Abstract
This review paper summarizes current understanding of the transport of organic carbon to, and the fate of organic carbon within, the East Siberian Arctic Shelf (ESAS), and of processes determining carbon dioxide (CO2) and methane (CH4) fluxes from the ESAS to the atmosphere achieved from analyzing the data sets obtained on 20 expeditions performed from 1999 to 2011. This study of the ESAS was aimed at investigating how redistribution of old carbon from degrading terrestrial and sub-sea permafrost and from coastal erosion contributes to the carbon pool of the ESAS, how changes in the hydrological cycle of the surrounding land and alteration of terrestrial carbon cycles affect the hydrological and biogeochemical parameters of shelf water masses, and which factors control CH4 and CO2 emissions from the ESAS. This report describes selected results achieved by a developing international scientific partnership that has been crucial at every stage of the study and will be even more important in the future.
Export citation and abstract BibTeX RIS
1. Introduction
The Arctic Ocean is surrounded by permafrost, which is being degraded at an increasing rate under conditions of warming which are most pronounced in Siberia and Alaska (ACIA 2004). The Arctic region contains a huge amount of organic carbon (OC) buried inland and within the Arctic Ocean sedimentary basin ('Arctic carbon hyper pool', Gramberg et al 1983), which could become involved in current biogeochemical cycling due to thawing of on-land and sub-sea permafrost (defined by the International Permafrost Association as ground that remains at 0 °C or below for at least two consecutive years), increasing coastal and bottom erosion, accelerating river discharge and carbon losses from soils (Semiletov et al 2000, Freeman et al 2001). Thaw and release of OC from Arctic permafrost is postulated to be one of the most powerful mechanisms causing a net redistribution of carbon from land and ocean to the atmosphere (Kelley and Gosink 1979, Oechel et al 1993, Zimov et al 1993, 1996, 1997, Semiletov 1999a, 1999b, ACIA 2004, Gruber et al 2004, IPCC 2007, Canadell and Raupach 2009). Determining the magnitude of particulate and dissolved fluxes of modern and old OC and other terrestrial material from land is critical to constraining a range of issues in the Arctic shelf-basin system, including carbon cycling, the health of the ecosystem, and the interpretation of sediment records. More than 90% of all OC burial in the Arctic Ocean occurs in sediments deposited on deltas, continental shelves, and upper continental slopes, of which the East Siberian Arctic shelf (ESAS) represents a significant fraction (Gramberg et al 1983, Vetrov and Romankevich 2004).
The shallow ESAS is a unique area of the World Ocean where ∼80% of predicted sub-sea permafrost exists (figure 1) (ACIA 2004). Sub-sea permafrost is much more vulnerable than its terrestrial counterpart, because it experiences a drastic change in its thermal regime due to inundation by the ocean and consequent warming by as much as 12–17 °C, prior to the current ongoing climate change (Soloviev et al 1987). It was therefore hypothesized that combined bottom-up geothermal and top-down seawater heat fluxes, possibly accelerated by amplified Arctic warming, have led to partial destabilization of sub-sea permafrost, causing methane (CH4) production within taliks (a term describing a thaw column or a thaw bulb of sediments within the permafrost) and release from seabed deposits that vent extensively to the Arctic atmosphere (Shakhova et al 2010a, 2010b, 2010c, Nicolsky and Shakhova 2010).
Figure 1. (a) Distribution of Arctic Ocean sub-sea permafrost (shown in purple); (b) springtime surface air temperature anomalies for 2000–5 relative to a 1968–96 base period (panels (a) and (b) modified from figure S1 of Shakhova et al (2010c)4); (c) predicted deposits of Arctic Ocean CH4 hydrates (shown in blue); (d) bathymetric map of the Arctic Ocean (depth ≤ 50 m shown in shades of red) (panels (c) and (d) modified from figure 1 of Shakhova et al (2010b)5).
