Abstract
The structural transformation of multi-walled carbon nanotubes (MWCNT) under electron beam (e-beam) irradiation at room temperature is studied, with respect to a novel passivation effect due to gold nanoparticles (Au NPs). MWCNT structural evolution induced by energetic e-beam irradiation leads to faster shrinkage, as revealed via in situ transmission electron microscopy, while MWCNT surface modification with Au NPs (Au-MWCNT) slows down the shrinkage by impeding the structural evolution process for a prolonged time under the same irradiation conditions. The new relationship between MWCNT and Au-MWCNT shrinking radii and irradiation time illustrates that the MWCNT shrinkage rate is faster than either theoretical predictions or the same process in Au-MWCNTs. As compared with the outer surface energy (positive curvature), the inner surface energy (negative curvature) of the MWCNT contributes more to the athermal evaporation of tube wall atoms, leading to structural instability and shrinkage under e-beam irradiation. Conversely, Au NPs possess only outer surface energy (positive curvature) compared with the MWCNT. Their presence on MWCNT surfaces retards the dynamics of MWCNT structural evolution by slowing down the evaporation process of carbon atoms, thus restricting Au-MWCNT shrinkage. Au NP interaction and growth evolves athermally on MWCNT surfaces, exhibits increase in their size, and indicates the association of this mechanism with the coalescence induced by e-beam activated electronic excitations. Despite their growth, Au NPs show extreme structural stability, and remain crystalline under prolonged irradiation. It is proposed that the surface energy of MWCNTs and Au NPs, together with e-beam activated soft modes or lattice instability effects, predominantly govern all the above varieties of structural evolution.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
MWCNT structural stability plays a crucial role in nanodevices and nanotechnology. The hollow, cylindrical, and distinctive flawless curved graphitic carbon nanotube (CNT) structure [1, 2] with sp2 hybridized atomic bonding makes them exceptional in mechanical strength [3–5], electronic properties [6], and a potential material candidate [7] for nanodevices and other areas of nanotechnology. Multiple attempts have been put forward for processing of single-walled carbon nanotube (SWCNT) [8–12], MWCNT [10, 13–15], and metal filled or modified MWCNT [13, 16–18] by in situ energetic electron beam (e-beam) irradiation in transmission electron microscopes (TEMs). The focus of previous studies [8–18] has been on classical knock-on mechanisms, natural surface healing processes and molecular dynamics simulations. Nevertheless, CNT processing and their associated structural evolutions are sensitive to the surface energy and e-beam activation at the nanoscale. It has been reported that SWCNT surface energy [19] accelerates its shrinkage faster, compared to theoretical predictions or simulations. In particular, nanotube inner surface energy and metal nanoparticle outer surface energy are size-dependent—becoming noticeable at the nanoscale, and accelerating their structural evolution [20]. Besides this, the ultrafast energy exchange during ultrafast irradiation [19–22] induces soft modes in solid matter at room temperature, and leads the solid structure (lattice) towards instability. These findings demonstrate that CNT and metal nanoparticle structural evolution is strongly sensitive to the surface energy effect at nanoscale, and to activation of soft modes under e-beam irradiation. In principle, theoretical or simulated predictions cannot account for the intrinsic nonlinear, disordered and non-equilibrium nature of surface energy effects and e-beam activated soft modes, because they focus on the symmetrical, periodical and linear nature of bulk crystalline solids or their approximations in equilibrium instead.
It can be stated under the above elaborations that both the surface energy effect and the e-beam-activated soft modes in MWCNT and gold nanoparticle (Au NP) will surely be different, due to their dissimilar surfaces and bonding structures, which leads to the prediction that Au NPs, with their strong metal bonds, can be a novel passivation candidate for MWCNT structural transformation under e-beam irradiation. Nevertheless, Au NPs' passivation effect on MWCNT structural transformation induced by e-beam irradiation has not been studied so far experimentally, as is imperative for their potential application in nanodevices and nanotechnology. Since MWCNTs exhibit inner and outer surface energy-dependent evolution with meta-stable structures under e-beam irradiation, proper control over their e-beam evolution process is crucial. Both MWCNTs and Au NPs are chemically inactive structures; they represent the most appropriate composite materials for novel structures in which e-beam induced structural transformation can be passivated depending on the surface energy and instability of the MWCNT and the Au NP. Au NPs, with their strong metal bonds and heavier Au atomic mass, are relatively more stable than MWCNTs, and do not undergo any other undesirable transformation; whereas the MWCNT structure is meta-stable under e-beam irradiation, e-beam processing is currently restricted to its higher surface energy and higher instability (strong activation of soft modes) to avoid any undesirable transformation. Thus, there is clear motivation for the expansion of new advancements that will widen the processing range of these structures, while gaining control over the MWCNT evolution and transformation, if the whole range of potential applications for MWCNT is to be grasped. This study aims at understanding the e-beam activated structural transformation process in MWCNT with novel passivation effects of Au NPs at room temperature, illustrating several mechanisms of MWCNT and Au NP structural evolution. A new hypothetical approach establishes a kinetic relationship between MWCNT and Au-MWCNT shrinking radii and irradiation time, which reflects that nanoscale surface energy effect on structural transformation of tubes becomes more pronounced than in theoretical predictions. The study demonstrates that MWCNT, Au-MWCNT and Au NP structural evolution processes depend on nanoscale surface energy effects and e-beam activated soft modes or lattice instability.
2. Experimental
MWCNTs grown using the graphitic arc discharge method were used in the following experiments. Ethanol-MWCNT suspensions were gently dispersed onto copper grid mounted holey carbon films, after agitation in an ultrasonic bath. MWCNTs were sputter coated with Au to deposit Au NPs of optimum size under suitable conditions. Individual, clean, straight, and uniform MWCNT and Au-MWCNT segments, approximately 14 nm in diameter, were preferred for irradiation. The in situ TEM irradiation experiments were performed at ambient room temperature in Tecnai F-30 field emission gun TEM operating at 300 KV, equipped with condenser with correction of the spherical aberration. Such an accelerated beam could easily pass through the sample dimension (i.e. diameter of MWCNT) [23]. To avoid any effect of the holey carbon support on MWCNT structural evolution, an e-beam with beam spot (∼160 nm) much larger than the segment length, and beam current density about 75 A cm−2, was constantly focused on individual segments protruding into the free space of copper grid mounted holey carbon films. In order to collect micrographs, the beam current density was reduced to 100 times weaker than that used for irradiation, which not only allowed a direct interpretation of the contrast, but also minimized the irradiation effects. The corresponding fast fourier transform (FFT) patterns were also recorded during irradiation. The series maps for MWCNT diameter (or radius) shrinkage under increased irradiation were measured after each succeeding dose. The changes in MWCNT and Au-MWCNT diameter as noted within the irradiated area, traced across the MWCNT axis along its length, are shown in figures 1 and 2. Notably, a minor change in e-beam flux or slight local MWCNT structural disorder could enhance the disorder with prolonged irradiation, and lead towards local non-uniformity in a shrunk MWCNT or Au-MWCNT. This enhanced disorder or non-uniformity in the structure (or radius) of a MWCNT or Au-MWCNT with each succeeding dose along its length was measured by taking the averaged sum of several small, uniform, and regular segmental parts. The corresponding error bars for such measurements (or calculations) represent an increase in error with the enhanced local structural disorder.
