Abstract
We have quantitatively investigated the oxidative inactivation process of Penicillium digitatum spores including intracellular nanostructural changes through neutral oxygen species with a flux-defined atmospheric-pressure oxygen radical source, using fluorescent confocal-laser microscopy and transmission electron microscopy (TEM). The results suggest that neutral oxygen species, particularly ground-state atomic oxygen [O(3Pj)], which is an effective species for inactivating P. digitatum spores, inhibit the function of the cell membrane of spores without causing major superficial morphological changes at a low O(3Pj) dose of ∼2.1 × 1019 cm−2 under an O(3Pj) flux of 2.3 × 1017 cm−2 s−1, following the oxidation of intracellular organelles up to an O(3Pj) dose of ∼1.0 × 1020 cm−2. Finally, intracellular nanostructures are degraded by excess oxygen radicals over an O(3Pj) dose of ∼1.0 × 1020 cm−2.
Export citation and abstract BibTeX RIS
1. Introduction
The applications of non-equilibrium cold plasma in biological fields, such as medicine, agriculture, and fisheries, have attracted much attention recently. There have been many reports on the inactivation of microorganisms by non-equilibrium atmospheric-pressure plasma (NEAPP).1–5) NEAPP simultaneously produces various factors, such as ultraviolet (UV) light, neutral and charged species, and electric fields, which may synergistically inactivate microorganisms, such as Bacillus spp,6–16) Escherichia coli,7,15,17–20) Staphylococcus aureus,7,21) Aspergillus niger,11) budding yeast,22) and Candida albicans.23) In particular, there have been many studies that showed that the inactivation is effectively carried out by reactive oxygen species (ROS), such as ground-state atomic oxygen [O(3Pj)],6,8,10,13,17,18,23) hydroxyl radicals ( OH),8,10,11,16) singlet oxygen molecules [O2(1Δg)],16,20,21,24) superoxide anions (
OH),8,10,11,16) singlet oxygen molecules [O2(1Δg)],16,20,21,24) superoxide anions ( O2−),16) and ozone (O3).14,20) However, in those studies, multiple ROS including charged species, in addition to UV radiation, were involved, and neither lifetime nor inactivation rate for individual species was determined. Therefore, it is important to discuss the inactivation effects of individual species quantitatively.
O2−),16) and ozone (O3).14,20) However, in those studies, multiple ROS including charged species, in addition to UV radiation, were involved, and neither lifetime nor inactivation rate for individual species was determined. Therefore, it is important to discuss the inactivation effects of individual species quantitatively.
In our previous works, we inactivated Penicillium digitatum spores by plasma treatment and investigated the inactivation mechanism from the point of view of superficial morphological and intracellular oxidative changes.25–27) P. digitatum spores cause the formation of green mold on citrus fruits, which is a difficult-to-inactivate postharvest disease. The spores, which are classified as fungi, differ from bacteria typically employed in plasma inactivation processes and have more resistant structures with different compositions and cell membrane functions. In addition, to clarify the inactivation effects of neutral oxygen species, we also developed an atmospheric-pressure oxygen radical source that supplies only neutral oxygen species.28,29) We measured the densities of O(3Pj), O2(1Δg), and O3 supplied from the radical source using vacuum ultraviolet absorption spectroscopy (VUVAS) and ultraviolet absorption spectroscopy (UVAS), and showed that the inactivation rate corresponded to the O(3Pj) density against the O2/(Ar + O2) gas flow ratio and exposure distance, while the inactivation efficiencies of O2(1Δg) and O3 are negligible.30) We concluded that O(3Pj) is an effective species for inactivating P. digitatum spores.30,31)
Moreover, we reported the possibility of the inactivation mechanism of P. digitatum spores with oxygen radical treatment. We observed the changes in spores of P. digitatum treated with an oxygen radical source, using in-situ fluorescent confocal-laser microscopy for investigating the biological responses causing the inactivation through neutral oxygen species.32) A confocal-laser microscope has a high resolution, especially in the depth direction. From fluorescent cross-sectional images with a z-axis resolution of approximately 0.2 µm, we can analyze the intracellular changes of the spores treated with oxygen radicals in detail. We showed that ROS, particularly O(3Pj), supplied from the atmospheric-pressure oxygen radical source, inhibit the function of the cell membrane for a short treatment time such as 0.5 min prior to the inactivation, and that no major superficial morphological variation occurs during the inactivation process from scanning electron microscopy (SEM) observations. Many reports have mentioned that microorganisms subjected to plasma treatment are inactivated with morphological changes such as etching, cracking, leakage, and size reduction.7,9,11,12,14,16,18,19,23) We also found that intracellular organelles are oxidized by oxygen radical treatment with the fluorescent dye diphenyl-1-pyrenylphosphine (DPPP). DPPP reacts with lipid peroxide in biomembranes and produces DPPP oxide.33,34) DPPP assay is usually used to probe the oxidation of microorganisms. Generally, lipid phosphates are oxidized by ROS to lipid peroxide through a chain reaction without the induction of additional ROS.35) The accumulation of lipid peroxide is considered to lead to cell death because of functional inhibition. It is, therefore, indispensable to analyze the degree of oxidation of P. digitatum spores in detail to elucidate the inactivation mechanism with oxygen radical treatment.