Download figure:
Standard imageBesides mobilization of old OC stored in sub-sea permafrost, there are terrestrial sources of OC delivered to the ESAS. It is highly likely that the Great Siberian Arctic Rivers (GSARs; Ob, Yenisey, Lena, Kolyma and Indigirka rivers) carry and bring to the shelf an integrated signal of terrestrial organic material (OM) released from the soil and the degrading terrestrial permafrost within their vast watersheds (Guo et al 2004, Dudarev et al 2006a, Alling et al 2010, Sánchez-García et al 2011). The ESAS acts as a Lena, Kolyma and Indigirka estuary; thus, it accepts the integrated signal and transfers it further via the signal carrier, which is shelf water, to the Arctic Ocean (Semiletov et al 2000, 2007, 2011, van Dongen et al 2008, Pipko et al 2010, 2011, Vonk et al 2010, Charkin et al 2011, Gustafsson et al 2011, Karlsson et al 2011). Another significant source of terrestrial carbon delivered to the ESAS is coastal erosion; on the shores of the ESAS the rates of erosion are the greatest on the globe and can reach up to 80 m yr−1 (figure 2; Grigoriev 1993, Are 1999, Rachold et al 2000). The latest estimate produced a value of 4 Tg C-OC released from the coastal ice complex into the ocean annually (Grigoriev et al 2006).
Figure 2. Process of coastal erosion as it is seen on the northern cape of Muostakh Island (September 2006, photo by I P Semiletov). Rates of coastal erosion can be as high as 20–30 m in two weeks.
Download figure:
Standard imageResults of initial offshore studies, performed in the 1990s, demonstrated that the biogeochemical consequences of terrestrial OM transformations within the ESAS could play a significant role in the Arctic marine carbon cycle (Semiletov et al 1994, 1996a, 1996b, Semiletov 1999a, 1999b). Further studies revealed that the eroded terrestrial OM is available for microbial oxidation; this oxidation is followed by a significant carbon dioxide (CO2) buildup in the water column, and consequent CO2 release into the atmosphere (Pipko et al 2005, 2008, 2011, Repina et al 2007, Semiletov and Pipko 2007, Semiletov et al 2007, Anderson et al 2009, 2011). Partitioning between the riverine and eroded terrestrial OM exported onto the shelf is still under discussion (Rachold et al 2000, Dudarev et al 2003, 2006b, Vetrov and Romankevich 2004, Semiletov et al 2005, 2011, Macdonald et al 2008, Vetrov et al 2008, Vonk et al 2010, Charkin et al 2011, Gustafsson et al 2011). Results presented in the current letter serve to contribute to this discussion.
Numerous important and newly emerging problems with potential influence on climate change arise from land–shelf interaction in the ESAS. Addressing these problems requires long-term international cooperation between Russia, to whom the ESAS belongs, and other countries. This brief overview describes the state of progress in establishing such cooperation by reporting results of field research accomplished during 1999–2011 in the ESAS. The objectives of this study were pursued in order to identify and quantify the main processes responsible for carbon cycling in the ESAS land–shelf–atmosphere system and factors altering them. These objectives included the following.
- To investigate how redistribution of old carbon from degrading terrestrial and sub-sea permafrost and from coastal erosion contributes to the carbon pool of the ESAS.
- To study how changes in the hydrological cycle of the surrounding land and alteration of terrestrial carbon cycles contribute to formation and propagation of halocline waters and affect the hydrological and biogeochemical parameters of shelf water masses, and to quantify the area-scaled ESAS contribution of CH4 and CO2 to the atmosphere.
- To define specific factors which control CH4 emission to the ESAS atmosphere in order to develop a conceptual model of CH4 propagation from the seabed to the atmosphere, and to assess the strength, type, and dynamics of the source.
2. Fieldwork and research platforms
The location of the main ESAS studies conducted from 2003 to 2009 is shown in figure 3. Vessels of different sizes, from small 15 m boats to mid-size ∼70 m hydrographic vessels and heavy diesel ice-breakers, were employed for the summer–fall work. The most comprehensive field research was accomplished during the 2008 International Siberian Shelf Study (ISSS) expedition. This cruise was initiated to alleviate the scarcity of observational data describing the transport and processing of water, sediment and carbon in the ESAS (Semiletov and Gustafsson 2009). The interlinked research themes such as biogeochemistry, geophysics/seismicity, marine chemistry, CH4 dynamics, physical oceanography and sedimentology guided research onboard the ice-strengthened hydrographic vessel Yakov Smirnitsky and two small vessels, the TB 0012 and the Orlan (Semiletov and Gustafsson 2009).