Figure 1. Sequential bright-field TEM micrographs (a)–(f) and corresponding FFT patterns (insets) representing structural evolution process in MWCNT with increased irradiation time (given at right bottom corner of each micrograph) as irradiated under uniform beam current density (≈75 A cm−2) of e-beam and e-beam spot size (≈160 nm) spreading over an area much larger than the observed area.
Download figure:
Standard image High-resolution imageFigure 2. Consecutive bright-field TEM micrographs (a)–(i) showing structural evolution process in 14 nm (Au NP size ≈2.3 nm and MWCNT size ≈11.7 nm) thick Au-MWCNT with increased time (given at left bottom corner of each micrograph) as irradiated under uniform beam current density (≈75 A cm−2) of e-beam and e-beam spot size (≈160 nm) spreading over an area much larger than the observed area. Micrographs indicate crystalline structure of Au NPs and their growth under irradiation.
Download figure:
Standard image High-resolution imageThe underlying key beam heating issue in the specimen is too intricate to estimate quantitatively, due to the parameter complexity of the beam energy deposition rate, as well as the thermodynamical non-equilibrium nature of nanostructured materials. However, recent reports have proposed that, even under similar irradiation conditions, the temperature rise in the nanostructure [13] is trivial due to its high surface-to-volume ratio, and cannot entirely account for the phenomena observed herein. Therefore, we can assume that structural transformation is predominantly driven by athermal activation effects of the e-beam.
3. Results and discussion
To investigate the Au (metal) passivation effect, e-beam irradiation-induced structural transformation of MWCNT and Au-MWCNT was compared. The irradiation was carried out at 75 Acm−2, a uniform beam current density under e-beam spot (∼160 nm) centered at the tubes axis position, extending over a region definitely larger than the observed area. Figure 1 presents the typical evolution induced by the e-beam in a straight, uniform, cylindrical MWCNT segment, irradiated for 600 s. Figure 1(a) shows that before irradiation, 14 nm thick pristine MWCNT possesses equally spaced multi-layered crystalline structure, with both ends fixed, and sticks out of the tube bundle. Figures 1(b)–(f) show detail of the modification (or evolution) process as sequentially monitored in situ with TEM. The TEM data analysis reveals that tube diameter shrinks from 14 to 9 nm with increasing irradiation time (or dose). The shrinkage entails arresting internal mass transport (atom evaporation) with reduction in surface energy, which essentially leads to structural instability of the tube. Figures 1(a)–(d) with the supporting FFT patterns make it clear that although structural defects and deformations increase cumulatively in the basal planes of MWCNT under irradiation, the inter-layered structural integrity is not fully lost, and the tubular (or cylindrical) shape remains virtually unaffected. Moreover, figures 1(d)–(f) with the supporting FFT patterns show that the atomic defect annihilation process supersedes the atomic defect generation process while competing dynamically, which clearly demonstrates the restoration ability in the curved sp2 hybridized multi-layered tubular structure through a self-healing process. As a consequence, this instability forces the tube structure to shrink at room temperature from one meta-stable state to another with smaller size. Obviously, figure 1 demonstrates that the evaporation (or escape) process is activated by the e-beam irradiation at room temperature and favored by the high surface energy (positive and negative curvature) of the MWCNT, as monitored in situ with TEM. Moreover, increasing tiny deformations or local structural defects and breakage of the innermost tube walls (only 1–2) are also noted. This could be due to the clustering of the local defects around the vacant positions (induced by e-beam or some intrinsic) left by the evaporated atoms, which could not be recovered with the increasing evaporation rate, since vacancies are immobile and trapping of the evaporated carbon atoms from the inner walls between the contiguous walls gaps can substantially limit their outward escape compared with the surface atoms. This explains why defects become more stable in MWCNT for a prolonged time at lower (or room) temperature. This is in accordance with the fact that interlayer atomic defects in the contiguous DWCNT walls induced by e-beam irradiation below 450 K are stable for a macroscopic time [24].
Au-MWCNT irradiation was also carried out with the same 300 KeV e-beam under exactly the same 75 Acm−2 irradiation, with beam current conditions as stated above for the MWCNT case. Figure 2 illustrates the typical transformation with successive evolutions in a smooth, uniform, and cylindrical Au-MWCNT induced by e-beam as irradiated in a sequence for 1260 s. Figure 2(a) shows that an equally spaced multi-layered crystalline structure of Au-MWCNT (see also figure 3) fixed at its both ends and sticking out of the tube bundle has 14 nm diameter on average prior to irradiation. It is to be noted that when separate, Au NP size and MWCNT diameter are approximated to 2.3 ± 0.3 nm and 11.7 nm respectively. Figures 2(b)–(i) illustrate the transformation (or evolution) process as successively monitored in situ with TEM. Examination of the micrographs reveals that tube diameter shrinks from 14 to 11.4 nm with increasing irradiation time (or dose). The Au-MWCNT shrinkage seems much slower than that of the MWCNT, as irradiation time is extended twice or even more. The Au-MWCNT retains its essential structural features almost unchanged (like the MWCNT) while shrinking, but the increase in local structural defects or tiny deformations appears to be much smaller compared with the MWCNT, as seen in figure 1. This essentially demonstrates that the Au-MWCNT structure is relatively more stable than the MWCNT structure under e-beam. The Au-MWCNT structural stability is dependent on its surface energy, which seems to be less than that of the MWCNT. This results in slower shrinkage, with less internal mass transport entailed, and hence lowered net reduction in the surface energy. In fact, the lower surface energy of Au-MWCNT is more closely associated with the lower surface energy of Au NPs than that of the MWCNT, as the following discussion demonstrates. In addition to an obvious passivation effect of Au NPs, an appreciable increase in their growth and size is also examined from the TEM micrographs, as shown in figure 2. It seems that coalescence process is proceeded via Au NP movement across the tube outer surface, induced with e-beam activation. Although interaction between Au NP and MWCNT is still questionable, in most cases a weak interaction between the metal NPs and CNT surface is considered. It can be expected that Au NP movement across the tube outer surface under e-beam is favored by the repulsion between the free electronic charges of the Au NPs (which, being metal, they are supposed to possess at their surfaces) and confined surface electronic charges due to shortened bond lengths of the carbon atoms of the curved graphitic layers of the MWCNT [13]. By increasing irradiation time (or doses), the coalescence process is enhanced further and the primarily mono-dispersed Au NPs of 2.3 ± 0.3 nm are transformed into 4.2 ± 0.5 nm, on average. Although nano-ripening (complete absorption of very small NPs by larger NPs) cannot be totally avoided, it seems that coalescence is the leading phenomenon herein, as marked by yellow arrows in figure 2. Au NPs advancing through their movement across the tube outer surface reduces the distance between them, and brings them close enough that their surfaces start to touch each other, energetically favoring their growth. More probably, Au atomic diffusion process across the in-contact surfaces of the Au NPs proceeds by d10 to d9s1 type electronic-excitation-induced Au atomic displacements activated by e-beam induced soft mode effect under Au NP positive curvature effect (see discussion). With scaling down, the natural tendency of crystalline NPs to expose the structural defects at their surfaces increases—and, moreover, their surfaces become mobile because of the e-beam activation effect and positive-curvature-induced phonon softening effect. Thus, Au atomic diffusion from a site of higher energy to a site of lower energy becomes very possible, while Au atoms try to decrease their energy (the system total energy) in order to gain a minimum energy state. In this way, Au NP number density is decreased, while their size is increased. Furthermore, under prolonged irradiation, it is observed that Au NPs try to move away from the irradiated tube surface, as a consequence of energy transfer by e-beam. Specifically, this can be attributed to the increasing surface energy of the shrinking tube because Au NPs, in an attempt to attain an equilibrium state, are moved from a smaller diameter site (irradiated zone) with higher surface energy towards a larger diameter site (non-irradiated zone) with reduced surface energy—i.e. in a direction leading them towards the tube fixed ends. Due to this effect, as well as coalescence, Au NP number density is reduced within the irradiated tube length, and their passivation effect is similarly weakened. As a consequence, to an extent, Au-MWCNT shrinkage speeds up after 1050 s irradiation. Figure 3 is a selected area magnified TEM image of the Au-MWCNT, as marked by the red rectangular area in figure 2(a), which demonstrates the typical crystalline features of the Au-modified tube before irradiation. This contains multiple layers, closely equivalent to the MWCNT shown in figure 1(a). Its walls are perfectly straight, and give well defined minute details of the structural transformation features during the successive irradiation doses, as shown in figure 2.
