Abstract
Nanomedicine for diagnosis and treatment of disease, as a discipline has been around for several years, with the first nanotherapeutic product being approved in 1995. Worldwide its importance was recognized with the setting up of several nanomedicine centres in 2004–2006. Many of these centres were set up to accelerate the speed of translation of the research. In this article we review, with a broad brush, the progress made in the last 15 years, and examine whether the translation efforts have been successful, and also evaluate whether such successes have changed the medical landscape. Possible reasons for the relatively long time to commercialization for nanomedicine products are also explored.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
In the 2004 National Institute of Health (NIH, USA) Roadmap of the Nanomedicine Intiative (as quoted in [1]), it was envisioned that 'this cutting-edge area of research will begin yielding medical benefits as early as ten years from now', specifically mentioning 'molecular machines' that will change the landscape of medicine. Eight centres were established in 2005 devoted to nanomedicine, with the concept of focusing on basic science in the first five years and on translation in the second five. Although products classified as nanomedicine products have indeed appeared by 2013, such products have not exactly revolutionized treatment paradigms as envisaged earlier. In particular no molecular machine or nanorobot has yet entered clinical trials, although research in these areas is picking up pace.
Nevertheless, it is time to take a look back and perhaps recalibrate our expectations of this technology. Historically, the approval of Doxil as the very first nanotherapeutic product in 1995 is generally regarded as the dawn of nanomedicine for human use. Since then, research activity in this area has been frenetic, with, for example, 2000 patents being generated in 2003, in addition to 1200 papers [2]. In the same time period, a total of 207 companies were involved in developing nanomedicinal products in diagnostics, imaging, drug delivery and implants. About 38 products loosely classified as nanomedicine products were in fact approved by 2004. Out of these, however, a number of products (five in all) were based on PEG-ylated proteins, which strictly speaking, are not so much nanomedicine products as molecular therapeutics. Nevertheless, the promise of nanomedicine was being translated into funding for small companies, and into clinical success, so that by 2013, the number of approved products had reached 54 in all, with another 150 in various stages of clinical trials [3]. The number of companies and institutions had risen to 241 (including research centres that were working on nanomedicine). A PubMed search on articles relating to nanomedicine shows 7400 hits over 10 years, of which 1874 were published in 2013 alone. Similarly, the US patent office database shows 409 patents (since 1976) that were granted in nanomedicine, with another 679 applications awaiting approval. So judging by research activity and funding the field of nanomedicine has been very fertile; however, when we use the yardstick of clinical success and paradigm shifts in treatment, the results appear more modest.
Nanotherapeutics: how far and how much further?
How far have we come in terms of truly revolutionary products in nanotherepeutics? Let us consider oncology as this is by far the most active area targetted by nanomedicine researchers. Doxil works by the principle of so-called passive targeting which uses the nanosize of the chemotherapeutic carrier to both circulate longer in the bloodstream and to reach tumour tissue via (nanosized) pores in leaky blood vessels. There is no doubt that Doxil is a commercial success: sales of liposomal nanomedicine products (which is dominated by Doxil) reached 1.84 billion in 2011 [4] and expected to grow to US$6.52 billion by 2017. Doxil did not revolutionize chemotherapy, but it led to substantially reduced side-effects and to better efficacy of action by increasing the tolerated dose levels. The same is true of other nanocarrier therapeutics, including Abraxane, Ambiosome and nanocrystalline drugs such as Rapamune. Such therapeutics are by and large administered intravenously, and exhibit either improved bioavalibility, longer circulation times and/or reduced side-effects. One product that has the promise to significantly alter clinical outcomes is BIND-014 (figure 1), which is a PLGA-PEG self-assembling nanoparticle system that is functionalized with a ligand targetted at a tumor antigen on prostate cancer cells, and incorporating docetaxel, another taxane derivative. As of this writing, this nanocarrier system is being tested in phase two trials for both prostate cancer and non-small cell lung cancer patients. If clinical outcomes show significantly better maximum tolerated doses and much greater reduction in side-effects (compared to passive targeting), this product would be the first example of successful selective targeting nanomedicine. Previous attempts to use ligands on nanocarriers to selectively target tumour tissue have not proved successful in the clinic.
One way to circumvent the issues relating to long blood lifetimes with intravenous administration is via direct intratumoral injection, utilizing the concept of tumour immunotherapy. In this approach, a nanoparticle (in this instance a protein 'cage' composed of a recombinant 'vault' protein) has been engineered to deliver a chemokine intratumorally to tumour cells; the chemokine then attracts T-cells to the tumour thus initiating tumour destruction [5]. The nanoparticle in this instance provides tumoral retention of the chemokine, as otherwise the injected chemokine would have been cleared within minutes.
The search for truly selective nanomedicine systems that can localize in tumours continues. To date, the use of monoclonal antibodies appears to be the best bet, and how well the antibody can be linked to a drug payload continues to be an important issue.
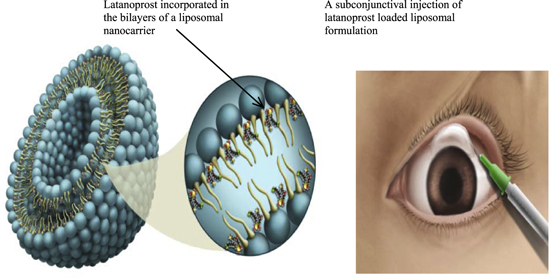
In non-cancer therapies, progress continues to be made. In ophthalmology, the nanosize confers the advantage of less scattering of light by injected nanoparticles (smaller the better), as well as of patient acceptance (less grittiness in the eye). Provided the injectable nanoparticles can sustain the release for a few months and have sufficient drug-carrying capacity (about 10–20% by eight), several ophthalmological conditions may be addressed, including front-of-the-eye diseases (glaucoma) as well as posterior eye segment conditions (macular degeneration). The glaucoma nanomedicine is a liposomal formulation (figure 2) that lowers intra-ocular pressure in patients for at least 3 months with a single injection [6].
