At its 24th meeting (2011) the General Conference on Weights and Measures noted the CIPM's intention to express new definitions of the kilogram, ampere, kelvin, and mole in terms of fixed numerical values of the Planck constant, elementary charge, Boltzmann constant, and Avogadro constant, respectively. The changes proposed for the International System of Units will not actually be adopted until the experimental results on the new definitional constants that are proposed have reached a further stage of refinement. This paper provides an overview of the activities and progress of the research groups who are carrying out experiments to determine the Boltzmann constant. The most promising methods, acoustic gas thermometry, dielectric-constant gas thermometry, Johnson noise thermometry and Doppler-broadening thermometry, are reviewed. The prospects for meeting the requirements of the Consultative Committee for Thermometry for a new definition of the kelvin are discussed.

The International Bureau of Weights and Measures (BIPM) was set up by the Metre Convention and has its headquarters near Paris, France. It is financed jointly by its Member States and operates under the exclusive supervision of the CIPM.
Its mandate is to provide the basis for a single, coherent system of measurements throughout the world, traceable to the International System of Units (SI). This task takes many forms, from direct dissemination of units (as in the case of mass and time) to coordination through international comparisons of national measurement standards (as in electricity and ionizing radiation).
The BIPM has an international staff of over 70 and its status vis-à-vis the French Government is similar to that of other intergovernmental organizations based in Paris.
Focus on the Boltzmann Constant
Guest Editors
Rod White , Measurement Standards Laboratory, New Zealand
Joachim Fischer , Physikalisch-Technische Bundesanstalt, Germany.

Figure. One of the acoustic resonators used to determine the Boltzmann constant by measuring the speed of sound at the temperature of the triple point of water. Image provided by the National Physical Laboratory, United Kingdom.
Scope
If the plans of the International Committee on Weights and Measures are fulfilled, in 2018 the 26th General Conference on Weights and Measures will redefine the kelvin by fixing the numerical value of the Boltzmann constant. This will replace the current definition of the kelvin based on the triple point of water, which has stood since 1954.
Always, when changes are made to the definitions of the SI base units, it is important that the changes are undetectable in the short term. Therefore, it is essential that the value of the Boltzmann constant to be adopted for the new definition of the kelvin is as consistent as possible with the current definition. Thus, the first step in the kelvin redefinition is to make the best possible measurement of the Boltzmann constant according to the current kelvin definition. Additionally, because there is a possibility of unknown systematic effects that might bias results, determinations should be made using different physical techniques to provide assurance that any unrecognised systematic effects must be small. The temperature measurement community is fortunate that we have four different techniques likely to produce results of sufficiently low uncertainty to contribute to the new definition:
- Acoustic gas thermometry
- Dielectric constant gas thermometry
- Johnson noise thermometry
- Doppler broadening thermometry
This focus issue collates papers from the dozen or so research groups, from different laboratories all around the world, who are making contributions to the Boltzmann constant determinations. Many of the papers report the latest determinations of the Boltzmann constant, while others report new developments in measurement technique or report supporting data from subsidiary experiments.
Introductory material
D R White and J Fischer 2015 Metrologia 52 S213
Acoustic Gas Thermometry
L Pitre et al 2015 Metrologia 52 S263
The Boltzmann constant k has been determined from a measurement of the speed of sound in helium gas in a quasi-spherical resonator (volume 0.5 l) maintained at a temperature close to the triple point of water (273.16 K). The acoustic velocity c is deduced from measured acoustic resonance frequencies and the dimensions of the quasi-sphere, the latter being obtained via simultaneous microwave resonance. Values of c are extrapolated to the zero pressure limit of ideal gas behaviour. We find  J⋅K−1, a result consistent with previous measurements in our group and elsewhere. The value for k, which has a relative standard uncertainty of 1.02 ppm, lies 0.02 ppm below that of the CODATA 2010 adjustment.
J⋅K−1, a result consistent with previous measurements in our group and elsewhere. The value for k, which has a relative standard uncertainty of 1.02 ppm, lies 0.02 ppm below that of the CODATA 2010 adjustment.