Figure 3. A selected area magnified TEM image of the Au-MWCNT before irradiation as marked by the red rectangular area in figure 2(a). This demonstrates the straight multiple walls and perfect crystalline features of the MWCNT with crystalline Au NPs of average size about 2.3 ± 0.3 nm residing at its outer surface.
Download figure:
Standard image High-resolution imageFigure 4 demonstrates the corresponding plots of MWCNT and Au-MWCNT radius shrinkage with increased irradiation time. By considering the above-described driving forces for radius shrinkage, the correlation between radius shrinkage and irradiation time can be established. From thermodynamics [19], the driving force for carbon atom transport and migration during MWCNT and Au-MWCNT shrinkage can be expressed as
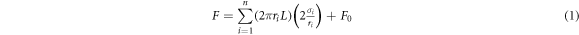
where the first term on the right-hand side is the composition of capillary forces for each individual wall and F0 corresponds to the van der Waals force between the contiguous tube walls, while  or
or  is the radius and
is the radius and  is the contiguous wall gap.
is the contiguous wall gap. 
 and
and  respectively stand for length, surface energy and surface area of each individual wall of the tube. Notably, both tensile stress on the outer surface and compressive stress on the inner surface of each individual wall of the tubes have been considered in equation (1), as will be explained in the following text. The nanocurvature effect in the normal shrinkage process of the MWCNTs has not been accounted for in the predictions of classical theory, where
respectively stand for length, surface energy and surface area of each individual wall of the tube. Notably, both tensile stress on the outer surface and compressive stress on the inner surface of each individual wall of the tubes have been considered in equation (1), as will be explained in the following text. The nanocurvature effect in the normal shrinkage process of the MWCNTs has not been accounted for in the predictions of classical theory, where  surface energy of the CNT is assumed to be independent of
surface energy of the CNT is assumed to be independent of  and
and  Nevertheless, our previous work [19] has demonstrated that when the SWCNT radius approaches the atomic bond length, the effect of curvature on both σ and r at the nanoscale increases drastically, and can be accounted for by the following additional term,
Nevertheless, our previous work [19] has demonstrated that when the SWCNT radius approaches the atomic bond length, the effect of curvature on both σ and r at the nanoscale increases drastically, and can be accounted for by the following additional term,
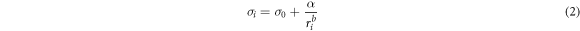
where  is a surface energy factor and
is a surface energy factor and  is a constant
is a constant  which is widely assumed to be greater than 2. Notably, the value of
which is widely assumed to be greater than 2. Notably, the value of  has been predicted to be 2 by the existing simulations [25]. This additional term
has been predicted to be 2 by the existing simulations [25]. This additional term  representing the increasing surface energy (or curvature) of each individual wall of the MWCNT and Au-MWCNT at the nanoscale is much greater than
representing the increasing surface energy (or curvature) of each individual wall of the MWCNT and Au-MWCNT at the nanoscale is much greater than  and thus
and thus
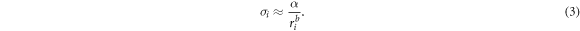
By kinetics, it is assumed that evaporation rate (loss in number of carbon atoms) in MWCNT and Au-MWCNT is proportional to the driving force of their shrinkage [19]. Thus, we have
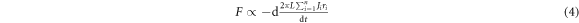
or
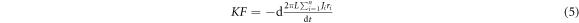
where  are the number of carbon atoms lost during the time interval
are the number of carbon atoms lost during the time interval  and
and  is the real density of surface atoms of each individual wall of the tubes (J is taken as constant temporarily as shrinkage is limited in this case). By equations (1), (3), and (5), we have
is the real density of surface atoms of each individual wall of the tubes (J is taken as constant temporarily as shrinkage is limited in this case). By equations (1), (3), and (5), we have
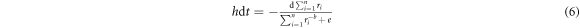
where  and
and  The thermodynamic reaction constant K in equation (6) is given as
The thermodynamic reaction constant K in equation (6) is given as  where
where 

 and
and  are standing as constant, gas constant, temperature, and the activation energy of the shrinkage respectively. As the MWCNT and Au-MWCNT radii are decreasing with irradiation time t; thus, the following condition can be defined,
are standing as constant, gas constant, temperature, and the activation energy of the shrinkage respectively. As the MWCNT and Au-MWCNT radii are decreasing with irradiation time t; thus, the following condition can be defined,
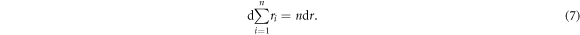
By substituting equation (7) in equation (6) and rearranging it, we get the following time dependent value of r, which is proportional to the irradiation dose (or time),
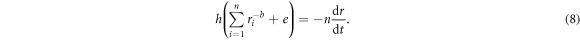
Thus, equation (8) establishes the kinetic relationship between radius shrinking of MWCNT and Au-MWCNT and the irradiation time, as tracked precisely for a limited shrinkage under high enough and constant beam current density. This reveals that physical insights of the underlying mechanism of shrinkage in the tubes with e-beam irradiation can be justified in terms of physical meaningful constants  and
and 
Figure 4. Radius (or diameter) shrinkage of MWCNT and Au-MWCNT with increasing irradiation time, as observed in figures 1 and 2. The MWCNT and Au-MWCNT radii are taken as the mean value of several representative sites across the tube axis. The solid lines are the fitted results according to equation (8), where b = 4.2 for the MWCNT and b = 3.1 for the Au-MWCNT.
