Abstract
Radiation dose is important in radiotherapy. Too little, and the treatment is not effective, too much causes radiation toxicity. A biochemical measurement of the effect of radiotherapy would be useful in personalisation of this treatment. This study evaluated changes in exhaled breath volatile organic compounds (VOC) associated with radiotherapy with thermal desorption gas chromatography mass-spectrometry followed by data processing and multivariate statistical analysis. Further the feasibility of adopting gas chromatography ion mobility spectrometry for radiotherapy point-of-care breath was assessed. A total of 62 participants provided 240 end-tidal 1 dm3 breath samples before radiotherapy and at 1, 3, and 6 h post-exposure, that were analysed by thermal-desorption/gas-chromatography/quadrupole mass-spectrometry. Data were registered by retention-index and mass-spectra before multivariate statistical analyses identified candidate markers.
A panel of sulfur containing compounds (thio-VOC) were observed to increase in concentration over the 6 h following irradiation. 3-methylthiophene (80 ng.m−3 to 790 ng.m−3) had the lowest abundance while 2-thiophenecarbaldehyde(380 ng.m−3 to 3.85 μg.m−3) the highest; note, exhaled 2-thiophenecarbaldehyde has not been observed previously. The putative tumour metabolite 2,4-dimethyl-1-heptene concentration reduced by an average of 73% over the same time. Statistical scoring based on the signal intensities thio-VOC and 3-methylthiophene appears to reflect individuals' responses to radiation exposure from radiotherapy. The thio-VOC are hypothesised to derive from glutathione and Maillard-based reactions and these are of interest as they are associated with radio-sensitivity. Further studies with continuous monitoring are needed to define the development of the breath biochemistry response to irradiation and to determine the optimum time to monitor breath for radiotherapy markers. Consequently, a single 0.5 cm3 breath-sample gas chromatography-ion mobility approach was evaluated. The calibrated limit of detection for 3-methylthiophene was 10 μg.m−3 with a lower limit of the detector's response estimated to be 210 fg.s−1; the potential for a point-of-care radiation exposure study exists.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Breath analysis as a supporting methodology for personalising treatment across a range of conditions is attractive to clinician and patient alike [1–3]. This work describes the association of exhaled sulphur containing volatile organic compounds (thio-VOC) with radiation exposure. Further, the levels of thio-VOC in exhaled breath in combination with the reduction of the exhaled abundance of 2,4-dimethyl-1-heptene(a previously proposed tumour marker), were studied to establish if there may be a basis for a radio-therapeutic biomarker.
This research arose from a study focussed on the detection of chemical, biological, radiation and nuclear injury associated with a mass casualty emergency [4]. Breath samples taken in a radiotherapy clinic, to verify and characterise previously reported volatile hydrocarbon markers of radiation exposure [5, 6], were unexpectedly found to contain elevated concentrations of five thio-VOC.
Radio-therapy is an important cancer treatment [7] that is accompanied by risk, as a therapeutic radiation dose damages cancer-cells and healthy tissues alike. The severity of damage to healthy tissues and associated acute and late-onset radio-toxicity effects varies between individuals and is difficult to predict [8]. Radio-toxicity is managed by limiting the radiation dose to avoid unsafe and intolerable side-effects; this affects the treatment outcome; the survival rate; and the patient's quality of life [9–11].
Previous studies on the effect of radiotherapy (stereotactic body radio-therapy ranging from 180 to 400 cGy d–1 to 700 to 1200 cGy d–1) on exhaled volatile organic compounds (VOC) n = 31, reported a panel of 15 VOC comprised of methylated-alkanes, alkenes and benzene [5]. This study identified radiation exposures ≥180 cGy with 99% accuracy, and ≥500 cGy with 78% accuracy. The decrease in the accuracy of the classification model from 99%–78% upon increasing the radiation dose was not explored, and this may be due to different radiation doses generating different VOC profiles. Follow on research used whole-body gamma irradiation in the Göttingen Minipig [6], and significant changes in breath concentrations of 58 VOC including alkanes, alkenes and benzene were claimed; apart from benzene, the identity and concentrations of the VOC in these two studies were not reported. Elevated levels of exhaled low molecular weight hydrocarbons have been attributed to lipid peroxidation: ethane following a total-body irradiation treatment for leukaemia and other malignancies [12]; ethene from UV exposure [13]; and, pentane following radiation therapy for lung cancer [14]. The inflammatory marker, nitric oxide, has also been invoked as a marker of radio-toxicity (radiation pneumonitis) following the observation of elevated expired concentrations observed after a radio-therapy treatment for oesophageal cancer [15].
Irradiation of meat (cooked ham [16], sausages [17], turkey-breast rolls [18], and beef [19]) also provides insight into possible radiation markers. The type of meat affects the VOC produced with hydrocarbons, (alkanes, alkenes, aromatic), aldehydes and thio-VOC all reported. These panels of VOC have also been attributed as markers of lipid peroxidation.