R M Gavioso et al 2015 Metrologia 52 S274
We have determined the acoustic and microwave frequencies of a misaligned spherical resonator maintained near the temperature of the triple point of water and filled with helium with carefully characterized molar mass  g mol−1, with a relative standard uncertainty
g mol−1, with a relative standard uncertainty  . From these data and traceable thermometry we estimate the speed of sound in our sample of helium at
. From these data and traceable thermometry we estimate the speed of sound in our sample of helium at  K and zero pressure to be
K and zero pressure to be  m2 s−2 and correspondingly deduce the value
m2 s−2 and correspondingly deduce the value  J mol−1 K−1 for the molar gas constant. We estimate the value
J mol−1 K−1 for the molar gas constant. We estimate the value  J K−1 for the Boltzmann constant using the currently accepted value of the Avogadro constant NA. These estimates of R and k, with a relative standard uncertainty of 1.06 × 10−6, are 1.47 parts in 106 above the values recommended by CODATA in 2010.
J K−1 for the Boltzmann constant using the currently accepted value of the Avogadro constant NA. These estimates of R and k, with a relative standard uncertainty of 1.06 × 10−6, are 1.47 parts in 106 above the values recommended by CODATA in 2010.
Inseok Yang et al 2015 Metrologia 52 S394
We determined accurate values of ratios among the average molar masses MAr of 9 argon samples using two completely-independent techniques: (1) mass spectrometry and (2) measured ratios of acoustic resonance frequencies. The two techniques yielded mutually consistent ratios (RMS deviation of 0.16 × 10−6 MAr from the expected correlation) for the 9 samples of highly-purified, commercially-purchased argon with values of MAr spanning a range of 2 × 10−6 MAr. Among the 9 argon samples, two were traceable to recent, accurate, argon-based measurements of the Boltzmann constant kB using primary acoustic gas thermometers (AGT). Additionally we determined our absolute values of MAr traceable to two, completely-independent, isotopic-reference standards; one standard was prepared gravimetrically at KRISS in 2006; the other standard was isotopically-enriched 40Ar that was used during NIST's 1988 measurement of kB and was sent to NIM for this research. The absolute values of MAr determined using the KRISS standard have the relative standard uncertainty ur(MAr) = 0.70 × 10−6 (Uncertainties here are one standard uncertainty.); they agree with values of MAr determined at NIM using an AGT within the uncertainty of the comparison ur(MAr) = 0.93 × 10−6. If our measurements of MAr are accepted, the difference between two, recent, argon-based, AGT measurements of kB decreases from (2.77 ± 1.43) × 10−6 kB to (0.16 ± 1.28) × 10−6 kB. This decrease enables the calculation of a meaningful, weighted average value of kB with a uncertainty ur(kB) ≈ 0.6 × 10−6.
Michael de Podesta et al 2015 Metrologia 52 S353
In 2013, a team from NPL, Cranfield University and SUERC published an estimate of the Boltzmann constant based on precision measurements of the speed of sound in argon. A key component of our results was an estimate of the molar mass of the argon gas used in our measurements. To achieve this we made precision comparison measurements of the isotope ratios found in our experimental argon against the ratios of argon isotopes found in atmospheric air. We then used a previous measurement of the atmospheric argon isotope ratios to calibrate the relative sensitivity of the mass spectrometer to different argon isotopes. The previous measurement of the atmospheric argon isotope ratios was carried out at KRISS using a mass spectrometer calibrated using argon samples of known isotopic composition, which had been prepared gravimetrically.
We report here a new measurement made at KRISS in October 2014, which directly compared a sample of our experimental gas against the same gravimetrically-prepared argon samples. We consider that this direct comparison has to take precedence over our previous more indirect comparison. This measurement implies a molar mass which is 2.73(60) parts in 106 lighter than our 2013 estimate, a shift which is seven times our 2013 estimate of the uncertainty in the molar mass.