Download figure:
Standard image High-resolution imageFollowing these elaborations, the respective shrinking radii of MWCNT and Au-MWCNT changing from 7 nm to 4.5 nm in 600 s and 7 nm to 5.7 nm in 1260 s are non-linearly fitted with equation (8), as shown in figure 4. The solid lines in figure 4 represent theoretical nonlinear well-fitting of the experimental data by equation (8) with  for the MWCNT and
for the MWCNT and  for the Au-MWCNT. Even when the uncertainties arising in measuring the tube radii are included, the fitted value of constant
for the Au-MWCNT. Even when the uncertainties arising in measuring the tube radii are included, the fitted value of constant  is still greater than 2
is still greater than 2  and
and  [25], implying that an addition to the curvature or surface energy of the MWCNT or Au-MWCNT at the nanoscale is proportional to
[25], implying that an addition to the curvature or surface energy of the MWCNT or Au-MWCNT at the nanoscale is proportional to  or
or  rather than
rather than  as calculated by the theory. This illustrates that, at the nanoscale, an increasing curvature increases both MWCNT and Au-MWCNT surface energy significantly more than predicted by the existing models. Quantitative analysis indicates that MWCNT net shrinkage is faster than that of the Au-MWCNT. MWCNT shrinkage and Au-MWCNT shrinkage can be averaged to 8.5 × 10−3 nm s−1 and 2.15 × 10−3 nm s−1 respectively, as drawn in the corresponding plots in figure 4. Overall, MWCNT shrinkage is estimated to be four times faster than Au-MWCNT shrinkage. MWCNTs are shrunk at a much faster rate, which speeds up further with the increasing irradiation time (or doses). In contrast, the shrinking of Au-MWCNTs occurs slowly, and is extended over a much prolonged irradiation period. However, a little increase, specifically after 1050 s, is noted when Au NP size is increased or their number desnsity is decreased. Furthermore, Au NP growth seems to be slower during the initial 250 s, as they are spotted apart from each at larger distances prior to the irradiation. But the distance among the Au NPs is reduced enough during this initial 250 s irradiation that their interaction and aggregation is triggered. From 250 s to 1260 s, the Au NP growth process is speeded up, eventually forming larger sized Au NPs as shown in figure 2.
as calculated by the theory. This illustrates that, at the nanoscale, an increasing curvature increases both MWCNT and Au-MWCNT surface energy significantly more than predicted by the existing models. Quantitative analysis indicates that MWCNT net shrinkage is faster than that of the Au-MWCNT. MWCNT shrinkage and Au-MWCNT shrinkage can be averaged to 8.5 × 10−3 nm s−1 and 2.15 × 10−3 nm s−1 respectively, as drawn in the corresponding plots in figure 4. Overall, MWCNT shrinkage is estimated to be four times faster than Au-MWCNT shrinkage. MWCNTs are shrunk at a much faster rate, which speeds up further with the increasing irradiation time (or doses). In contrast, the shrinking of Au-MWCNTs occurs slowly, and is extended over a much prolonged irradiation period. However, a little increase, specifically after 1050 s, is noted when Au NP size is increased or their number desnsity is decreased. Furthermore, Au NP growth seems to be slower during the initial 250 s, as they are spotted apart from each at larger distances prior to the irradiation. But the distance among the Au NPs is reduced enough during this initial 250 s irradiation that their interaction and aggregation is triggered. From 250 s to 1260 s, the Au NP growth process is speeded up, eventually forming larger sized Au NPs as shown in figure 2.
To examine the validity of our results, several other similar MWCNT and Au-MWCNT segments were irradiated under the same irradiation conditions as stated above, and this has ensured that the typical transformation with successive evolutions, as demonstrated in figures 1 and 2, are duplicable. That experimental series demonstrates that MWCNT structural transformation proceeds at a faster rate than that of the Au-MWCNT due to faster evaporation of carbon atoms driven by the strong effect of high surface energy under e-beam activation. In contrast, Au-MWCNT structural transformation proceeds at a slower rate than that of the MWCNT, due to slower evaporation of carbon atoms driven by the weaker effect of lower surface energy under e-beam activation. Importantly, numerous MWCNTs and Au-MWCNTs with layer numbers less (or equal or more than shown in figures 1 and 2) and Au-MWCNTs with Au NP sizes ranging from 1–10 nm (or even larger) were irradiated, and their shrinking tendencies carefully noted. Eventually, it has been concluded that the passivation effect of Au NPs with optimal size ranging between 2–5 nm on Au-MWCNTs having layer numbers comparable (or even equal) to a given MWCNT (shown in figure 1) is in a good agreement under our defined experimental conditions, as shown in figure 2. Furthermore, regardless of the coalescence, a very fast movement of very small sized (<2 nm) Au NPs across the tube outer surface leads them quickly towards the tube fixed ends. This drawback weakens their passivation effect in a limited irradiation time, and speeds up the structural transformation process in Au-MWCNTs, whereas larger sized Au NPs (>7 nm) cover more than 50% of the tube outer surface area, which slows down the coalescence process and Au-MWCNT structural transformation process for a much prolonged irradiation period by limiting Au NP movement across the tube outer surface. This illustrates that an optimal Au NP size (2–5 nm), which can offer obvious intense resistance to the MWCNT structural transformation at room temperature under e-beam due to its predominant and novel passivation effect, is indispensable. Notably, any evolution in MWCNT, Au-MWCNT, and Au NP coalescence cannot proceed without e-beam activation. In order to test the e-beam activation effect, the e-beam has been switched off numerous times during the experiments; the results of this check indicate that micrographs in figure 1 or figure 2 represent the true and predominant e-beam activation effect, like the SWCNT [19] or other nanostructures [10] (see discussion).
Figure 1 illustrates that MWCNT fixed at both ends with uniform multi-layered structure possesses uniform curvature (high surface energy). Thus, it is more likely that uniform curvature with e-beam activation can apply an inward pulling force on the tube walls to shrink them to the smaller curvature site of less surface energy with continuous loss of carbon atoms. Probably, a large fraction of the surface atoms can be evaporated outward (into free space) and a small fraction (especially those evaporated from the inner walls) can be trapped between the contiguous walls gaps or in the inner hollow. The overall mechanism is illustrated with schematics presented in figures 5(a)–(c). In contrast, the vacant positions and dangling bonds left momentarily by the evaporated atoms are instantly refilled by the contiguous carbon atoms through a surface healing process, as the MWCNT possesses a strong capability to restore its curved, graphitic, and tubular structure with sp2 hybridized atomic bonding [26, 27]. This eventually causes faster shrinkage. Nevertheless, the inter-layered weak interactions can limit the shrinkage process in MWCNTs compared with that in SWCNTs [19], as the surface vacancies [28] can only provide the outward pathways for those atoms evaporating from the inner walls as shown in figures 5(a)–(c). It is likely that surface vacancies are partially refilled by the atoms escaping from the inner walls, and hence MWCNT shrinkage is delayed. In contrast, Au-MWCNT with crystalline Au NPs at its surface possesses non-uniform curvature (less surface energy), as demonstrated in figure 2. Thus, it can be expected that crystalline Au NPs decrease the curvature effect or reduce the inward driving force on the tube walls, so that both loss of carbon atoms as well as shrinkage are suppressed for a prolonged period. Meanwhile the strong passivation effect of Au NPs slows down the defect generation (or deformation) rate and structural transformation process. As a consequence, only a small fraction of atoms are evaporated outward and trapped between the contiguous walls gaps or in the inner hollow compared with the MWCNT. The detailed mechanism is demonstrated with schematics in figures 5(d)–(f). Besides passivation, Au NP increasing interaction and growth lead them towards an increased size. As described in literature, an outward atomic diffusion from a cluster of a few atoms to a larger sized particle is called nano-ripening, whereas inward/outward atomic diffusion between two NPs via their in-contact surfaces followed by particle free mobility over the host surface is called coalescence [29]. While interacting, softening of phonons in the shortened bond lengths of the Au surface atoms induced by positive curvature effect of the Au NP at nanoscale plays a crucial role and e-beam activates atomic displacements through d10 to d9s1 type electronic excitations (pseudo-Jahn-Teller type interactions, see section 3.2). This energetically promotes inward/outward atom diffusion leading towards their growth, while they are going through a thermodynamic non-equilibrium process in an attempt to achieve equilibrium. Such a growing tendency always exists when Au NP surfaces come in contact with another NP or they get close enough that it is energetically favored by high surface energy and e-beam activation. Furthermore, as metals possess a strong natural intrinsic capability to restore from the broken bonds, and this causes them to restore vacancies and dangling bonds generated momentarily through the contiguous atoms, while undergoing a self-healing process and decreasing the surface energies [30] in order to achieve equilibrium. It is more likely that new Au NP orientations are insensitive to the old orientations during coalescence, whereas the larger sized Au NP retains its orientation during ripening [31]. Overall, in this thermodynamical non-equilibrium growth process, number of density Au NPs decreases while their size increases.