Levels of sulfur containing compounds such as glutathione (GSH) are thought to increase in the liver following radiation exposure, providing protection against oxidative stress [20]. GSH is regulated by de novo synthesis or by thioredoxin metabolism [20] and is thought to act by binding to reactive oxygen species, or by reducing hydrogen peroxide as a substrate for GSH peroxidase enzyme [21]. Irradiating a rabbit right hemicerebrum (n = 4) with a dose of 2000 cGy caused peroxide-induced DNA damage, accompanied by a 173% increase in GSH levels, GSH synthesis was proposed as a protective response [22].
Adoption of thio-VOC as alternative markers for assessing radiation exposure has potential operational benefits too, because thio-VOC are amenable to detection and quantitation by gas chromatography-ion mobility spectrometry (GC-IMS), offering sensitive and fast point-of-care detection in the clinic [23].
The aim of the research was to undertake an untargeted metabolomic study, using thermal-desorption/gas-chromatography/mass-spectrometry [24] (TD-GC-MS), to isolate and characterise changes in VOC in breath following radiotherapy. A subsequent consequential aim was to establish the feasibility of using a single-breath point-of-care GC-IMS for continuation studies.
2. Materials and methods
2.1. Breath sample collection for TD-GC-MS analysis
This study was approved by the South East Scotland Research Ethics Committee 01 (16/SS/0059) and all clinical staff were trained, and proficiency tested for breath analysis prior to the start of patient recruitment. The study included a total of 62 participants receiving radiotherapy for: prostate cancer (n = 40, mean age = 68.63 yr, mean radiotherapy dosage = 6000 cGy in 20 fractions); breast cancer (n = 18, mean age = 56.88 yr, mean radiotherapy dosage = 4005 cGy in 15 fractions + 1000 cGy in 4 fractions (boost to tumour bed)); and, lung cancer (n = 4, mean age = 58.25 yr, mean radiotherapy dosage = 5500 cGy in 25 fractions). Each of 62 participants were asked to provide (if possible) a breath sample before radiotherapy, and then at 1, 3, and 6 h post-exposure (240 breath samples with 58 environmental and 55 air-supply samples were acquired). Figures S1 and S2 (available online at stacks.iop.org/JBR/15/016004/mmedia) summarise participant numbers and the subsequent numbers of samples analysed, data-processed and modelled within the study. Breath samples were collected with a respiration collector for in vitro analysis (ReCIVATM) device (Owlstone Medical, Cambridge, UK) supplied with clean air provided from room-air purified with an activated-carbon scrubber and a HEPA filter, at an average flow of 35 dm3 min−1. The air-supply unit was built and tested by the Centre for Analytical Science, Loughborough, UK. End-tidal breath was sampled for 900 s, or for a total sample volume of 1000 cm3 whichever condition was achieved first, onto a Tenax®/Carbotrap 1TD hydrophobic adsorbent tube (Markes International Ltd, Llantrisant, UK). All materials were vacuum and temperature conditioned before use to reduce exogenous VOC artefacts [25]. Training, proficiency tests and protocol checklists were used throughout to ensure compliance with the study protocol. Environmental and air-supply samples were collected with each set of breath samples (if possible). Samples were sealed and chilled to ca. 4 °C immediately after collection, and transported to Loughborough Centre for Analytical Science within 48 h. On receipt all samples were dry-purged with a 120 cm3 of purified nitrogen at a flow rate of 60 cm3 min−1. During the dry-purge a six-port valve was used to load Toluene-D8 (69 pg) and trichloromethane-d (280 pg) internal standards onto thesample tube. All samples were then sealed and stored at 4 °C prior to analysis.
2.2. TD-GC-MS and GC-IMS operating conditions
Samples were analysed with thermal desorption (Unity-2, Markes International) interfaced to a GC (Agilent, 7890 A) coupled to a quadrupole mass spectrometer (Agilent, MS 5977 A), see table S1 for operating details.
A GC-IMS (Breathspec, GAS) was used to evaluate the operational feasibility of monitoring thio-VOC breath biomarkers at point-of-care in the clinic, see table S2 for operating details.
2.3. Chemicals
Calibration/verification standards of 3-methylthiophene (CAS:616-44-4), and 2-thiophenecarbaldehyde (CAS:98-03-3) were obtained (Avocado Research Chemicals Ltd, Lancaster, UK).
2.4. Statistical process control
Continuous instrument condition monitoring was carried out by analysing 0.2 µl of a reference mixture containing 20 standards (table S3) daily before analysis. Multivariate (Principal Component Analysis (PCA) and univariate ( -scores) statistical process control approaches were used to evaluate instrument performance with respect to peak area, height, width, symmetry and signal-to-noise ratio for the 20 standards in the reference mixture. Analysis was undertaken when instruments were within
-scores) statistical process control approaches were used to evaluate instrument performance with respect to peak area, height, width, symmetry and signal-to-noise ratio for the 20 standards in the reference mixture. Analysis was undertaken when instruments were within  = ± 3 for more than 80% of the 100 quality control parameters [26]. Responses from the gaseous internal standards were also monitored continuously to track the combined stability of the TD-GC-MS analysis and dry purging system, see figures S3 and S4.