In this paper we review the procedures used in our 2013 estimate of molar mass; describe the 2014 measurement; highlight some questions raised by the large change in our estimate of molar mass; and describe how we intend to address the inconsistencies between them. We also consider the effect of a new estimate of the low pressure thermal conductivity of argon at 273.16 K. Finally we report our new best estimate of the Boltzmann constant with revised uncertainty, taking account of the new estimates for the molar mass and the thermal conductivity of the argon.
Fernando J Pérez-Sanz et al 2015 Metrologia 52 S257
An acoustic gas thermometer was used to achieve a determination of the Boltzmann constant, kB, using a misaligned stainless steel (316L) spherical cavity with an internal volume of approximately 268 cm3. Measurements of the speed of sound while the cavity is filled with argon at the temperature of the triple point of water, 273.16 K, and at different pressures between 78.2 kPa and 0.9 MPa, were used to extrapolate the value of the speed of sound in argon at zero pressure. The internal volume of the resonator was accurately determined by measuring microwave resonance frequencies at the same temperature and pressure conditions as for the acoustic measurements. The measurements were taken at pressures from 78.2 kPa up to 901.3 kPa, and at 273.16 K. As the results of the measurements, we determined kB = (1.380 644 1 ± 0.000 022 1) × 10−23 J K−1 which means a relative standard uncertainty of 16 parts in 106.
X J Feng et al 2015 Metrologia 52 S343
We report progress toward determining the Boltzmann constant kB using the concept of a virtual acoustic resonator, a hypothetical resonator that is mathematically equivalent to a cylindrical cavity with periodic boundary conditions. We derived the virtual resonator by combining the measured frequencies of the longitudinal acoustic modes of two argon-filled, cylindrical cavity resonators in such a way to minimize the effects of the cavities' ends, including transducers and ducts attached to the ends. The cavities had lengths of 80 mm and 160 mm and were operated in their longitudinal (ℓ,0,0) modes. We explored virtual resonators that combine modes of the two resonators that have nearly the same frequencies. The virtual resonator formed from the (2,0,0) mode of the 80 mm resonator combined with the (4,0,0) mode of the 160 mm resonator yielded a value for kB that is, fractionally, only (0.2 ± 1.5) × 10−6 larger than the 2010 CODATA-recommended value of kB. (The estimated uncertainty is one standard uncertainty corresponding to a 68% confidence level.) The same virtual resonator yielded values of the pressure derivatives of the speed of sound c in argon, (∂c2/∂p)T and (∂c2/∂p2)T, that differed from literature values by 1% and 2%, respectively. By comparison, when each cavity was considered separately, the values of kB, (∂c2/∂p)T, and (∂c2/∂p2)T differed from literature values by up to 7 ppm, 10%, and 5%, respectively. However, combining the results from the (3,0,0) or (4,0,0) modes of shorter resonator with the results from the (6,0,0) or (8,0,0) modes of the longer resonator yielded incorrect values of kB that varied from run-to-run. We speculate that these puzzling results originated in an unmodeled coupling, either between the two cavities (that resonated at nearly identical resonance frequencies in the same pressure vessel) or between the cavities and modes of the pressure vessel.
James B Mehl 2015 Metrologia 52 S227
Finite-element calculations of electromagnetic eigenvalues are known to converge to the exact solutions in the limit of vanishing element sizes. In an extension of previous work (Mehl 2009 Metrologia 46 554–9) the eigenfrequencies of the TM1n and TE1n (n = 1, 2, ··· 6) modes of triaxial ellipsoids were calculated as a function of mesh size. Higher-accuracy eigenvalues were obtained through a limiting process as the mesh size was reduced; the extrapolation was based on the theoretical convergence rate. The difference between the finite-element eigenfrequencies and the eigenfrequencies predicted by shape perturbation theory is found to be proportional to the cube of the fractional deformation parameter  for all investigated modes. For ellipsoids with axes proportional to 1 : 1.0005 : 1.0010, the cubic term represents a fractional perturbation of the average TM16 eigenvalue k2 by −0.16 × 10 − 6 and the average TE16 eigenvalue by −0.22 × 10 − 6. This work adds support to the correctness of the analytic second-order formula derived in the previous work, and also demonstrates the usefulness of finite-element methods for investigating the quasi-spherical resonators (QSRs) used in measurements of the Boltzmann constant. In principle, the method can be extended to QSRs whose shape differs from triaxial ellipsoids.