The biggest breakthrough in ophthalmology will come from the development of a long-lasting nanomedicinal formulation for treating or indeed reversing angiogenesis in the posterior eye segment, the root cause of at least one type of macular degeneration. Such work is ongoing in several laboratories around the world, including industrial laboratories.
Imaging: nanotechnology has a big role to play
In imaging, nanotechnology is poised to make significant inroads (and bring substantial patient benefits), particularly in cardiovascular medicine. The age-old issue of the causes of atherosclerosis is being addressed with better imaging techniques; in turn this will lead to better treatment options including pre-empting the onset of disease. Currently, the approved nanomedicinal imaging products are based on iron oxide particles for MRI imaging: Ferumoxsil, Feridex, and OMP50, all based on superparamagnetic iron oxide (SPIO) particle of about 300 nm diameter that is ingested orally for image contrast enhancement of liver, bowel tissue and the spleen. It is generally agreed that early identification of the so-called 'vulnerable' atherosclerotic plaque is a critical factor in control of cardiovascular arterial disease progression; constitutents of this plaque include macrophages, endothelial cells, collagen, fibrin as well as markers of angiogenesis following an ischemic event or condition. These targets and some of the work towards the targeting of these tissues/ECM components is reviewed by Lobatto et al [7]. Interestingly, the vascular endothelium around such plaques is also 'leaky', hence one can capitalize on the knowledge gained for passively targeting tumour tissue using nanoparticles; the main difference in this case would be the attachment of an imaging agent. Imaging agents that have been tried to date include nanoliposomes, nanoemulsions, SPIO and CLIO (crosslinked iron oxide NPs) particles. Nanoliposomes are particularly interesting for two reasons: first, their ability to incorporate cholesterol in the bilayer leads to reduced aortic cholesterol and even lesion thickness in hyperlipidemic rabbits [8]. Second, these nanoliposomes can be made to bear ligands such as PEG to enable long blood-circulation lifetimes, thus leading to preferential accumulation in plaques [9] and/or incorporate drugs such as anti-inflammatories thus acting as a theranostic agent. This avenue of research seems poised for human studies, although clinical endpoints may be more difficult to define especially if theranostic considerations are involved.
In vitro diagnostics: earlier, and faster
Finally, some thoughts about in vitro diagnostics, which also has its fair share of nanomedicine product approvals. The 'gold standard' in this segment is the use of gold nanoparticles decorated with nucleic acids, also termed SNAs (spherical nucleic acids). Such particles are approximately 5–15 nm in diameter, and can be functionalized to enable sensitive detection of both nucleic acids and proteins unique to pathogens [10]. This technology has led to the development of microarray detection modules based on simple optical methods such as absorption changes caused by aggregation of the gold nanoparticles [11], trademarked Verigene, and commercialized by Nanosphere. The main advantages of this technology are the short detection times, selectivity of the assays (especially for identification of bacteria) and fairly simple sample preparation usually without the need for extraction and amplification. These advantages are critical in the choice of the appropriate treatment regimen, including the class of antibiotic, which has important ramifications for minimizing bacterial antibiotic resistance as well as for reducing hospital stays by accelerating the treatment time. The use of this technology is not limited to pathogen detection (in blood or in stool samples) but can be extended to sensitive and early detection of biomarkers in cardiovascular disease, for example. Although currently not geared towards point-of-care use, this technology in principle could be developed for disease detection in the field, especially of infectious diseases, so that containment of such diseases is made easier.
Prognosis
At this time, it is evident that the use of nanotechnology in medicine is widespread, and spans in vitro diagnostics, in vivo imaging and injectable therapeutics. Although nanotechnology has not yet led to complete shifts in treatment paradigms, it is beginning to do so. Why has this technology taken so long to bear fruit in medicine? Possibly the uncertainty over the environmental consequences of the use of nanotechnology (in handling during manufacture, for example) is one contributing factor; this has necessitated more elaborate safety testing, and consequently led to a longer pre-clinical phase of development, and thus to higher costs of development [12]. Scaling up of nanomedicine products has also not been straightforward, and has cost time and money. Financing of such projects (with long commercialization times) is subject to a cost-to-benefit analysis, which may have been marginal for many current nanomedicine concepts.
Figure 1. A multi-functional nanocarrier of promise (as shown on the Bind Therapeutics website, www.bindtherapeutics.com/technology/accurins.html; last accessed 31 March 2014).
Download figure:
Standard image High-resolution imageFigure 2. Sub-conjuctival instillation of nanocarriers incorporating latanoprost lowers eye pressure in glaucoma patients for up to three months. Reproduced with permission from [6]. Copyright 2014 by the American Chemical Society.
Download figure:
Standard image High-resolution imageWhat are the major obstacles to be overcome? Given the more stringent safeguards for handling nanoparticles and their perceived toxic effects, I believe that nanotoxicology must take a more central role in product development. Another important advance is to establish sufficient control over drug efflux from nanocarriers, which is not a trivial task. We must also explore routes of administration other than intra-venous injections. Additionally, it is important that truly multidisciplinary research drives the solutions to current medical needs, with active participation from clinician scientists at every stage of the process. Such multidisciplinary nanomedicine centres have been set up worldwide (for example at Northwestern University, University of California at San Diego and Nanyang Technological University, to name a few), and it will be exciting to follow their translational success rates in the future. In the final analysis, only with demonstrable and superior patient benefits, and overall cost reductions in healthcare management, can nanomedicine make bigger inroads.