= ± 3 for more than 80% of the 100 quality control parameters [26]. Responses from the gaseous internal standards were also monitored continuously to track the combined stability of the TD-GC-MS analysis and dry purging system, see figures S3 and S4.
2.5. Data processing & statistical modelling
210 TD-GC-MS data sets obtained from 62 participants including associated environmental and control samples were deconvolved (AnalyzerPro Spectral Works, UK) and 250 to 400 VOC features per sample were recorded. These features were retention index aligned and sorted and grouped using the VOCCluster algorithm [27]. Each feature was assigned an identifier of (BRI -

 ...
...
 ). BRI indicated the retention index for the VOC breath feature and
). BRI indicated the retention index for the VOC breath feature and  ...
...
 are the nominal masses of the ion-fragments in decreasing order of abundance needed to uniquely define the feature. A preliminary untargeted metabolomic study (n = 31 participants) was used to isolate and identify candidate VOC, and a validation pilot study (n = 31 participants) tested the findings from the preliminary study.
are the nominal masses of the ion-fragments in decreasing order of abundance needed to uniquely define the feature. A preliminary untargeted metabolomic study (n = 31 participants) was used to isolate and identify candidate VOC, and a validation pilot study (n = 31 participants) tested the findings from the preliminary study.
Candidate markers were isolated and identified with an eight-step multi-variate analysis data-workflow based on the use of SIMCA-P + software with integrated 7-fold cross validation groups to protect against overfitting (Version 16.1, Umetrics, UK [28]). The work-flow comprised of: removal of rare data; identification and removal of potential environmental contaminants; data-scaling; removal of non-discriminatory features; identification of discriminatory features, compound identification; statistical characterization of candidate markers; and, scoring and classification modelling, see figure S5.
2.5.1. Removal of rare data and potential environmental contaminants for untargeted metabolomics survey
TD-GC-MS data sets obtained from 31 participants with prostate cancer were used and, for the 6 h samples: 1858 features were discarded from modelling as they were present in less than 20% of breath samples; and, 104 features were removed from consideration because they were present in the environmental sample at an abundance greater than 5% of their exhaled level. Ubiquitous siloxane compounds, arising from analytical components, were also removed. The peak areas of all remaining features (n = 206) from the 31 participants in the discovery pilot were normalised to the corresponding peak area value of trichloromethane-d- internal standard (m/z 84, retention index window = 690 to 713) and consolidated into a 'Breath-Matrix' that contained features that could be reliably classed as endogenous.
2.5.2. Scaling, and Removal of non-discriminatory features
All the Breath-Matrix features were log10 transformed and Pareto scaled before multivariate analysis to test for the presence of significant radio-therapy discriminators [28, 29]. Orthogonal partial least squares-discriminant analysis (OPLS-DA) was applied to the data from the 31 participants with prostate cancer to reduce data dimensionality by removing VOC unrelated to group classification. A total of 118 compounds were removed by this step see, figure S5.
2.5.3. Identification of discriminatory features
Unsupervised principle components analysis (PCA) was applied to the remaining 88 features in the breath matrix to indicate candidate VOC that discriminated between pre- and post-exposure, at 6 h post radiotherapy (figure S5). These compounds were identified (See metabolites identification below) and assessed for their suitability for adoption as breath-biomarkers.
2.5.4. Validation pilot study
Four compounds 1-(methylsulfanyl)propane, 1-(methylsulfanyl)-1-propene isomers, 3-methylthiophene and 2,4-dimethyl-1-heptene were identified as potential markers of radiotherapy. Their abundances were aggregated into a score  ; the ratio of the aggregated peak areas of the three thio-VOC (
; the ratio of the aggregated peak areas of the three thio-VOC ( to the peak area for 2,4-dimethyl-1-heptene (
to the peak area for 2,4-dimethyl-1-heptene ( ) (equation (1)).
) (equation (1)).  ; was used in a logistic regression model ('fitglm') with a 5-fold cross-validation. to determine a binary classification between radiated (post 6 h) and non-radiated data (MATLAB, MathWorks, USA) (equation (2)). Untargeted metabolomics survey data was used for the training set and the candidate marker data from the validation pilot study used for the testing set.
; was used in a logistic regression model ('fitglm') with a 5-fold cross-validation. to determine a binary classification between radiated (post 6 h) and non-radiated data (MATLAB, MathWorks, USA) (equation (2)). Untargeted metabolomics survey data was used for the training set and the candidate marker data from the validation pilot study used for the testing set.
Equation (1).