Figure 5. Schematic diagram illustrating step wise structural transformation, atomic evaporation, and atomic diffusion in MWCNT (a)–(c) and metal passivation effect on structural transformation, atomic evaporation, and atomic diffusion in Au-MWCNT (d)–(f). Schematic diagram shows interactions between Au NPs and structural evolution process with their growth in detail.
Download figure:
Standard image High-resolution imageThe e-beam excitation induced MWCNT or Au-MWCNT shrinkage with evaporation, metal passivation effect and Au NPs growth with atom transport by inward/outward diffusion at room temperature, described above, is not expected to occur in bulky solids. The arresting size-dependent structural disparity between the MWCNT and crystalline Au NPs cannot be compared with the current understanding. Our results clearly demonstrate that the size-dependent high surface energies of MWCNTs and Au NPs, and e-beam activated soft modes, are characteristic features of the structural transformations and evolutions, in contrast to those in bulky solids. This challenges the present thought. Although a wide study has been carried out on similar e-beam activation effects in CNTs [10, 15, 18] and other one dimensional nanostructures [32, 33], the underlying nanoscience concept, typical evolutions or transformations in MWCNTs and metal NPs, and their nanoprocessing with e-beam activated soft modes under high surface energy effects, have not been focused on previously. The present classical concept standing on knock-on mechanism explicates the structural transformation phenomena with molecular dynamics simulations, and treats one dimensional nanostructure systems or their approximations with equilibrium, symmetry, periodicity and linear nature. At the nanometer scale, size effect has been mostly linked with the increased surface area and number of surface atoms. The classical knock-on mechanism and simulation, with a focus on existing size effect concept [8–18], cannot elucidate the soft modes activated by high surface energy (positive and negative curvature effect in MWCNT or Au NP) together with e-beam irradiation. Careful consideration of the the present results, specifically faster shrinkage with swift atom evaporation driven by the MWCNT high surface energy effect under strong e-beam activation, metal passivation with slower shrinkage and impeded atom evaporation driven by suppressed surface energy under weak e-beam activation, and Au NP growth activated with atom transport by inward/outward diffusion at room temperature, associates them with the size effect at nanometer scale and ultrafast e-beam energy transfer rate.
3.1. Negative and positive curvature effect (bond length shortening)
It is a well established fact that, as the characteristic dimensions of nanostructures shrinks to nanoscale, their surface/volume ratio, higher than the bulk counterpart, changes the energetic states of surface atoms, which not only causes an abnormal increase in the surface energy, but also leads towards meta-stable states [20]. Obviously, surface energy effect on the instabilities of nanostructures, and hence evolutions in their structure, cannot be disregarded. In CNTs, size effect (nanoscale dimensions) alters the equilibrium, periodicity, and linearity (as compared with graphene layer(s)) of carbon atoms, and hence their energetic states, as a consequence of their bending at an angle (theta) from the normal positions. The carbon atoms lose symmetries, and bonds no longer remain parallel to each other; their rearrangement in tubular form generates positive and negative curvature with distortion of the electron cloud, and hence thermodynamical non-equilibrium, non-periodical and nonlinear states of the atoms are introduced. These new atomic states cannot be predicted or simulated with previous theories, as they treat nanostructures with equilibrium, symmetry, periodicity, and linearity like their bulk counterparts. This process is schematically illustrated in figure 6(a), which represents SWCNT (the simplest form of CNT) cross-sectional view with radius r, as drawn by black solid line (circular). In MWCNT, for sure, similar bending would happen but bending angle may increase further from outer-to-inner wall due to further size reduction of each individual wall. An intriguing feature of the size effect in CNTs (we can consider rolling up of a graphene sheet(s) into CNT(s) herein) is that bond lengths shorten, and bond angles change spontaneously [34], as illustrated in the schematics in figure 6(b). These changes not only increase the associated bond energies, but also strengthen the bonds, and depress the pair potentials, as compared with flat graphene layer(s). As a consequence, the increased bond strains and bond strengths induced by quantum confinement of local electronic charges or dangling bonds localize and densify the energy, which leads to an excess storage of energy within the surface or at locations surrounding the structural defects (if any), a factor softening the phonons in addition to perturbing some other related effects. For example, frequency of phonons (198 cm−1) [34] is much suppressed in MWCNT (8–50 walls) in contrast to that (850 cm−1) in graphite [21]. An arresting difference between CNTs and nanowire, NPs, nanocrystals and bulk is that the former has an inner surface with negative curvature, in addition to the outer surface with positive curvature, as mentioned with two dotted circles, drawn inside/outside the black solid circle in figure 6 (a). Although each wall of CNT holds two surfaces and the combined effect of both plays its role during their structural evolutions, the effect of negative curvature at the inner surface becomes prominent at extremely small scales. Theoretically, the surface energies of the nanostructures holding positive and negative curvatures increase with decreasing size, and atomic vibrations exhibit different size effects for systems possessing positive and negative curvatures [20, 35]. Owing to this particular feature—that CNTs possess two distinct kinds of surface—the normal atomic vibrations or phonon modes at the two surfaces become dissimilar; that is, the strength of phonons at the inner surface becomes weaker than at the outer surface, and both become smaller than in the bulk interior. This reveals that the system holding negative curvature has a higher surface energy than that of the system holding positive curvature, and both surface energies are higher than that of the bulk counterpart when their sizes are extremely small. In fact, the less-coordinated inner surface with negative curvature exhibits diverse bonding interaction (or surface energy) than that of the nanowire (amorphous and crystalline) interior or the NP interior or nanocrystal interior or the bulk interior. This unique increase in the inner surface energy of nanostructures with inner surfaces can be attributed to the size-dependent negative curvature of the nanostructures, which tends to shrink nanostructures by applying an inward pulling force, as shown with green arrows in figure 6(a). It has been demonstrated that the Debye or 'melting temperature' of a nanotube becomes lower than that of a nanowire due to the nanoscale negative curvature [20, 36]. It can be expected that inclination of MWCNT could also be similar. This states that the negative curvature effect suppresses atomic vibrations (phonon softening) at the inner surface, which not only lowers the Debye temperature of MWCNTs appreciably, but also reduces the energy barrier of strong structural constraint and favors kinetic evaporation or sputtering of carbon atoms under e-beam activation (see next section). This affirms that surface energy of a CNT or other nanotube is much higher than that of a nanowire or NP of the same size (diameter), and both of them are higher than their bulk counterparts. It is noteworthy that contributions by the gains in bond strain, bond strength and increasing density of dangling bonds in the inner surface (increased negative curvature effect) become crucial as the tube size decreases. Additionally, it has recently been reported that inner surface energy of a nanotube increases, and outer surface energy decreases, below certain diameter ranges [20, 36]. In fact, this decreased outer surface energy is a consequence of the decreasing contribution of the bond strain energy or strength (increasing strength of amplitudes of atomic vibrations at the outer surface compared with that of the inner surface) and density of dangling bonds to the outer surface.