Figure 3. Location of the ISSS (2003–9). More than 1100 hydrological stations, more than 700 sediment coring and grab sites, and four coastal erosion study areas were investigated (2003–7, white circles; 2008, yellow triangles; Nansen and Amundsen Basin Observational System (NABOS) 2009, magenta circles; TB-012 2009, red circles).
Download figure:
Standard imageAdditional biogeochemical work was done onboard the commercial ice-breaker Kapitan Dranytsin in cooperation with the Nansen and Amundsen Basin Observational System (NABOS) project (www.iarc.uaf.edu/nabos) and onboard the passenger vessel Moskovsky-11, which was utilized for water sampling and taking onboard measurements from the mid-stream to the mouth of the Lena River (for details see Semiletov and Gustafsson 2009). In the winters of 2002, 2007 and 2011 we used a transport caravan and cross-country vehicles provided by Tiksi Hydrobase of the Northern Sea Route service. Both summer and winter helicopter surveys to study the short-term spatial–temporal variability and vertical distribution of the three main greenhouse gases (CH4, CO2 and water vapor) and the thickness of the meteorological boundary layer were conducted in 2006, 2010 and 2011. More than 20 expeditions were accomplished during the last 10 years.
Since 1999 the parameters of the carbonate system have been measured onboard; since 1999 stable C, N, O and H isotopes in the particulate OC (POC) and sediment OM have been measured in the lab. From summer 1999 to summer 2002 we utilized techniques and equipment described in detail in Semiletov et al (1996a, 1996b, 2005, 2007), Semiletov (1999b), Pipko et al (2002, 2011). Since summer 2003 our field activities (figure 3) have included making 'traditional' measurements such as in situ temperature, conductivity (salinity), colored dissolved OM, turbidity and photosynthetically active radiation; these parameters were measured using the Seabird SBE 19plus Profiler. Utilizing the same instruments and analyses across years improves our understanding of linkages between various physical and biogeochemical processes and their inter-annual variability in the Arctic land–shelf system, which is an important objective for the ESAS carbon study. All the methods have been described in detail in dedicated papers cited throughout this letter.
3. Selected results
3.1. Western and eastern geochemical provinces of the ESAS
A notable characteristic of the ESAS is large gradients of hydrological and biogeochemical parameters that correspond to geographically critical contrasts in the Arctic system where the Pacific and local shelf waters interact over the shelf. That is reflected in the sedimentation regime (Semiletov et al 2000, 2005, van Dongen et al 2008, Gustafsson et al 2011).
The distribution of δ13C-OC in the surface bottom sediments was used to distinguish between the two biogeochemical provinces, western and eastern, in the ESAS (Semiletov et al 2005). This approach was later confirmed with more detailed considerations using biomarkers, isotopes, and C/N ratios (Vetrov et al 2008, Vonk et al 2010). The western geochemical province extends from Vilkitsky Strait (∼120E) in the west to Cape Chukochiy (roughly 172E) in the east, where it is approximately bounded by the isolines of OC and nitrogen isotope ratios: δ13Corg =− 24.5‰ and δ15Norg = 7‰. The western province is strongly influenced by particulate material (PM) transport induced by coastal erosion and fresh water (FW) transport by Lena runoff. PM concentrations in the western province are as much as ten times higher than in the eastern geochemical province, which extends from Cape Chukochiy to the Bering Strait and is highly affected by Pacific inflow (Semiletov et al 2005).