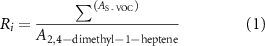
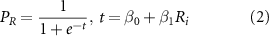
Generalized linear regression model = fitglm(X, Y, 'linear','Distribution', 'binomial') [30]
Estimated coefficients:
| Estimate | Standard Error | tStat | pValue | |
|---|---|---|---|---|

| 2.2939 | 0.7237 | 3.1696 | 0.0015 |

| −0.3505 | 0.1136 | −3.0848 | 0.0020 |
Here  is the probability of radiation exposure and
is the probability of radiation exposure and  and
and  are the logistic regression parameters. The accuracy of the class boundary was assessed using the area under a receiver operator characteristic curve (AUROC).
are the logistic regression parameters. The accuracy of the class boundary was assessed using the area under a receiver operator characteristic curve (AUROC).
2.6. Metabolites identification
The Metabolomics Standards Initiative was adopted [31] and 3-methylthiophene and 2-thiophenecarboxaldehyde were identified by obtaining the retention indices and mass spectra of pure reference standards along with their calibration data (Level 1 identification, Supplementary document Figure S6). Other putative Level 2 identities were based on retention-index and mass-spectral library matches.
3. Results
3.1. VOC profiles and candidates for identification of radio-therapy response
A total of 240 breath, 58 environmental and 55 air supply (control) samples were obtained from 62 participants. For the first 31 participants (pilot set) the number of VOC isolated and placed in the breath matrices for 1, 3, and 6 h post-radiation exposure were 230, 278 and 207 respectively (Not all of the participants were able to provide breath samples at all the time points, figures S1 and S2 in supplementary data document). Table 1 lists the discriminating VOC that were identified at Level 2 or Level 1 from the prostate multi-variate PCA analysis (figure 1 shows the PCA analysis for 3 and 6 h post radiotherapy, and figure S5 shows the OPLS-DA supervised modelling analysis and data modelling workflow). There were 188 other compounds that were difficult to identify at Level 2 and the study identifiers are listed in table S4. which is accompanied by a spreadsheet containing all of the breath matrices for the 1, 3 and 6 h post-radiation therapy.
Figure 1. Pre-radiation (blue squares), post-radiation (red triangles) unsupervised PCA scoring plots for prostate cancer patients. Left PCA (pareto scaling, 3 principle components) and dendrogram plots of prior to radiation (C, red triangles) and post 3 h exposure (R-3. Right PCA-(pareto scaling, 5 principle components) and dendrogram plots for post 6 h exposure (R-6). Note there is some misclassification associated with participants with low thio-VOC scores, see Discussion, and figure 5. For pre-radiation and 1 h post radiation exposure modelling is shown in supplementary data figure S5, which also shows workflow steps in the data modelling starting from supervised OPLS discriminant analysis for noise reduction using the S-plot (unrelated to group classification), to unsupervised PCA modelling and loading scores plot.
Download figure:
Standard image High-resolution imageTable 1. A List of radiation associated VOC, identified at Level 2 (Library spectrum match and retention index match), at 1 h, 3 h and 6 h. CAS number per each compound presented in brackets. (o Indicates a compound identified against a reference standard, Level 1 identification).
| 1 h post radiation | 3 h post radiation | 6 h post radiation |
|---|---|---|
| 3-methylthiopheneo (616-44-4) | ||
| 1-(methylsulfanyl)propane (3877-15-4) | ||
| (1Z)-1-(methylsulfanyl)-1-propene (52195-40-1) and/or (E)-1-(methylsulfanyl)-1-propene (42848-06-6) | ||
| dimethylsulfone (67-71-0) | ||
| 2-thiophenecarbaldehydeo (98-03-3) | ||
| 2,4-dimethyl-1-heptene (19549-87-2) | 2,4-dimethyl-1-heptene (19549-87-2) | 2,4-dimethyl-1-heptene 19549-87-2) |
| cyclohexanone (108-94-1) | cyclohexanone (108-94-1) | cyclohexanone (108-94-1) |
| 3-furaldehyde (498-60-2) | ||
| 2-methylfuran (534-22-5) | 2-methylfuran (534-22-5) | |
| ethanoic acid (64-19-7) | ethanoic acid (64-19-7) | |
| 3-buten-2-one (78-94-4) | 3-buten-2-one (78-94-4) | |
| 2-propanone (67-64-1) | ||
| 2(5 H)-furanone (497-23-4) | 2(5 H)-furanone (497-23-4) | |
| 1-ethylcyclohexene, (1453-24-3) | ||
| 2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane (79-92-5) | ||
| 1-methyl-4-methylethenylcyclohexene (138-86-3) | ||
| (1 R,5 R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (80-56-8) and/or 6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane (127-91-3) | ||
| 33 x branched hydrocarbons | 8 x branched hydrocarbons | 32 x branched hydrocarbons |
Note:provisional identification of the hydrocarbons was limited by the resolution of the mass spectrometry and reliability of NIST library search matches, a list of the breath matrix entries may be found in table S4 [32].