Figure 6. Schematic representation of (a) carbon atoms bending at an angle theta from their normal equilibrium positions in a single wall of MWCNT, where contribution by the inner surface energy (or negative curvature) to the driving force of shrinkage exceeds that of the outer surface energy (or positive curvature), (b) bond length shortening in the MWCNT structure under positive and negative curvature effect in comparison to the bond lengths in graphene layer(s), and (c) surface atoms bending at an angle theta from their normal equilibrium positions characterized by the positive curvature effect, which shortens the atomic bond lengths of the shell (2–3 atomic layered surface structure) structure compared to that of the core (inner structure), and facilitates Au atom inward/outward diffusion (Au NPs growth) during surface contact with e-beam activated soft modes and instability effects.
Download figure:
Standard image High-resolution imageAs far as the Au NP, figure 6(c) demonstrates that its shell (2–3 atomic layers) is under-coordinated, while the core underneath the outer surface is under the effect of strong structural constraints, like the interior of bulk Au. The outer surface and interior structures have been respectively called as shell and core structures, in order to distinguish their contributions in the surface energy of the Au NP. The bonding interactions in the shell structure are dissimilar compared with the core structure, as bending of the outer surface atomic layers at an angle (theta) distorts the outer surface electron cloud, giving rise to a positive curvature, as shown with dotted circle in figure 6(c), and thermodynamically leads the atomic states towards non-equilibrium, non-periodicity and non-linearity. Furthermore, the positive curvature effect stores an excess energy in the shell structure via shortening the bond lengths and changing the bond angles [20] spontaneously, which eventually softens the phonons, as described above in detail. Intriguingly, the core structure remains intact during the whole process. It is stated above that a system holding positive curvature possesses less surface energy than that of a system holding negative curvature. In Au NPs, the atomic facets and some intrinsic structural defects at the outer surface with its metal bonded crystalline structure somehow decrease the effect of positive curvature or the surface energy. Despite these two facts, which can cause some reduction in its surface energy, it still possesses much higher surface energy than that of its own core (interior), bulk surface or bulk interior; but, in fact, less than that of a CNT or amorphous nanowire, which makes it stable compared with an MWCNT or amorphous nanowire under e-beam irradiation. In addition, Au (or metal) NPs have a natural tendency to throw out structural defects at their surfaces [29]. This tendency of exposing defects (increasing dangling bonds) at the surface, together with higher surface energies, facilitates inward/outward atomic diffusion or material transportation under external stimulus (e-beam) while their surfaces are in contact, which eventually activates their growth as shown in figure 2. Consequently, it can be affirmed from the above discussion that MWCNTs, with positive and negative curvature effects, possess a much higher surface energy (or possibility of higher instability under e-beam) compared with the crystalline Au NP with positive curvature (or possibility of lowered instability under e-beam), and vice versa, as will be explained next.
3.2. E-beam activated soft modes (ultrafast lattice instability)
It is presented above that energetic e-beams induce great structural instability in MWCNTs. To evaluate this in more detail, it is essential to know the mechanism of energy transfer from such electrons to the MWCNT. Obviously, the possibility that the energetic electron directly interacts with the internal core of the carbon atoms of the MWCNT seems very low. Furthermore, as electrons possess low mass, there is a high probability that energy can be transferred to the valence electrons in the vicinity of the trajectory of the electron. Under negative (and positive) curvature effects, gain in the bond strains and bond strengths by spontaneous change in the bond lengths/angles confines the bond energies (phonon softening). This confinement makes the bonds very sensitive and active sites, and their dynamical response to the electrons (e-beam) becomes crucial during irradiation. The dynamical response is in fact associated with the excitation of valence electrons, which represents the bonding character for typical carbon materials (including MWCNTs) [21] and metals. As a consequence, the energetic states of electronic charges change such that generation of soft modes or bond distortions via excitation by an external exciting source (e.g. e-beam) becomes very possible. In fact, soft mode or bond distortion induced lattice instability in crystalline nanostructures or condensed matter is a widespread phenomenon, as this is not only limited to the e-beam irradiation—ion and laser beam irradiation can also produce such effects [19, 21, 22]. By definition, 'that particular excitation, which crosses over the bond stability limit due to unstable phonon mode, is called as soft mode' [37]. This phenomenon is quite different from the thermal response or the knock-on response. Herein, this can be named as e-beam induced athermal activation. For soft mode occurrence, energy transfer by the e-beam must be ultrafast—and, moreover, frequencies of its electrons must compete with or exceed that of the solid (or condensed) matter. Figure 7 demonstrates that energy transfer  by an energetic electron of 300 KeV e-beam is more likely to take place on an ultra-short timescale
by an energetic electron of 300 KeV e-beam is more likely to take place on an ultra-short timescale  during a single inelastic event, like the energy transfer by an ultra-short (10−15 s or even less) laser pulse. This energy transfer by e-beam to the solid matter occurs in an ultrafast, non-equilibrium, and highly localized manner. Moreover, it is very possible that frequencies of ultrafast (nearly 80% of the speed of light) electrons of 300 KeV e-beam can easily compete with (or even exceed) the natural phonon frequencies (normal atomic vibrations) in MWCNTs or Au NPs. Thus, ultrafast, non-equilibrium and highly localized energy transfer by the electrons in passing through the MWCNT or Au NP structure generates a high density of soft modes, which weaken the bonding structure and eventually leads to lattice instability. At the point of instability, the electrons and atomic lattice are at different temperatures. For covalent structures and metals, the timescale for electron−phonon coupling (lattice heating) is relatively slow (10−12 s or even longer, depending on increasing excitation density) compared with that of the lattice instability (10−15 s) [38]. Therefore, heat transfer between the electron and the atomic lattice during the instability can be disregarded. This clearly reveals that athermal activation may overcome the electron−phonon coupling (lattice heating), and atoms in the solid matter lose their bound states—eventually affecting the stability of the nanostructure. This lowers (makes disappear) the energy barrier. At this point, vibrational modes (complex frequencies of phonon) approach zero
during a single inelastic event, like the energy transfer by an ultra-short (10−15 s or even less) laser pulse. This energy transfer by e-beam to the solid matter occurs in an ultrafast, non-equilibrium, and highly localized manner. Moreover, it is very possible that frequencies of ultrafast (nearly 80% of the speed of light) electrons of 300 KeV e-beam can easily compete with (or even exceed) the natural phonon frequencies (normal atomic vibrations) in MWCNTs or Au NPs. Thus, ultrafast, non-equilibrium and highly localized energy transfer by the electrons in passing through the MWCNT or Au NP structure generates a high density of soft modes, which weaken the bonding structure and eventually leads to lattice instability. At the point of instability, the electrons and atomic lattice are at different temperatures. For covalent structures and metals, the timescale for electron−phonon coupling (lattice heating) is relatively slow (10−12 s or even longer, depending on increasing excitation density) compared with that of the lattice instability (10−15 s) [38]. Therefore, heat transfer between the electron and the atomic lattice during the instability can be disregarded. This clearly reveals that athermal activation may overcome the electron−phonon coupling (lattice heating), and atoms in the solid matter lose their bound states—eventually affecting the stability of the nanostructure. This lowers (makes disappear) the energy barrier. At this point, vibrational modes (complex frequencies of phonon) approach zero  , as shown in figure 7.