Recent studies indicate a connection among the atmospheric circulation and the hydrological and geochemical characteristics of the water masses in this region (Semiletov et al 2005, Anderson et al 2009, 2011, Porcelli et al 2009). Multi-year variability of the carbonate system and dissolved CH4 dynamics and efflux of CO2 into the atmosphere confirm the significance of changing atmospheric circulation patterns (Pipko et al 2005, 2008, 2011, Shakhova and Semiletov 2007, 2008). An important effect on distribution of Atlantic and Pacific waters in the upper Arctic Ocean could be exerted by a change in direction of water transport associated with a shift from the cyclonic (Zn) mode to the anticyclonic (Az) mode of atmospheric circulation over the Arctic basin. Under Az mode conditions the trans-Arctic current (which transports FW northward from the Asian shelf) is intensified and shifted toward Siberia (Proshutinsky and Johnson 1997). The switch of atmospheric circulation regimes (from Az to Zn mode) causes a significant inter-annual shift in the position of the trans-Arctic current, and its correlation (0.78) with hydrological conditions (anomalies of temperature and salinity in the East Siberian Sea) is quite high (Shpaikher and Yankina 1969). The river runoff also determines development of the low salinity Siberian coastal current (SCC) that follows the coasts from west to east (Weingartner et al 1999). Due to wind forcing the SCC is highly variable. Under cyclonic atmospheric conditions the SCC exists as a narrow jet along the coast (for example, in summer 2003), while under anticyclonic atmospheric circulation it was less confined and was not observed in the easternmost part of the ESAS (Weingartner et al 1999, Savelieva et al 2008, Pipko et al 2011). Thus, the variability in position of the frontal zone (FZ) between the 'Pacific waters' and 'local shelf waters' could be considered an indicator of regional climate change as reflected in alternations of the Zn and Az circulation regimes (Semiletov et al 2005). Further comprehensive studies are required to establish process models with different time-scale resolutions, from intra-seasonal to inter-annual to decadal.
3.2. Specific features of sedimentation in the western and eastern provinces
The sediment OM is generally a mixture between marine and terrigenous material that is characterized by a range of C/N ratios between 6 and 15; typically the lower values characterize marine OM, and the higher values characterize the terrigenous material (Stein and Macdonald 2004). The distribution of δ15Norg also shows an eastward change from its 'terrestrial' value of less than 6 to the 'marine' value of more than 8 (Walsh et al 1989). The C/N ratio is negatively correlated with δ13Norg (r =− 0.73) and δ15Norg (r =− 0.77). The spatial pattern of δ13Corg distribution in the bottom sediment was used to quantitatively estimate the contribution of terrestrial OM (CTOM) to the East Siberian Sea sediment west and east of the geochemical FZ. Following Walsh et al (1989), the amount of OC derived from the terrestrial end-member (δ13Cter) can be calculated from the data as
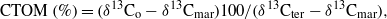
where 'o', 'mar', and 'ter' refer to the δ13Corg values of observed, marine and terrestrial sediment, respectively. Given that the two end members are δ13Cter of − 27‰, typical of higher plants, and δ13Cmar of − 21‰, typical of phytoplankton (Walsh et al 1989, Naidu et al 2000), the CTOM values for the western and eastern geochemical provinces (bounded by CTOM = 70%) become 86% and 52% of OCter, respectively (Semiletov et al 2005). Calculations indicate that there is a significant amount of OCter stored within sediments, especially in the nearshore zone most strongly influenced by coastal erosion; between the Dmitry Laptev Strait and the Kolyma River mouth, the OC is almost all of terrestrial origin (from 81% to 100%, mean CTOM = 93%). In contrast, almost half of the sediments underneath transformed Pacific-origin water (the eastern province) are marine in origin. To illustrate the anomalous export of terrestrial OM into the ESAS we show the integrated plume of PM and terrestrial OM in POC for the period 2003–7 (figure 4) from data obtained east of the Lena Delta.
Figure 4. Secondary role of the Lena River and dominating role of coastal erosion in ESAS sedimentation is illustrated by surface distribution of (a) particulate materials (mg l−1): (1) <2, (2) 2–12, (3) 13–24, (4) >24; (b) contribution of terrestrial OM to POC (%): (1) <25, (2) 25–49, (3) 50–75, (4) >75.
Download figure:
Standard imageThese observations support the hypothesis of a dominant role for coastal erosion (Semiletov 1999a, 1999b) in ESAS sedimentation and in the dynamics of the OC and carbonate system. It can be seen that the PM and the terrestrial OM/POC plume from the Lena is limited to the narrow nearshore zone where most of the Lena River burden settles. The pattern also shows a negligible direct influence of Lena transport of PM into the East Siberian Sea as was earlier suggested (Semiletov et al 2005).