It has not yet been indicated that oxygen radicals oxidize intracellular organelles on the inactivation process and cause the intracellular structural changes. Besides, it is not clear how the oxidation process is related to the inactivation. In this study, we have observed P. digitatum spores stained with DPPP for various treatment times, and simultaneously indicated a correlation between the degree of oxidation, which was determined from the result of DPPP staining, and the number of survivors. Besides, we have observed the intracellular nanostructural changes for various treatment times using transmission electron microscopy (TEM). Moreover, we have quantitatively evaluated these results on the basis of the dose of neutral oxygen radicals, which is estimated from the radical density, and proposed the inactivation mechanism of P. digitatum spores treated with oxygen radicals as a function of the dose.
2. Experimental procedure
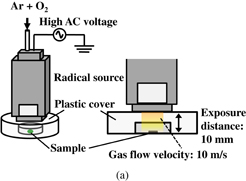
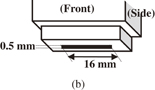
Figure 1(a) shows a schematic diagram of the experimental setup of the spores treated with oxygen radicals using an atmospheric-pressure oxygen radical source. The oxygen radical source (Fuji Machine; Tough Plasma) was described in detail in Refs. 28 and 29. The radical source is based on atmospheric-pressure high-density O2/Ar plasma, employing a 60 Hz–10 kV power source, which generates electrons with a high density of about 1016 cm−3. Charged species and optical radiation from the O2/Ar plasma are blocked by electrodes and the exit aperture of the radical source, so that only neutral species are supplied to the samples. The exit configuration of the radical source used in this study was a slit aperture of 0.5 × 16 mm2, as shown in Fig. 1(b). The treatment area was purged with Ar gas from the radical source using a plastic cover to eliminate the influence of atmospheric gases. The flow rate of Ar gas with a small amount of O2 gas was sufficient to purge the ambient gas from the area surrounded by the plastic cover. A P. digitatum spore suspension of 10 mg/ml was prepared from distilled water, 1% surfactant (Tween20), and spores. One microliter of the suspension was spotted on a 35-mm-ϕ dish and dried.
Download figure:
Standard image High-resolution imageFig. 1. Schematic diagram of (a) experimental setup of oxygen radical treatment using an oxygen radical source and (b) radical source head.
Download figure:
Standard image High-resolution imageFluorescent images were obtained using an inverted confocal-laser microscope (Olympus FV1000-D) equipped with a 100× oil immersion objective lens (Olympus UPLSAPO100×O). A fluorescent dye was employed for investigating the effects of oxygen radicals. The staining solution consisted of a mixture of 1 mg of DPPP aqueous solution (Dojindo Laboratories) and 1 ml of dimethylsulfoxide. DPPP reacts with lipid peroxide and forms DPPP oxide. Lipid peroxide is produced via the oxidation of lipids in biomembranes, which also exist in intracellular organelles. Thus, DPPP assay can be used to probe the oxidation of P. digitatum by radical treatment. The DPPP oxide excited at a wavelength of 352 nm produces blue fluorescence at 380 nm. In this study, a diode laser with a wavelength of 405 nm was used for exciting the DPPP oxide, and a dichroic beam splitter (DM405/473) and an emission filter (Olympus BA430-455) were used for observing the fluorescence.
For transmission electron microscopy (TEM) observation, spores were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), and then postfixed with potassium permanganate. The samples were subsequently dehydrated with an ethanol solution series and embedded in epoxy resin (Nisshin EM Quetol 812) at 60 °C for 48 h. Ultrathin sections of 80–90 nm thickness were prepared, stained with uranyl acetate and lead, and mounted on copper grids. Observations were performed with a JEOL JEM-1200EX.