The exhaled profiles changed over the 6 h of the study with different VOC panels discriminating between the pre- and post-radiation states at 1, 3 and 6 h. This was not surprising for the exhaled VOC was envisioned to reflect: the development of a clinical exposome; hunger and ketosis; the consumption of drinks and snacks; the washout of radiolysis products; the cascade of VOC associated with the progression of a radiation injury from the initial targeted site; and, systemic catabolism of radiation injury products. The data from the 6 h samples was selected for more detailed study as they showed the greatest differentiation from pre-radiation samples and it was also considered that the 6 h time point was most likely to be representative of a fully developed radiation VOC panel for all the participants.
Exhaled VOC from radiated participants may be classified with non-supervised PCA, and like previous studies, methylated alkanes appear to be associated with radiotherapy exposure. Methylated hydrocarbons have been associated with reactive oxidative stress (ROS), and ionising radiation increases free-radical ROS production causing oxidative damage that may spread to neighbouring cells activating protective metabolic processes [33, 34]. The radiation induced in vivo formation kinetics of methylated hydrocarbons, and their subsequent washout, has yet to be described, and such a study would be confounded by exogenous sources of hydrocarbons. Other challenges associated with the adoption of methylated hydrocarbons as radiation biomarkers derive from the difficulty in establishing an unambiguous identity, particularly in the presence of straight chain isomers (c.f. the mass spectra and retention indexes of octane and 2,4-dimethyl heptane [32]). An additional factor in selecting a target molecule as a radiation exposure marker is the ease with which it may be detected, at low concentrations, using portable instruments; alkanes are problematic in this regard.
Seven other compounds identified at the 6 h end point as potentially discriminating were excluded from further study as it was possible that they had arisen from exogenous sources, or as a consequence of the study design. Cyclohexanone is an industrial solvent (it is also a systemic inflammatory marker [35]). Methyl furan is a fragrance/flavour ingredient, and acetic acid is a food ingredient as likely to come from a packet of potato chips in this study as an endogenous process. 2-propanone is ubiquitous and was attributed to hunger and appetite/ketosis; this is likely identifying the 6 h duration of the study. 2(5H)-furanone is a flavour component of a wide range of foodstuffs including confectionary and baked goods. 2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane (camphene), (1R,5R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (pinene) and/or 6,6-dimethyl-2-methylenebicyclo [3.1.1] heptane (beta-pinene) have extensive exogenous sources in a clinical/healthcare facility.
Surprisingly, five exhaled thio-VOC were observed at elevated levels that could not be attributed to an exogenous source; and thio-VOC have been reported previously to be associated with radiation [36]. It should also be noted that the instrumentation and components were not selected for sulfur inertness. Consequently, some surface adsorption artefacts were evident at the lowest concentrations used in the calibrations, and as such adsorption artefacts may be assumed to have supressed thio-VOC signals for the lowest intensity features.
Dimethylsulfone, and 2-thiophenecarbaldehyde were observed after 6 h. 2-thiophenecarboxaldehyde has not been previously identified in human breath [37, 38], however, 2-thiophenecarbaldehyde was excluded from further study as it was observed in less than 20% of breath samples. Dimethylsulfone was observed in 36 participants however, the differences in abundance at pre and post exposure were not statistically significant at 6 h post exposure and therefore dimethylsulfone was excluded from further study.
42 (out of the 48 participants that provided samples at 6 h, figure S1) had detectable levels of 1-(methylsulfanyl)propane in their breath, and 32 out of these 42 participants had an increase in abundance following radiation treatment. A total of 12 participants had detectable levels of 1-(methylsulfanyl)propane only after irradiation had occurred After 6 h the average change across the cohort was a 3-fold increase with a t-value statistic of 4.20 with a one-tailed critical t-value of 1.67, p = 4.08 × 10−5 with 66 degrees of freedom.
(1Z)-1-(methylsulfanyl)-1-propene and (E)-1-(methylsulfanyl)-1-propene were also present in the 6 h post radiotherapy exhaled breath at elevated levels. The retention index for (1Z)-1-(methylsulfanyl)-1-propene was found to be 753, while the retention index of (1E)-1-(methylsulfanyl)-1-propene was found to be 754. The mass spectra obtained for the two isomers are essentially the same on the quadrupole instrument used. In the absence of further verification of correct isomer classification, the abundances for these two isomers were combined and recorded as a single thio-VOC entity.
46 participants had detectable levels of exhaled 1-(methylsulfanyl)-1-propene isomers that increased in abundance in 32 of them following exposure to radiation. 15 participants had detectable levels of 1-(methylsulfanyl)-1-propene isomers only after radiation had occurred After 6 h the average change in exhaled abundance across the cohort was 2.4-fold with a t-value statistic of 3.28 with a one-tailed critical t-value of 1.66, p = 0.0007 with 89 degrees of freedom.
3-methylthiophene was detected in the breath of 44 participants and 22 of the participants had elevated levels of 3-methylthiophene following exposure to radiation. 11 participants had detectable levels of 1-(methylsulfanyl)propane only after radiation had occurred. The 3-methylthiophene was significantly elevated at 3 h, however, after 6 h the average fold-change across the cohort was a 1.2-fold increase, and this difference was not statistically significant across the whole cohort.