General differences between the oceanographic and geochemical regimes of the western and eastern provinces in the East Siberian Sea are reflected in the size fraction distribution of the surface sediments. The mean psammite (sand) fraction more than doubled from the western province to the eastern province, while the aleurite (silt) fraction was nearly halved (Semiletov et al 2005). The percentage contribution of the pelite (clay) fraction (particle size = <0.01 mm) is almost the same in the western and eastern provinces; however, the pelite fraction is almost completely terrestrial in origin in the western province, but exhibits a mix of marine and terrestrial origins in the eastern province. The highest OC concentration (up to 1.9%) exists in the pelite fraction. The dominant presence of terrestrial eroded material in ESAS sedimentation is illustrated by the distribution of surface sediments in the ESAS (figure 5); >95% of the entire ESAS area is covered by clayey silt, the main product of coastal erosion (Dudarev et al 2006b).
Figure 5. Surface sediments of the ESAS: 1—gravel and pebble, 2—coarse and medium sand, 3—fine sand, 4—silty sand, 5—sandy silt, 6—muddy sand, 7—clayey silt, 8—silty clay, 9—clay, 10—sandy sand–silt–clay, 11—silty sand–silt–clay, 12—clayey sand–silt–clay.
Download figure:
Standard image3.3. Molecular and radiocarbon compositional variations of the terrestrial OM exported by coastal erosion and the GSARs
It is necessary to quantitatively assess the fraction of terrestrial OC (OCterr) contributed by riverine runoff and the fraction from coastal erosion; in order to provide a benchmark of the composition, provenance, and extent of degradation of the OCterr, it is very important to distinguish between these two sources. The molecular and radiocarbon composition of the OCterr released by the GSARs indicate clear patterns relatable to vegetation and climate variations across the 4000 km continent-scale climosequence of the Siberian Arctic (Guo et al 2004, van Dongen et al 2008, Vonk et al 2010, Gustafsson et al 2011). Black carbon ranged from 3 to 10% of total OC; the highest fractions in the permafrost-covered East Siberian region showed a 14C (radiocarbon) mass balance, suggesting about 20% from biomass burning and the balance from fossil fuel combustion or relict black carbon in uplifted source rocks (Elmquist et al 2008). Among the lipid biomarkers, there is a large contribution of C23 and C25 n-alkanes relative to other homologs, suggesting a substantial contribution from sphagnum, with a decreasing trend eastward. Increasing concentrations of both high-molecular-weight (HMW) n-alkanoic acids and beta-sitosterol relative to HMW n-alkanes toward East Siberia is consistent with increasing permafrost and a shorter annual thaw period (Vonk et al 2010, Gustafsson et al 2011).
Compound-specific radiocarbon analysis yielded similar modern 14C fraction values for long-chained n-alkanes and n-alkanoic acids, which increased toward the east. The 14C signal of the bulk OC exhibited an opposite spatial trend. We suggest that these gradients reflect decoupled hydraulic conduits for fresh and aged OCterr. Since this decoupling is related to the extent of permafrost, there may be repercussions for OCterr remobilization under a warming climate (van Dongen et al 2008, Vonk et al 2010, Gustafsson et al 2011). If the climate in the ESAS region becomes more like the current climate in the West Siberian region, these results would predict a greater degree (compared to that in western Siberia) of decomposition of the old terrestrial OM released by coastal erosion (figure 2) and the Lena and other eastern GSARs, and thus greater remineralization and release as CO2.