for all investigated modes. For ellipsoids with axes proportional to 1 : 1.0005 : 1.0010, the cubic term represents a fractional perturbation of the average TM16 eigenvalue k2 by −0.16 × 10 − 6 and the average TE16 eigenvalue by −0.22 × 10 − 6. This work adds support to the correctness of the analytic second-order formula derived in the previous work, and also demonstrates the usefulness of finite-element methods for investigating the quasi-spherical resonators (QSRs) used in measurements of the Boltzmann constant. In principle, the method can be extended to QSRs whose shape differs from triaxial ellipsoids.
M R Moldover et al 2015 Metrologia 52 S376
We review correlated uncertainties among the accurate determinations of the Boltzmann constant  that used the techniques of primary acoustic gas thermometry (AGT). We find correlated uncertainty contributions from four sources: (1) the uncertain chemical and isotopic compositions of the test gases that lead to an uncertain average molar mass, (2) measurements of the temperature, (3) measurements of the shape and dimensions of the cavity resonators, and (4) fitting acoustic resonance frequencies as a function of the pressure. Molar-mass-dependent uncertainties are correlated among those measurements that used argon with isotopic abundances determined using an isotopic standard prepared at the Korea Research Institute of Standards and Science in 2006. Correlated, cavity-dependent uncertainties result from using the same cavity for more than one measurement. Small, correlated uncertainties propagate into all the AGT determinations of
that used the techniques of primary acoustic gas thermometry (AGT). We find correlated uncertainty contributions from four sources: (1) the uncertain chemical and isotopic compositions of the test gases that lead to an uncertain average molar mass, (2) measurements of the temperature, (3) measurements of the shape and dimensions of the cavity resonators, and (4) fitting acoustic resonance frequencies as a function of the pressure. Molar-mass-dependent uncertainties are correlated among those measurements that used argon with isotopic abundances determined using an isotopic standard prepared at the Korea Research Institute of Standards and Science in 2006. Correlated, cavity-dependent uncertainties result from using the same cavity for more than one measurement. Small, correlated uncertainties propagate into all the AGT determinations of  when acoustic resonance frequencies are fit for
when acoustic resonance frequencies are fit for  using uncertain literature data for the Avogadro constant and for the thermal conductivity and the higher acoustic virial coefficients of helium or argon.
using uncertain literature data for the Avogadro constant and for the thermal conductivity and the higher acoustic virial coefficients of helium or argon.
Johnson Noise Thermometry
Jifeng Qu et al 2015 Metrologia 52 S242
The unit of thermodynamic temperature, the kelvin, will be redefined in 2018 by fixing the value of the Boltzmann constant, k. The present CODATA recommended value of k is determined predominantly by acoustic gas-thermometry results. To provide a value of k based on different physical principles, purely electronic measurements of k were performed by using a Johnson noise thermometer to compare the thermal noise power of a 200 Ω sensing resistor immersed in a triple-point-of-water cell to the noise power of a quantum-accurate pseudo-random noise waveform of nominally equal noise power. Measurements integrated over a bandwidth of 575 kHz and a total integration time of about 33 d gave a measured value of k = 1.3806513(53) × 10−23 J K−1, for which the relative standard uncertainty is 3.9 × 10−6 and the relative offset from the CODATA 2010 value is +1.8 × 10−6.