, as shown in figure 7.
Figure 7. Schematic illustration of the e-beam activated soft modes, where ultrafast and highly localized energy exchange between a single energetic electron of the e-beam and single covalent or metal bond leads to an individual lattice (structural) instability in the solid matter, without transferring a part of energy to the surroundings.
Download figure:
Standard image High-resolution imageIn MWCNTs, the transversal optical phonons expand the bond lengths to a maximum value at the Γ-point as carbon atoms in the curved graphitic carbon layers tend to move in alternate perpendicular directions (out of the graphitic planes). An increase in the bond length owes to atom displacement S from the equilibrium position A to  =
=  [21]. As a consequence, energy gap between the valence band (bonding character) and conduction band (anti-bonding character) lowers significantly, and density of states becomes finite everywhere. This decreases the energy of electronic excitations with increasing displacement S. Thus, phonons become soft, which makes the lattice unstable under increasing density of e-beam induced electronic excitations. Each electronic excitation contributes approximately equally to the repulsive force between the carbon atoms; thus, the relation between the square of reducing phonon frequency and increasing electronic excitation density becomes linear. Notably, there always exists a certain threshold (or minimum) value of excitation density, above which phonons become unstable. This is because electron−electron scattering determines the relaxation time for the electrons, which decreases strongly with the increase in excitation density. In contrast, at lower excitation density, electron−phonon scattering becomes prominent. Around the equilibrium positions, atomic vibrations turn into an exponentially rising instability, which ruptures the graphitic bonding structure, thereby increasing the kinetic energy and velocity of atoms. It is expected that with a 300 KeV e-beam, a high density of electronic excitations much exceeding the threshold can be attained easily.
[21]. As a consequence, energy gap between the valence band (bonding character) and conduction band (anti-bonding character) lowers significantly, and density of states becomes finite everywhere. This decreases the energy of electronic excitations with increasing displacement S. Thus, phonons become soft, which makes the lattice unstable under increasing density of e-beam induced electronic excitations. Each electronic excitation contributes approximately equally to the repulsive force between the carbon atoms; thus, the relation between the square of reducing phonon frequency and increasing electronic excitation density becomes linear. Notably, there always exists a certain threshold (or minimum) value of excitation density, above which phonons become unstable. This is because electron−electron scattering determines the relaxation time for the electrons, which decreases strongly with the increase in excitation density. In contrast, at lower excitation density, electron−phonon scattering becomes prominent. Around the equilibrium positions, atomic vibrations turn into an exponentially rising instability, which ruptures the graphitic bonding structure, thereby increasing the kinetic energy and velocity of atoms. It is expected that with a 300 KeV e-beam, a high density of electronic excitations much exceeding the threshold can be attained easily.
It has been predicted theoretically that in graphite, structural transition from graphite to diamond is possible under irradiation with x-ray or laser beams [39, 40]. These theoretical predictions show that a greater instability in the graphitic planar structure due to the excitement of holes in the valence band can cause transition from sp2 to sp3 [39]. It may seem doubtful that the structural transition that destroys a covalent bond can be activated by an excited hole in the valence band. Nevertheless, the graphitic planar structural stability is crucially dependent on the valence band (bonding character). In graphitic structures, although the network of three-fold electron bonding (which represents the bonding character) keeps the planar structure stable, irradiation can introduce two holes in the valence band through the final states in the Auger effect. The excitement of holes makes the planar graphitic structure instable, whereby carbon atoms can be displaced in a perpendicular direction, form strong covalent bonds, and transform the local structure from graphite (sp2 hybridized bonding) to diamond (sp3 hybridized bonding). As far is concerned with the core-excited state, the graphitic structure is stable in energy compared with that of diamond, because it is expected that an electron excited from the core occupies the lowest energy state in the conduction band—whereas, in diamond, an electron excited from the core possesses a much higher energy than that in the graphitic structure due to its larger band gap energy. Although such instabilities can be induced under intense irradiation, an extreme high pressure and temperature is more favorable for graphite to diamond transition [13, 21, 39]. At room temperature and normal atmospheric pressure, a liquid-like flow or atom evaporation is more likely to occur, as seen in figures 1 and 2. Thus, in our results, e-beam induced electronic excitations might transit to the graphitic domains and cause selected instability through several intermediate transitions in the atomic structure—a totally different mechanism from the beam heating effect.
Furthermore, the covalent bonding network of each individual sp2 hybridized wall of MWCNT is composed of lightweight carbon atoms, wherein the sputtering coefficient, the dangling bond energy, and the covalent bond strength are relatively lower than in Au. These factors make it easy for carbon atoms to carry away (sputtering) the ultrafast and extremely localized energy transferred by energetic electrons, without delivering it to the surroundings. Increasing irradiation doses (time) increase the evaporation rate, which in turn enhances the structural defects, thereby contributing to the deformation. Thus, the atom evaporation process induced by e-beam activation triggers the structural instability in the MWCNT, which causes shrinkage, and leads towards the structural transformation.