3.4. Transport of terrestrial OM to the ESAS and dynamics of the carbonate system
Rivers transport large quantities of both dissolved and particulate matter and therefore they are carriers of a wide variety of chemical signals to the sea margin (Semiletov 1999a, 1999b, Guo et al 2004, Semiletov et al 2007, Pipko et al 2008, 2010, 2011, van Dongen et al 2008, Anderson et al 2009, 2011, Vonk et al 2010, Charkin et al 2011, Karlsson et al 2011). The degradation of particulate OM (POM) causes the formation of an anomalously high partial pressure of CO2 (pCO2) and nutrient concentrations in the nearshore zone, where the river signal overlaps with the biogeochemical signal from highly eroded coastal ice complexes enriched by old terrestrial organics (Semiletov 1999a, 1999b, Semiletov et al 2007, 2011). The POM signal of coastal erosion leads to a pCO2 increase in bottom water near the highly eroded ice complexes by up to ∼4000 µatm, while pCO2 values in the surface water strongly impacted by river runoff were usually observed to be ∼1000 µatm or less (figure 6).
Figure 6. Distribution of partial pressure of CO2 (pCO2, µatm) in the surface and bottom layers in August–September 1999 (a) and in 2005 (b). Numbers refer to station numbers. Note the difference in X axis scale.
Download figure:
Standard imageThis is consistent with levels of pCO2 measured in carbon plumes from the GSARs along the northern sea route (figure 7); pCO2usually appears in surface water at levels <500 µatm (Semiletov et al 2007, 2011).
Figure 7. Distribution of pCO2 (a), dissolved oxygen (b), and sum of nitrites plus nitrates (c) in the nearshore zone along the Northern Sea Route in 2000.
Download figure:
Standard imageNote that rates of oxidation of POM and OM from surface sediments must be quite high to produce a homogeneous distribution of anomalously high pCO2 values ranging between 2000 and 3000 µatm in the completely mixed water column (figure 6(a), Stations 13–15); the highest values were found in the bottom water of the Buor-Khaya Gulf (figure 6(b), Stations 123–125) where the water column is strongly stratified (Charkin et al 2011). High rates of eroded OM oxidation were confirmed by direct measurements of CO2 release (measured in September 2006 and 2008 using the Li-Cor chamber technique) in the air above the bluff of the highly eroded ice complex at the northern cape of Muostakh Island in the Laptev Sea; CO2 efflux to the atmosphere usually ranged from tens up to hundreds of mM per square meter per day.
Recycling of limiting nutrients from old terrestrial OM could influence the element stoichiometry (and 'new production') (Codispoti and Richards 1968, Cauwet and Sidorov 1996, Jones et al 1998, Kattner et al 1999). Our data show that the decay of POM and OM at the sediment surface results in low in situ pH values (as low as 7.24, total scale) and a naturally acidified marine environment. The ESAS exhibits the lowest values of calcium carbonate saturation ever reported for the open marine environment. In some areas of the Laptev Sea (such as at Stations 123–125, figure 6(b)) acidification lowers the saturation state of calcite and aragonite in the surface waters to 0.4 and 0.2, and in the bottom water to 0.2 and 0.1, respectively; these values are even lower than those measured in the East Siberian Sea (Anderson et al 2011). In contrast to eroded POM, dissolved organic carbon (DOC) was believed to be bio-unavailable (Dittmar and Kattner 2003). However, the DOC concentrations measured in samples from the ISSS 2008 cruise showed a strong non-conservative pattern in areas with FW residence times of several years (Alling et al 2010). The average DOC losses during mixing along the ESAS shelf were estimated to be 30–50%, suggesting an additional and unaccounted source of CO2 to the atmosphere.