3. Results and discussion
Fluorescent and transparent microscopy images of P. digitatum spores stained with DPPP are shown in Fig. 2. The O2/(Ar + O2) flow rate ratio and total flow rate were 0.6% and 5 slm, respectively. These conditions correspond to the maximum O(3Pj) density and maximum inactivation rate of P. digitatum spores.30,31) The spores were exposed to radicals 10 mm downstream from the radical head for 1.5, 3, 5, 7, and 10 min. After the treatment, the spores were recovered with 300 µl of 1% Tween20 solution. Sixty microliters of the suspension was mixed with 6 µl of 1 mg/ml DPPP solution (Dojindo Laboratories) and incubated at 37 °C for 60 min. One microliter of the mixture was spotted on a glass-bottom dish and dried. Transparent and fluorescent images of the control spores and the spores treated with oxygen radicals for 1.5, 3, 5, 7, and 10 min are shown in Figs. 2(a)–2(f), respectively. We previously measured the density of neutral oxygen radicals, such as O(3Pj), O2(1Δg), and O3 using VUVAS and UVAS under nearly the same condition with radical treatment.31,32) From the O(3Pj) density of 2.3 × 1014 cm−3 at an exposure distance of 10 mm and a flow velocity of 10 m/s, O(3Pj) flux can be estimated to be 2.3 × 1017 cm−2 s−1. In addition, from the flux and treatment time, we can quantitatively determine the dose of O(3Pj), which has the most effective and fastest inactivation rate (∼10−17 cm3 s−1) of P. digitatum spores in neutral ROS.30,31) From these results, we can quantitatively discuss the effects of oxygen radicals on the inactivation process of P. digitatum spores. No or little emissions were observed from the control spores or the spores treated with oxygen radicals at an O(3Pj) dose of 2.1 × 1019 cm−2, as shown in Figs. 2(a) and 2(b), respectively. Then, the spores treated with O(3Pj) at doses of 4.2 × 1019 and 7.0 × 1019 cm−2 fluoresced slightly, as shown in Figs. 2(c) and 2(d), respectively. The spores treated with O(3Pj) at a dose above 9.8 × 1019 cm−2 showed that fluorescent light was clearly emitted from intracellular organelles under an O(3Pj) flux of 2.3 × 1017 cm−2 s−1, as shown in Figs. 2(e) and 2(f). DPPP reacts with lipid peroxide and forms DPPP oxide. Lipid peroxide is produced via the oxidation of lipids in biomembranes, which also exist in the intracellular organelles. These results indicate that intracellular membranes in spores are increasingly oxidized by oxygen radicals with an increase in treatment time. Next, we estimated the degree of oxidation of these spores by quantifying the intensity from the emission in the area of the spores.
Fig. 2. Fluorescent and transparent microscopy images of P. digitatum spores stained with DPPP: (a) control spores and (b)–(f) spores treated with oxygen radicals for 1.5, 3, 5, 7, and 10 min, respectively. The doses of O(3Pj) for various treatment times are also expressed under a flux of 2.3 × 1017 cm−2 s−1.
Download figure:
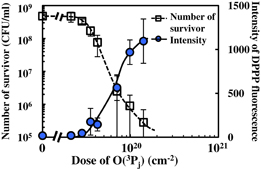
Standard image High-resolution imageFigure 3 shows the variation of the degree of oxidation in spores with oxygen radical treatment on the basis of quantification of the DPPP staining intensity. The results indicated that the spores were hardly oxidized at an O(3Pj) dose less than 2.1 × 1019 cm−2. Then, the degree of oxidation steadily increased with the treatment of O(3Pj) dose from 2.1 × 1019 to 9.8 × 1019 cm−2. Finally, the increase turned to be saturated at a dose over 9.8 × 1019 cm−2. We have investigated the number of survivors as a function of treatment time at the same time. The number of surviving spores rapidly and exponentially decreased with the treatment with O(3Pj) at a dose from 2.1 × 1019 to 9.8 × 1019 cm−2, and more than 99.9% of the spores were inactivated at a dose of 1.2 × 1020 cm−2. The decimal reduction time (D-value) at O(3Pj) doses from 2.1 × 1019 to 9.8 × 1019 cm−2 was estimated to be 1.8 min, which nearly replicated our previous results.30) These results suggested that the degree of oxidation corresponded to the inactivation of the spores. We previously reported that the function of cell membranes is inhibited by oxygen radical treatment for 0.5 min, which corresponds to an O(3Pj) dose of 7.0 × 1018 cm−2, using the fluorescent dye 1,1'-dioctadecyl-3,3,Y,3'-tetramethylindocarbocyanine perchlorate (DiI).32) Oxygen radicals, mainly O(3Pj), interacted with the cell membrane and cell wall, and penetrated into spores even for a short treatment time. In this study, the intensity of DPPP staining rapidly increased at an O(3Pj) dose over 2.1 × 1019 cm−2. Therefore, the results of our present work and our previous work using DiI suggest that the inhibition of cell membranes leads to the penetration of oxygen radicals into spores, resulting in an increase in the degree of oxidation. Then, the spores are inactivated together with an increase in the degree of oxidation.
Fig. 3. Intensity of DPPP staining and number of surviving spores with radical treatment as a function of O(3Pj) dose under a flux of 2.3 × 1017 cm−2 s−1.