Pure standards for 3-methylthiophene and 2-thiophenecarbaldehyde were commercially available and these were used to verify their identification and calibrate the instruments enabling the limits of detection to be estimated (following European Medicines Agency International Conference on Harmonization Tripartite Guidelines [39]) as 120 pg and 110 pg on-column masses respectively (supplementary data, figure S6).
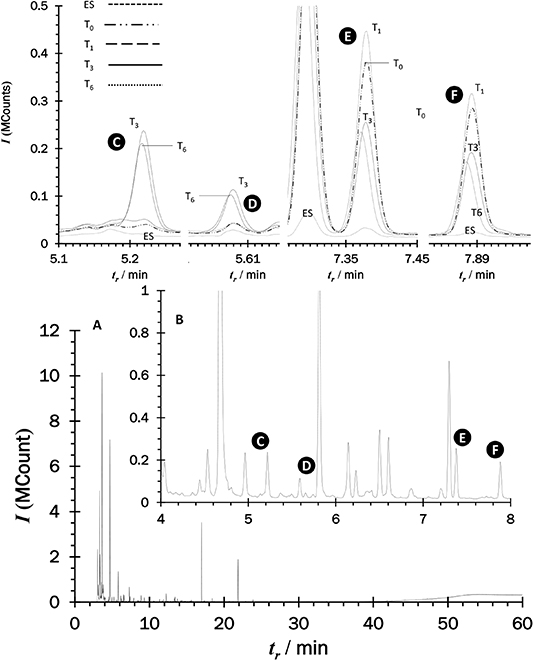
Their concentration ranges observed in breath samples were between 80 ng m−3 and 790 ng m−3 for 3-methylthiophene and 380 ng m−3 and 3.85 μg m−3 for 2-thiophenecarbaldehyde, the responses obtained for 1-(methylsulfanyl)propane and 1-(methylsulfanyl)-1-propene isomers, were comparable; indicative of similar concentrations. Figure 2 is an overlaid set of chromatograms from a participant that highlight the nature and scale of the radiation associated changes in the VOC profiles observed over 6 h following irradiation.
Figure 2. TD-GC-MS features from clinical breath samples taken before and after radiation exposure at 1, 3 and 6 h. (A) full chromatogram, (B) close-up of A identifying: (C) 1-(methylsulfanyl)propane; (D) 1-(methylsulfanyl)-1-propene; (E) 2,4-dimethyl-1-heptene and (F) 2,4-dimethyl-heptane, prior to radiation (T0) and post-radiation therapy at 1 (T1), 3 (T3) and 6 (T6) hour as well as an environmental sample (ES).
Download figure:
Standard image High-resolution image2,4-dimethyl-1-heptene (Level 2 identification [31]) was found to differentiate post radiation breath at 1 h, 3 h and 6 h from pre-radiation breath. 2,4-dimethyl-1-heptene has been reported to increase in concentration in cancer cell lines, and has an association with cancer cell metabolism [40]. A total of 48 out of the 62 participants were able to provide a 6 h post-exposure breath sample and the mean reduction in exhaled abundance was 72%, with a t-value statistic of 5.6 with a one -tailed critical t-value of 1.68, p = 2.19 × 10−7, n = 48 with 67 degrees of freedom. In seven participants, the level of 2,4-dimethyl-1-heptene fell below detectable levels. The question of whether this is a general radiation exposure/ROS response, or a marker of radiation damage to a tumour is one to be addressed in future work.
The reduction in the abundance of 2,4-dimethyl-1-heptene following irradiation was marked. This could be due to tumour damage and as such a marker of treatment efficacy, or it could be a generalised marker for radiation exposure. The incorporation of the thio-VOC and 2,4-dimethyl-1-heptene ratios provides a radiation exposure classification approach for this cohort and by implication people with cancer receiving radiotherapy. The average calculated  -value of all the data (both discovery and validation pilots) was 3.71 at 0 h and 54.37 at 6 h with a t-value statistic 3.21 with a one -tailed critical t-value of 1.68, p = 0.001 n = 48 with 47 degrees of freedom). The area under the receiver operator characteristic (AUROC) value for the training (discovery) and testing (validation) sets were 0.91 and 0.75, respectively (figure 3).
-value of all the data (both discovery and validation pilots) was 3.71 at 0 h and 54.37 at 6 h with a t-value statistic 3.21 with a one -tailed critical t-value of 1.68, p = 0.001 n = 48 with 47 degrees of freedom). The area under the receiver operator characteristic (AUROC) value for the training (discovery) and testing (validation) sets were 0.91 and 0.75, respectively (figure 3).