3.5. Factors controlling CH4 release and atmospheric emissions from the ESAS
Permafrost degradation takes place during climate warming and marine transgression. At present, the mean annual onshore permafrost temperature ranges from − 11 °C in the coastal zone (Grigoriev et al 2006, Rachold et al 2007), to − 12 to − 15 °C in the northern ESAS (Novosibirsky Islands, 73 °N–76 °N), giving a mean latitudinal gradient of about 1.5 °C/100 km (Romanovskii et al 2005). The bed of the high-latitude, shallow ESAS has been alternately exposed to the air and inundated with seawater during glacial and interglacial periods, respectively. Because of its shallowness and location, the ESAS experiences unique climatological transitions. During previous glacial periods, when sea level was more than 100 m lower than at present, the coastline extended up to 1000 km further northward, exposing the continental shelf above the sea surface and, thus, increasing the area of the Siberian coastal accumulative plain by a factor of five (Romanovskii et al 2005). During the last glaciations, the exposed ESAS permafrost was 4–6 °C colder than now (Romanovskii et al 2000); therefore, the mean annual permafrost temperature ranged from − 15 °C in the southern part of the ESAS to − 19 to − 21 °C at its northern periphery. Extensive permafrost formation occurred because freezing extended hundreds of meters deep. Upwardly migrating CH4 became trapped below the impermeable permafrost and was transformed into permafrost-related Arctic hydrates (Soloviev et al 1987, Kvenvolden 1988). Because they are permafrost-controlled, these hydrates destabilize when sub-sea permafrost experiences warming (Makogon et al 2007). The transition from the last glacial period to the current warm Holocene was accompanied by a sea level rise that inundated the previously exposed shelf area 5–12 kyr ago (Fleming et al 1998). Shallow shelf water annual mean temperatures ranged from − 1.8 °C to slightly above 0 °C; therefore, inundation resulted in a warming at the seafloor boundary by up to 13–20 °C (versus ∼12–17 °C suggested by Soloviev et al (1987) relative to the temperatures under which permafrost originated in cold epochs when sediments were not covered with water. Over time, ocean heat together with geothermal heat flux-drive submerged permafrost to a new equilibrium state and cause it to thaw. This process first occurs in the areas of fault zones and river estuaries where geothermal heat flux and annual water temperatures are the greatest (Romanovskii et al 2005, Nicolsky and Shakhova 2010, Charkin et al 2011). Thus, as permafrost degradation takes place, thaw bulbs, called taliks, form in limited areas (Soloviev et al 1987, Kvenvolden 1988). Thaw bulbs that penetrate through permafrost are called open taliks (Romanovskii et al 2005, 2000). Taliks could be considered, first, to be an environment favorable for modern methanogenesis from thawed old carbon and, second, to form gas migration pathways by which CH4 can be released from seabed deposits to the water column and further to the atmosphere (Shakhova et al 2010b, 2010c). The discontinuous presence of taliks is manifested by extremely high spatial variability in concentrations of dissolved CH4, which correlate with spikes in mixing ratios of CH4 in the atmosphere like those shown in figure 8.
Figure 8. Distribution of mixing ratios of CH4 in the atmospheric layer above the sea water (a) and concentrations of dissolved CH4 in the surface layer of shelf water (b) (September 2005).
Download figure:
Standard imageAdditional destabilization of seabed CH4 deposits could be caused by sediment settlement and adjustment caused by partial hydrate destabilization, seismic and tectonic events, and formation of drainage features in permafrost, where they are interpreted as providing possible migration pathways (Hyndman and Dallimore 2001, Wright et al 2008). Recently detected warmer water temperatures near the seabed may also impact the stability of the ESAS shelf's submarine permafrost (Holemann et al 2011, Shakhova and Semiletov 2007, 2008).
Our measurements of CH4 taken in 1994–9 and 2003–10 over the ESAS demonstrate that the system is in a destabilization period (Semiletov 1999a, Shakhova et al 2010a, 2010b, 2010c). Moreover, unlike most other sub-sea locations where CH4 oxidizes within the water column, a substantial amount of CH4 released at the seafloor in the shallow ESAS is delivered to the atmosphere (Shakhova et al 2010b, 2010c). Among the factors controlling the fluxes of CH4 to the atmosphere over the ESAS, we discovered seasonal features such as ice coverage, wind speeds and wave heights (frequency of storm events); depth of the water column, which affects transition time from the bottom to the surface and the amount of CH4 removed by oxidation as well as water mixing during deep fall convection; and fraction of CH4 transported by bubbles relative to that transported through diffusion, because bubble-mediated CH4 transport is far more efficient than diffusion (Shakhova et al 2010b, 2010c). We suggest that fluxes from the ESAS could contribute to rising atmospheric CH4 because the ESAS serves as a source of CH4, annually releasing ∼8 Tg CH4 to the atmosphere. A number of flux components remain to be quantified and added to the existing evaluation (Shakhova et al 2010c).