Dielectric-Constant Gas Thermometry
Christof Gaiser et al 2015 Metrologia 52 S217
The principles, techniques and results from dielectric-constant gas thermometry (DCGT) are reviewed. Primary DCGT with helium has been used for measuring T–T90 below the triple point of water (TPW), where T is the thermodynamic temperature and T90 is the temperature on the international temperature scale of 1990 (ITS-90), and, in an inverse regime with T as input quantity, for determining the Boltzmann constant at the TPW. Furthermore, DCGT allows the determination of several important material properties including the polarizability of neon and argon as well as the virial coefficients of helium, neon, and argon. With interpolating DCGT (IDCGT), the ITS-90 has been approximated in the temperature range from 4 K to 25 K. An overview and uncertainty budget for each of these applications of DCGT is provided, accompanied by corroborating evidence from the literature or, for IDCGT, a CIPM key comparison.
Thorsten Zandt et al 2015 Metrologia 52 S305
For the determination of the Boltzmann constant by dielectric-constant gas thermometry, the uncertainty of pressure measurements in helium up to 7 MPa has been decreased compared with previous achievements (Sabuga 2011 PTB-Mitt. 121 247–55). This was possible by performing comprehensive cross-float experiments with a system of six special pressure balances and the synchronization of their effective areas. It is now possible to measure a helium pressure of 7 MPa with a relative standard uncertainty of 1.0 ppm applying a 2 cm2 piston-cylinder unit, the calibration of which is traceable to the SI base units.
Doppler Broadening Thermometry
Eugenio Fasci et al 2015 Metrologia 52 S233
We report on complementary tests and measurements regarding our recent determination of the Boltzmann constant, kB, by means of Doppler broadening thermometry, also providing additional information as compared to previous articles. A revised uncertainty budget is illustrated, including some new components that were ignored in previous spectroscopic experiments, and better quantifying other components that were estimated to be negligible. In particular, we consider the relativistic Doppler effect, the perturbation caused by the finite bandwidth of the detection system and the influence of the spontaneous emission content of the probe laser. These new components do not increase the global uncertainty which still amounts to 24 ppm. Our value for the Boltzmann constant is 1.380 631 (33) × 10−23 J K−1, which is the best determination reported so far by using an optical method.
S Mejri et al 2015 Metrologia 52 S314
We report on our ongoing effort to measure the Boltzmann constant,  , using the Doppler broadening technique on ammonia. This paper presents some of the improvements made to the mid-infrared spectrometer including the use of a phase-stabilized quantum cascade laser, a lineshape analysis based on a refined physical model and an improved fitting program increasing the confidence in our estimates of the relevant molecular parameters, and a first evaluation of the saturation parameter and its impact on the measurement of
, using the Doppler broadening technique on ammonia. This paper presents some of the improvements made to the mid-infrared spectrometer including the use of a phase-stabilized quantum cascade laser, a lineshape analysis based on a refined physical model and an improved fitting program increasing the confidence in our estimates of the relevant molecular parameters, and a first evaluation of the saturation parameter and its impact on the measurement of  . A summary of the systematic effects contributing to the measurement is given and the optimal experimental conditions for mitigating those effects in order to reach a competitive measurement of
. A summary of the systematic effects contributing to the measurement is given and the optimal experimental conditions for mitigating those effects in order to reach a competitive measurement of  at a part per million accuracy level are outlined.
at a part per million accuracy level are outlined.
G-W Truong et al 2015 Metrologia 52 S324
Spectroscopy has been a key driver and motivator of new understanding at the heart of physics. Here we describe high-precision measurements of the absorption lineshape of an atomic gas with an aim towards primary thermometry. We describe our progress in pushing this type of spectroscopy to the ultimate limit, in particular in describing experimental work with Rubidium and Cesium, although we also consider the potential for other elements in expanding the precision, accuracy and range of the approach. We describe the important technical and theoretical limits which need to be overcome in order to obtain accurate and precise results—these challenges are not unique to atomic spectroscopy but are likely to afflict all high precision spectroscopy measurements. We obtain a value for  J K
J K where the 71 ppm uncertainty arises with difficulties in defining the Lorentzian component of the lineshape.
where the 71 ppm uncertainty arises with difficulties in defining the Lorentzian component of the lineshape.