Figure 3. The ratio  score calculated using equation (1) enabled a benchmark assessment of radiation exposure as shown in the box plot above for 0 and 6 HR post-radiotherapy. This may either be a combination of radio-sensitivity and therapeutic efficacy, or a candidate for a generalised radiation exposure marker. The AUROC (highlighted with blue) for the logistic regression model with five folds cross-validation for the training (ROC curve presented in this figure) and testing data were 0.91 and 0.75, respectively. Classifier accuracy for the training set was calculated using the optimum point (classifier point) and it was found to be 84.1% and the classifier point (0.09, 0.77) is also shown (orange circle) on the ROC curve above.
score calculated using equation (1) enabled a benchmark assessment of radiation exposure as shown in the box plot above for 0 and 6 HR post-radiotherapy. This may either be a combination of radio-sensitivity and therapeutic efficacy, or a candidate for a generalised radiation exposure marker. The AUROC (highlighted with blue) for the logistic regression model with five folds cross-validation for the training (ROC curve presented in this figure) and testing data were 0.91 and 0.75, respectively. Classifier accuracy for the training set was calculated using the optimum point (classifier point) and it was found to be 84.1% and the classifier point (0.09, 0.77) is also shown (orange circle) on the ROC curve above.
Download figure:
Standard image High-resolution image3.2. Evaluation of GC-IMS for point-of-care sampling and analysis
Further studies into the verification, development and biokinetics of thio-VOC production after radiotherapy will require point-of-care monitoring and GC-IMS is a candidate technology for this role. Details about GC-IMS and potential applications in different clinical settings are available in the book 'Ion Mobility Spectrometry' [23] and a recent review [41]. A single breath GC-IMS approach has recently been benchmarked following the 'Peppermint Protocol' [42] and this methodology was assessed for at-patient exhaled thio-VOC determination. The limit of the GC-IMS detector's response was estimated to be 210 fg s−1. TD-GC-MS generates a 104 enrichment from a 1 dm3 breath sample while the GC-IMS does not use an enrichment stage with an injected volume of 0.5 cm3 sample of end-tidal breath. Assuming a Gaussian elution profile with a 6 s peak width concentrations of 10 μg m−3 may be detected currently; with some evidence that sulfur-surface interactions inhibit detection at lower levels with the existing instrument configuration and method [39], figure 4.
Figure 4. GC-IMS calibration for 3-methylthiophene protonated monomer. Top Left: normalised responses to a 81 pg injection, used to acquire instrument calibration showing projection of IMS spectrum and chromatographic peak. Top right: method calibration from 0.5 cm3 injection volume, indicating a current limit of detection of 10 μg m−3; non-linear sulfur-interaction artefacts are apparent at low concentrations (negative y-axis intercept), curtailing the lower end of the linear dynamic range to an on-column mass of 10 pg. Bottom: instrument calibrations from the deconstructed chromatographic peak [38] indicating that the detector ceases to respond reliably to mass fluxes of less than 210 fg s−1.
Download figure:
Standard image High-resolution imageFurther refinement and optimisation of sample volume and sampling materials/handling along with chromatography parameters are likely to be sufficient to deliver the analytical performance needed. Using a single breath sampling approach is likely to be tolerable for most radiotherapy patients during an extended breath sampling study to detect and monitor thio-VOC profiles during treatment.
4. Discussion
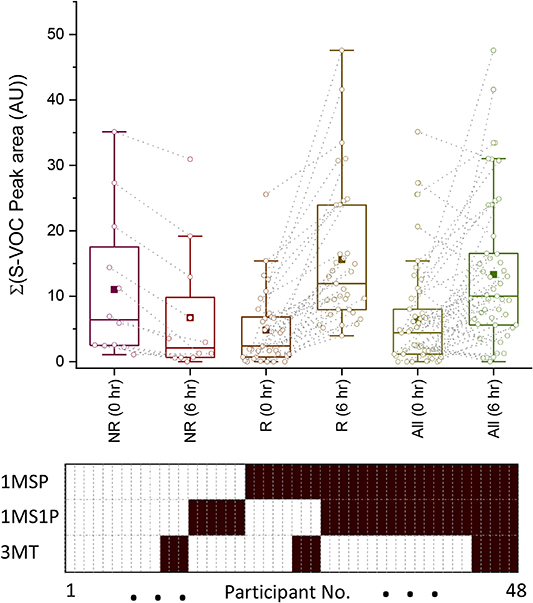
A study of the aggregated thio-VOC responses for each participant revealed a pattern of thio-responders and non-thio-responders following radiation exposure, figure 5.
Figure 5. Comparison of thio-non-responders (NR) verses thio-responders (R) for elevated levels of 1-(methylsulfanyl)propane (1MSP), 1-(methylsulfanyl)-1-propene isomers (1MS1P), and 3-methylthiophene (3MT). Top: aggregated peak areas at 0 h and 6 h after radiation exposure. NR did not show a significant change in abundance in S-VOC levels at 6 h, while R had an average 3.2-fold increase (t-value statistic of 5.16 with a one-tailed critical t-value of 1.67, p = 2 × 10−6 with 52 degrees of freedom. The whole cohort had an average 2.03-fold increase (t-value statistic of 2.55 with a one-tailed critical t-value of 1.66, p = 0.006 with 94 degrees of freedom Bottom Panel showing the distribution of elevated levels at 6 h post radiation for 1MSP, 1MS1P and 3MT across the 48 participants where a solid cell indicates a participant had elevated thio-VOC at 6 h.