4. Summary statements and future work
To investigate processes of carbon transport and fate in the ESAS, we employed the ESAS as an integrator of ongoing changes in the surrounding land, which create a terrestrial or exogenous signal, and in situ changes that present a marine or endogenous signal which is generated by submarine permafrost destabilization, increasing coastal and bottom erosion, and involvement of old carbon in the modern biogeochemical cycle. Because two different hydrologic and biogeochemical regimes exist in the western and eastern provinces of the ESAS, the features of carbon cycling are different and specific to each province. In the western province the main processes that drive the marine carbon cycle (except for CH4 emissions) are determined by the combined influence of coastal erosion and riverine runoff from the GSARs. Transformation of the OCterr leads to oversaturation of shelf waters in CO2; CO2 is highest where the contribution of coastally eroded OCterr prevails. The decay of OCterr also lowers pH leading to the acidification of shelf water in the ESAS, which is especially pronounced in the western biogeochemical province. In the eastern province, which is largely influenced by Pacific Ocean inflow, the contribution of OCterr is much lower and marine primary production dominates; there, pCO2 indicated under-saturation of shelf water in CO2.
Since the compound-specific signal of the bulk OC is related to the extent of permafrost, there may be repercussions for OCterr remobilization under a warming climate. Observations showed a highly mosaic pattern of dissolved CH4 spatial distribution in the shelf water. The ESAS serves as a source of CH4 to the atmosphere. CH4 release from the ESAS is determined by the current state of sub-sea permafrost, which has failed to seal ancient carbon pools sequestered in seabed deposits within and beneath permafrost, and which provides CH4 with migration pathways; among such pathways taliks could be considered to be primary. The resulting CH4 emissions to the atmosphere are determined primarily by seasonal features specific to the ESAS such as ice cover, water mixing during storm events, and deep fall convection, rather than by factors influencing the type and the strength of the source. We can conclude that the ESAS accumulates the integrated biogeochemical signal produced by terrestrial and marine ecosystems in response to ongoing climate change. To further assess and quantify the contribution of the ESAS to the regional and global carbon cycle, we plan to obtain new data to answer the following overarching questions.
- (1)How does the contribution of the ESAS affect the role of the Arctic Ocean in influencing the regional CO2 balance?
- (2)How do processes of terrestrial OM decay in the ESAS water contribute to Arctic Ocean acidification?
- (3)How much CH4 could be released to the atmosphere from the ESAS due to degradation of sub-sea permafrost and decay of seabed deposits? What is the current state and projected future dynamics of sub-sea permafrost?
To answer these questions we call for extended international cooperation in studying the ESAS. Such study will require multiple year-round exploration campaigns, including drilling of sub-sea permafrost to evaluate the sediment CH4 potential and comprehensive atmospheric measurements to assess the ESAS strength as a greenhouse gas source. International effort should also be joined in order to quantitatively assess future changes in greenhouse gas emissions in response to ongoing climate change by establishing and developing regional numerical models with the aim of incorporating them into global climate models.
Acknowledgments
We thank two anonymous reviewers for helpful comments and criticism. This work was supported by the International Arctic Research Center (IARC) of the University of Alaska Fairbanks; the Far Eastern Branch of the Russian Academy of Sciences (FEBRAS); the Cooperative Institute for Arctic Research, through NOAA Cooperative Agreement NA17RJ1224; the US National Science Foundation (Nos OPP-0327664, OPP-0230455, ARC-1023281, ARC-0909546); the NOAA OAR Climate Program Office (NA08OAR4600758); and the Russian Foundation for Basic Research (Nos 07-05-64819, 08-05-00184, 08-05-00191, 10-05-00996, 11-05-00781, 11-05-12021, 11-05-12027, 11-05-12028, 11-05-12032). The ISSS-2008 cruise was carried out with logistic support from the Knut and Alice Wallenberg Foundation and from the Swedish Polar Research Secretariat. Other cruises and winter campaigns were carried out with logistic assistance from the Tiksi Hydrobase (Dmitry Mel'nichenko) and the Scientific Agency Arctic Marine Studies (Elena Streletskaya). We thank Candace O'Connor for English editing.