For e-beam irradiation effects in Au NPs, phonon softening induced by positive curvature effects in the shell structure can facilitate the e-beam to activate Au atomic displacements via d10-to-d9 type electronic excitations. The tetragonal d9 systems (Cu(II) and Au(II)) with elongated bonds in z-axis direction are expected to undergo Jahn−Teller distortions [41–44]. As compared with Cu, d-orbital splitting is much more probable in Au. For d9 Au(II), the last electron should occupy the very high energy  orbital in order to attain square planar geometry, which is unfavorable. The maximum crystal field splitting energy (Δ) of Au(II) makes it highly unstable, and it thus undergoes disproportionation via gaining an electron, forming Au(I), a d10-system (particularly in pure gold form as in our case), where linear geometry with coordination number 2 is most favorable. In the force constant model, the Au ion is treated as a deformable ion. The relaxation energy needed to undergo an intermediate interstitial site defines barrier height. By classical mechanics, it can be assumed that relaxation energy determines the barrier height reasonably [41]. Nevertheless, quantum mechanically, Au atom displacements can be easily associated with dynamic coupling of atom movements to the electronic excited states, i.e. Jahn−Teller interactions [41]. This can justify reduction in the energy barrier, allowing Au atoms to diffuse despite their chemical character. Furthermore, Au—being a metal—possesses the natural tendency of self healing to recover from the structural defects induced during atom displacements. Thus, it is expected that dynamic competition between atomic displacement process and atomic annihilation process is overcome by the atomic annihilation process eventually. During this, atom mobility at the Au NP surface is enhanced, and as a consequence structural fluctuations increase. To minimize these structural fluctuations, the unstable (displaced) Au atoms undergo disproportionation to attain the d10 state, which promotes their diffusion across the boundary via in-contact surfaces, thereby enhancing the coalescence process. In Au, due to much heavier atomic mass and strong metal bonding, its sputtering coefficient and dangling bond energy are expected to be very high. Although Au metal undergoes a self mending process through bond restoration as it is exceptionally flexible [30], a direct sputtering (or evaporation) of Au atoms, or its amorphisation, seems not so easy. This clearly indicates its novel passivation effect under e-beam irradiation. It is noteworthy that an ultrafast quenching of the molten Au NP, or cascade effect under ion beam irradiation, can cause its amorphisation; however, at room temperature, e-beam irradiation is unable to amorphise it [45]. (Besides this work, we have irradiated several Au NPs with multiple sizes on various supporting materials under e-beam, and studied the growth processes. More detailed explanation will be given later (manuscripts in progress or submitted) separately, wherein precise evaluation of the Au NPs growth kinetics with modeling describing the pronounced effect of positive curvature and e-beam activated soft modes or lattice instability will be presented). This reveals that the difference between the two structures (MWCNT and Au) is fundamentally crucial to the activation of two dissimilar e-beam irradiation effects in the two structures. The above discussion illustrates that carbon atom evaporation or Au atom diffusion is dependent on the bonding network and energetic states of atoms. In fact, it is easy for the e-beam to activate the evaporation process in MWCNTs, as compared with Au NPs. This shows that activation of soft mode and lattice instability under e-beam irradiation is more pronounced in MWCNTs than in Au NPs. The underlying reasoning could be the lower dangling bond energy or the surface energy of the covalently bonded MWCNT compared with the metal bonded Au NP. The lower dangling bond energy can decrease the melting temperature and mechanical strength of the MWCNT, and thus the tube structure becomes able to accommodate a large fraction of defects (dangling bonds or vacancies). This can also be attributed to the higher surface energy, which generates a strong tendency of shrinkage in the MWCNT structure.
orbital in order to attain square planar geometry, which is unfavorable. The maximum crystal field splitting energy (Δ) of Au(II) makes it highly unstable, and it thus undergoes disproportionation via gaining an electron, forming Au(I), a d10-system (particularly in pure gold form as in our case), where linear geometry with coordination number 2 is most favorable. In the force constant model, the Au ion is treated as a deformable ion. The relaxation energy needed to undergo an intermediate interstitial site defines barrier height. By classical mechanics, it can be assumed that relaxation energy determines the barrier height reasonably [41]. Nevertheless, quantum mechanically, Au atom displacements can be easily associated with dynamic coupling of atom movements to the electronic excited states, i.e. Jahn−Teller interactions [41]. This can justify reduction in the energy barrier, allowing Au atoms to diffuse despite their chemical character. Furthermore, Au—being a metal—possesses the natural tendency of self healing to recover from the structural defects induced during atom displacements. Thus, it is expected that dynamic competition between atomic displacement process and atomic annihilation process is overcome by the atomic annihilation process eventually. During this, atom mobility at the Au NP surface is enhanced, and as a consequence structural fluctuations increase. To minimize these structural fluctuations, the unstable (displaced) Au atoms undergo disproportionation to attain the d10 state, which promotes their diffusion across the boundary via in-contact surfaces, thereby enhancing the coalescence process. In Au, due to much heavier atomic mass and strong metal bonding, its sputtering coefficient and dangling bond energy are expected to be very high. Although Au metal undergoes a self mending process through bond restoration as it is exceptionally flexible [30], a direct sputtering (or evaporation) of Au atoms, or its amorphisation, seems not so easy. This clearly indicates its novel passivation effect under e-beam irradiation. It is noteworthy that an ultrafast quenching of the molten Au NP, or cascade effect under ion beam irradiation, can cause its amorphisation; however, at room temperature, e-beam irradiation is unable to amorphise it [45]. (Besides this work, we have irradiated several Au NPs with multiple sizes on various supporting materials under e-beam, and studied the growth processes. More detailed explanation will be given later (manuscripts in progress or submitted) separately, wherein precise evaluation of the Au NPs growth kinetics with modeling describing the pronounced effect of positive curvature and e-beam activated soft modes or lattice instability will be presented). This reveals that the difference between the two structures (MWCNT and Au) is fundamentally crucial to the activation of two dissimilar e-beam irradiation effects in the two structures. The above discussion illustrates that carbon atom evaporation or Au atom diffusion is dependent on the bonding network and energetic states of atoms. In fact, it is easy for the e-beam to activate the evaporation process in MWCNTs, as compared with Au NPs. This shows that activation of soft mode and lattice instability under e-beam irradiation is more pronounced in MWCNTs than in Au NPs. The underlying reasoning could be the lower dangling bond energy or the surface energy of the covalently bonded MWCNT compared with the metal bonded Au NP. The lower dangling bond energy can decrease the melting temperature and mechanical strength of the MWCNT, and thus the tube structure becomes able to accommodate a large fraction of defects (dangling bonds or vacancies). This can also be attributed to the higher surface energy, which generates a strong tendency of shrinkage in the MWCNT structure.
4. Conclusions
MWCNTs and Au-MWCNTs with almost equal diameters are presented as functional structures to investigate metal passivation effects under e-beam irradiation. The direct triggering of pure carbon atoms from the contiguous tube walls of MWCNT facilitates a faster evaporation, and promotes a faster shrinkage, while Au NPs suppress the predominant evaporation process, thereby restricting Au-MWCNT shrinkage. The new relationship established between shrinking radius and irradiation time in MWCNTs and Au-MWCNTs demonstrates that actual MWCNT shrinkage is faster compared with both theoretical predictions and Au-MWCNTs. in situ TEM investigation allows the following two key points to be put together regarding Au-MWCNTs (or Au NPs): (i) they have lower surface energy; and (ii) they experience weak soft modes or lattice instability effects. The first of these is attributed to the Au NPs' non-uniform surface curvature, and the second is associated with their significantly heavier atomic mass, higher sputtering coefficient and dangling bond energy, and strong metallic bonding. The free movement of Au NPs across the tube surface increases Au NP interaction, and hence their coalescence with e-beam energy transfers. The study offers a new strategy to improve structural stability and functionality of the MWCNT, which is otherwise not a stable structure under e-beam irradiation. Furthermore, MWCNT electronic properties might be changed with the above-described e-beam processing, which could be useful for MWCNT derived electronic devices. The study reflects the underlying physics of thermodynamic non-equilibrium and nonlinear natured surface energy (negative and positive curvature) effects and e-beam activated soft modes or lattice instability effects in one dimensional nanostructures, wherein the classical knock-on mechanism and molecular dynamics simulations are unable to explain these phenomena.
Acknowledgments
We highly acknowledge the support and suggestions for TEM observation by Qiming Hong from electron microscopy centre, Xiamen University. This work was supported by the National Key Research and Development Program of China (2016YFB0400801), 863 program (2014AA032608), National Natural Science Foundation of China (U1405253, 61227009, 90921002), Natural Science Foundation of Fujian Province of China (2016J01265), and Fundamental Research Funds for the Central Universities (20720160015).