Download figure:
Standard image High-resolution imageParticipants either had a significant increase in the aggregated exhaled levels of thio-VOC 6 h after irradiation (thio-responders) or exhibited no significant change (thio-non-responders). The panel at the bottom of figure 5 shows the distribution of thio-responses across 48 participants (see figure S2) for the three thio-VOC included in the panel, and the box plot in figure 4 summarises the changes in thio-VOC levels observed at 6 h.
GSH reacts with glucose to produce Maillard-type volatile products including furans, carbonyl and sulfur-containing compounds such as thiophenes [43]. The discovery of an thio-VOC panel suggests a GSH/Maillard mechanism for radiation markers in breath. A concentration rise in exhaled thio-VOC may indicate radiation damage, and consequently a radiotherapy effect for an individual receiving treatment. For the patient receiving radiotherapy a non-response could suggest hypoxia and a radiotherapy resistance. An alternative hypothesis is that elevated thio-VOC levels are a measure of a systemic hepatic-protective response to an ROS/inflammatory insult.
The dynamic development of an exhaled VOC panel following radiotherapy raises further questions: If the VOC panel develops over time then when is the best time to sample breath to assess changes in breath bio-chemistry due to radio-therapy and/or radio-toxicity responses? Are changes in thio-VOC exhaled concentration dose related, or do different doses engender the formation of different thio-VOC species? and is the engagement of a GSH response specific to a radiation insult or, as has been noted above, a more general systemic hepatic-protective response? Note that this study cohort was not stratified for GSH levels, and questions about the relationship between GSH and the types and levels of thio-VOC in breath following radiotherapy with a range of radiation doses remain to be answered.
Arriving at the answers to such questions needs significantly more breath samples, and at asignificantly higher frequency, than was ethically and practically feasible in this study. The preparatory work reported above with GC-IMS sought to address this requirement.
5. Conclusion
These observations are encouraging for a test to support personalised radiation therapy would enable the balance between therapeutic and radio-toxic effects to be better managed. In this study, the combination of a previously reported volatile tumour metabolite with a panel of thio-VOC appears to offer a potential biomarker for therapeutic radiation exposure at levels between 4005 cGy and 6000 cGy Ideally, classification of radio-therapy exposure needs to be based on readily detectable biomarkers that are associated specifically with radio-sensitivity, and the compounds identified during this study appear to have these characteristics.
A previously unreported thio-VOC in breath, 2-thiophenecarbaldehyde has been identified. The thio-VOC discriminators were part of a larger radio-sensitive panel of VOC that includes methylated alkanes, alkenes, ketones, furans. Putative identities have been proposed (Level 2), and previously reported observations of hydrocarbons as candidate radiation markers have been repeated.
The reduction in exhaled concentration of a previously reported tumour metabolite, 2,4-dimethyl-1-heptene, following radiotherapy raises the potential of non-invasive assessment of: the whole-body tumour activity; the surviving fraction following a radiotherapy session; and hence the efficacy of the therapy. Combining a thio-VOC panel with 2,4-dimethyl-1-heptene appears to offer the prospect of a measure of a radio-therapeutic effect. The extension of this approach to a larger cohort with a wider range of radio-therapy targets combined with radio-toxicity follow through will be a logical continuation of this research; supported with companion in vitro studies. The end-point of such research would include a description of the relationship, if any, between radio-toxicity in individual participants undergoing radio-therapy and the levels of thio-VOC in their breath. To this end the adoption of at-patient single breath sample GC-IMS based analysis has been evaluated and appears to be of sufficient analytical sensitivity to support adoption, adaption and optimisation for use in further studies. The GC-IMS method evaluated in this study appears to be a practicable proposition for undertaking continuation studies in clinic.
Acknowledgments
This study was part of an interdisciplinary European project TOXI-triage, which aims to characterise of radiation biomarkers in exhaled breath for mass casualty triage.
The authors of this manuscript gratefully acknowledge: European Union funding for the TOXI-triage project (H2020 funded project 653409), the clinical research nurses, radio-therapy staff and cancer patients of Edinburgh General Western Hospital for their help with obtaining the clinical data sets; G.A.S. Gesellschaft für analytische Sensorsysteme GAS for support with the GC-IMS; and, SpectralWorks limited for support for the AnalyzerPro software used to deconvolve the clinical breath data used in this study; Andrea Soltoggio, Department of Computer Science, Loughborough University, UK for supervisory support to Yaser Alkhalifah and, Melissa Pearson, Pharmacology, Toxicology & Therapeutics Unit, University of Edinburgh who was the clinical study manager for our research. Table S5 summarises the contributions of the authors to this study.