Download figure:
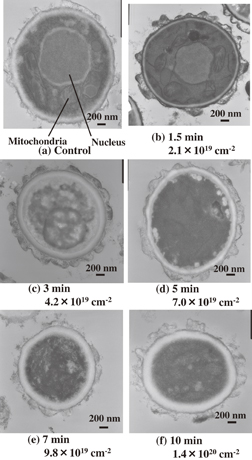
Standard image High-resolution imageWe previously showed that P. digitatum spores are inactivated with radical treatment without major superficial morphological changes, using SEM.32) However, the structural change of intracellular membranes has not been clarified yet. Thus, we investigated the change of the intracellular nanostructure in the spores treated with oxygen radicals on the inactivation process using TEM. Figures 4(a)–4(f) show TEM images of cross sections of the control spore and the spores of P. digitatum with radical treatment for 1.5, 3, 5, 7, and 10 min, respectively. The control spore clearly shows the intracellular structure and organelles such as the nucleus and mitochondria, as shown in Fig. 4(a). Those membrane structures in the spores treated with O(3Pj) at doses of 2.1 × 1019 and 4.2 × 1019 cm−2 were relatively maintained, as shown in Figs. 4(b) and 4(c), respectively. On the other hand, the intracellular structure was degraded at an O(3Pj) dose over 7.0 × 1019 cm−2, as shown in Figs. 4(d)–4(f). Cerioni et al. also reported that excess oxidative stress with hydrogen peroxide causes severe damage in the intracellular structure of P. digitatum spores, as determined by TEM.36) These indicate that oxygen radicals gradually causes the decomposition of the intracellular membrane structure with an increase in an O(3Pj) dose. Considering the results shown in Figs. 3 and 4, the increase in the degree of oxidation enhances the accumulation of lipid peroxide. In mammalian cells, lipid peroxidation reactions can occur in cell membranes and mitochondria membranes, and trigger programmed cell death such as apoptosis and autophagy.37) P. digitatum spores as well as mammalian cells might exhibit an apoptotic-like reaction at an O(3Pj) dose below 4.2 × 1019 cm−2, where the decomposition of intracellular structures was not observed.
Fig. 4. TEM images of cross sections of P. digitatum spores: (a) a control spore and (b)–(f) spores treated with oxygen radicals for 1.5, 3, 5, 7, and 10 min, respectively. The doses of O(3Pj) for various treatment times are also expressed under a flux of 2.3 × 1017 cm−2 s−1.
Download figure:
Standard image High-resolution imageFrom the above results, we propose the inactivation mechanism of P. digitatum spores treated with oxygen radicals as follows: First, oxygen radicals affect the cell wall and cell membrane of P. digitatum spores and inhibit their functions without causing major superficial morphological changes with the treatment at a low O(3Pj) dose of ∼2.1 × 1019 cm−2. Next, intracellular organelles are also oxidized and degraded dose-dependently up to a dose of ∼1.0 × 1020 cm−2. At this stage, most spores are inactivated. Finally, a high O(3Pj) dose of over 1.0 × 1020 cm−2 completely decomposes intracellular organelles including the membrane structure under an O(3Pj) flux of 2.3 × 1017 cm−2 s−1.
4. Conclusions
To investigate the inactivation process of P. digitatum spores by neutral oxygen species, the spores were treated with a flux-defined atmospheric-pressure oxygen radical source, which eliminates the effects of radiation and charged species. To analyze the degree of oxidation of the spores in the process of oxygen radical treatment in detail, we have observed P. digitatum spores treated with oxygen radicals and stained with the fluorescent dye DPPP, using a fluorescent confocal-laser microscope, and quantified the degree of oxidation. The increase in the degree of oxidation of the spores correlated with the decrease in the number of surviving spores. Moreover, we have observed the changes of the intracellular nanostructure of the spores treated with oxygen radicals, using TEM. The intracellular organelles were degraded by oxygen radicals, which corresponded to the increase in the degree of oxidation. From the results, we have elucidated the mechanism of the effects of oxygen radicals on the inactivation process of P. digitatum spores on the basis of the O(3Pj) dose. Oxygen radicals, mainly O(3Pj) which is the dominant factor for inactivating P. digitatum spores, inhibit the function of the cell membrane of spores without causing major superficial morphological changes at a low O(3Pj) dose of 2.1 × 1019 cm−2, following the oxidation of intracellular organelles up to an O(3Pj) dose of 1.0 × 1020 cm−2. Most spores are inactivated at this stage. Finally, intracellular nanostructures are degraded by excess oxygen radicals over an O(3Pj) dose of 1.0 × 1020 cm−2 under an O(3Pj) flux of 2.3 × 1017 cm−2 s−1.
Acknowledgement
This work was partly supported by a Grant-in-Aid for Scientific Research on Innovative Areas, "Frontier Science of Interactions between Plasmas and Nano-interfaces" (No. 21110006) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.