which may facilitate cell adherence and growth. Furthermore, it also possesses the crucial bioinductive property owing to release of growth factors such as VEGF and TGF-β. The strength of this scaffold also lies in its sound in vitro cytocompatibility, hemocompatibility and histocompatibility. For its advantages over current approaches, this SIS/PHBHHx-PLGA scaffold may provide an option for clinical treatment of full circumferential esophageal defects. For the next step, we shall use an animal model to demonstrate its potential value for clinical application.
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China (31271058, 81100327) and the National High Technology Research and Development Program of China (2012AA020503).