C-F Cheng et al 2015 Metrologia 52 S385
A Doppler broadening thermometry (DBT) instrument is built based on cavity ring-down spectroscopy (CRDS) for the precise determination of the Boltzmann constant. Compared with conventional direct absorption methods, the high-sensitivity of CRDS allows one to reach a satisfactory precision at lower sample pressures, which reduces the influence due to collisions. By recording the spectrum of C2H2 at 787 nm, we demonstrate a statistical uncertainty of 6 ppm (part per million) in the determined linewidth values by several hours' measurement at a sample pressure of 1.5 Pa. As for the spectroscopy-determined temperatures, although with a reproducibility better than 10 ppm, we found a systematic deviation of about 800 ppm, which is attributed to 'hidden' weak lines overlapped with the selected transition at 787 nm. Our analysis indicates that it is feasible to pursue a DBT measurement toward the 1 ppm precision using cavity ring-down spectroscopy of a CO line at 1.57  m.
m.
Selected articles previously published in Metrologia related to the Boltzmann constant:
M R Moldover et al 2014 Metrologia 51 R1
We review the principles, techniques and results from primary acoustic gas thermometry (AGT). Since the establishment of ITS-90, the International Temperature Scale of 1990, spherical and quasi-spherical cavity resonators have been used to realize primary AGT in the temperature range 7 K to 552 K. Throughout the sub-range 90 K < T < 384 K, at least two laboratories measured (T − T90). (Here T is the thermodynamic temperature and T90 is the temperature on ITS-90.) With a minor exception, the resulting values of (T − T90) are mutually consistent within 3 × 10−6 T. These consistent measurements were obtained using helium and argon as thermometric gases inside cavities that had radii ranging from 40 mm to 90 mm and that had walls made of copper or aluminium or stainless steel. The AGT values of (T − T90) fall on a smooth curve that is outside ±u(T90), the estimated uncertainty of T90. Thus, the AGT results imply that ITS-90 has errors that could be reduced in a future temperature scale. Recently developed techniques imply that low-uncertainty AGT can be realized at temperatures up to 1350 K or higher and also at temperatures in the liquid-helium range.
Michael de Podesta et al 2013 Metrologia 50 354
The Comité international des poids et mesures (CIPM) has projected a major revision of the International System of Units (SI) in which all of the base units will be defined by fixing the values of fundamental constants of nature. In preparation for this we have carried out a new, low-uncertainty determination of the Boltzmann constant, kB, in terms of which the SI unit of temperature, the kelvin, can be re-defined. We have evaluated kB from exceptionally accurate measurements of the speed of sound in argon gas which can be related directly to the mean molecular kinetic energy,
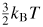 . Our new estimate is kB = 1.380 651 56 (98) × 10−23 J K−1 with a relative standard uncertainty uR = 0.71 × 10−6.
. Our new estimate is kB = 1.380 651 56 (98) × 10−23 J K−1 with a relative standard uncertainty uR = 0.71 × 10−6.
Christof Gaiser et al 2013 Metrologia 50 L7
Fellmuth et al (2011 Metrologia 48 382–90) published the first value of the Boltzmann constant k determined by dielectric-constant gas thermometry at the triple point of water (k = 1.380 654 × 10−23 J K−1, standard uncertainty 9.2 parts per million (9.2 ppm)). Since that time, essential progress of this primary thermometry method has been achieved concerning the design and the assembly of the measuring capacitor, the determination of its effective compressibility, the sensitivity of the capacitance bridge, the influence of stray capacitances, the purity of the measuring gas, the pressure measurement, and the scattering and the evaluation of the data. The resulting new k value amounts to k = 1.380 650 9 × 10−23 J K−1 with a standard uncertainty of 4.3 ppm. This value is about 1.5 ppm larger than the CODATA 2010 one, which has a relative uncertainty of 0.9 ppm.
Read the Metrologia Highlights of 2015 here.