Aptamers and pathogen-based carriers
Published December 2018
•
Copyright © 2018 Morgan & Claypool Publishers
Pages 6-1 to 6-15
You need an eReader or compatible software to experience the benefits of the ePub3 file format.
Download complete PDF book, the ePub book or the Kindle book
Abstract
DNA and RNA are two of the four major types of macromolecules that can act as an enormous hard disk for information storage. They also have great potential to be used as diagnostic gadgets and therapeutic doers. Aptamers are known as an alternative for antibodies because they not only have all of the advantages of antibodies, but also they prevail over the weaknesses of antibodies. Aptamers are low toxic molecules and they have low immunogenicity, because nucleic acids usually are not recognized as exterior agents by the human immune system. Here, we review the application of aptamers and pathogen-based carriers in many fields such as diagnosis and treatment of pathogens, modification of nanoparticles, antibiotic delivery and gene delivery. Pathogenic diseases are the most prevalent diseases that threaten many people all over the world and are caused by microorganisms such as viruses, bacteria, protozoa, prion, fungi, etc. Today, incompetent delivery of antibiotics to the infection cells, side effects of high dosage usage of them and the slow rate of developing new and improved anti-pathogenic drugs are important global issues that need to be solved.
6.1. Aptamers
The nucleic acids DNA and RNA possess exquisitely specific recognition abilities that make them suitable for use as artificial memory for information storage. They can also be applied in promising approaches in diagnosis and therapy [1].
Aptamers are oligonucleotides, including RNA, ssDNA (single-strand DNA) or peptides, that can be selected to bind to selected targets with high affinity and specificity, because of their highly variable three-dimensional structures [2]. Aptamers are used as an alternative to antibodies, because not only do they have all of the advantages of antibodies, but they also can overcome some of the weaknesses of antibodies [3].
Oligonucleotides are more thermally stable than proteins, so they can preserve their structures over frequent thermal cycles of denaturation and renaturation. Thus one of the greatest benefits of aptamers compared to antibodies is their better stability at elevated temperatures [4, 5].
Aptamers are generally non-toxic molecules with low immunogenicity, because nucleic acids are usually not recognized as foreign substances by the human immune system in the same way as proteins are [6]. Aptamers are isolated by SELEX (Systematic Evolution of Ligands by Exponential Enrichment) which can be regarded as an in vitro evolutionary process. Because cells and animals are not employed, toxins and other molecules with low immunogenicity can be used as targets against which to develop aptamers. Aptamers are considered to be more adaptable for different applications. Aptamer-based hybrid molecules have been studied as diagnostic and therapeutic tools, and in drug development, drug delivery, gene delivery and antibiotic delivery systems, etc [7].
In this section, we review the application of aptamers in fields such as the diagnosis and treatment of pathogens, modification of NPs, antibiotic delivery and gene delivery. Pathogenic diseases threaten many people all over the world, caused by micro-organisms such as viruses, bacteria, fungi, protozoa, prions, etc. Today, rapidly growing antibiotic resistance, intolerable side effects from the high doses necessary, and the slow rate of discovery of novel and improved anti-pathogen drugs are important global health issues that are still to be solved [8].
In 2003, Vivekananda et al designed aptamers (ssDNA) that bind to Francisella tularensis subspecies [9]. F. tularensis is a pathogenic aerobic Gram-negative bacterial species that causes tularemia. They disassembled a set of 25 aptamer candidates that could specifically bind to F. tularensis. When these aptamers were applied in a cocktail they showed unique specificity to bind only to tularemia bacterial antigens, making them a promising diagnostic choice for F. tularensis. In 2012, Duan et al reported the first use of whole-bacterium SELEX to identify specific DNA aptamers to recognize Vibrio parahemolyticus. A set of FAM-labeled ssDNA aptamer molecules showed specific binding to V. parahemolyticus. Identification was carried out by flow cytometric analysis. The A3P aptamer showed significant binding affinity (76% to V. parahemolyticus). This system could be used for detection of this bacterium, even in complex sample matrices such as food. Moreover, linking of this aptamer sequence to magnetic NPs could assist in controlling food safety [10].
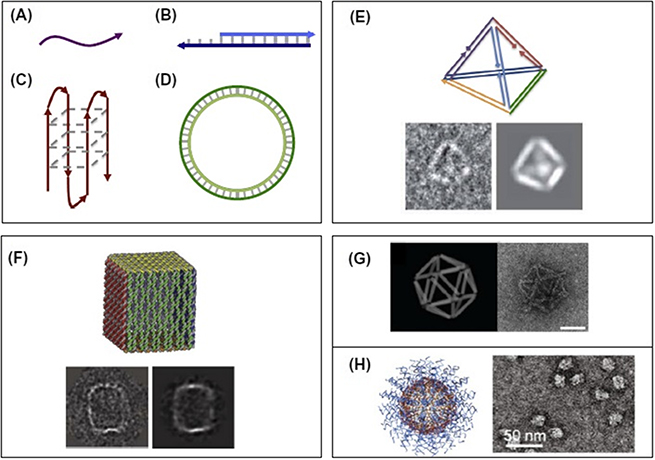
In 2014, Charoenphol et al investigated aptamer-targeted DNA nanostructures for therapeutic delivery purposes [11]. DNA nanostructures have been used as delivery vehicles to carry several therapeutic cargoes such as aptamers and siRNA without any chemical modification. The chemical and physical properties of these nanostructures depend on their size and shape. For example building blocks and recognition sequences can be assigned along the oligonucleotide blocks or longer ssDNA sequences (figure 6.1) [12]. In one study, they merged AS1411 aptamers into DNA pyramids and observed selective growth inhibition of HeLa cancer cells. It was found that aptamer-displaying DNA pyramids were more stable to nuclease degradation than single stranded aptamers. Although, in this study a single cargo type was tested, DNA nanostructures can also be used in combination therapy [13].
Figure 6.1. Computational design of various structural forms of DNA nanocarriers; linear (A) ssDNA and (B) dsDNA, (C) quadruplex DNA, (D) circular double-stranded DNA, (E) wireframe DNA, (F) origami box of DNA with a lid, (G) orthographic model and TEM of DNA icosahedron origami, and (H) coreless and spherical polyvalent nucleic acid nanostructures. Reprinted from [12], with permission from Elsevier.
Download figure:
Standard image High-resolution imageThe Gram-positive pathogenic bacterium Staphylococcus aureus is a common threat to public health [14]. Detection of S. aureus through molecular recognition procedures mediated by aptamers is extremely effective [15]. On the other hand, enrichment and separation procedures using magnetic particles and optical detection by gold nanoclusters are also powerful tools because of their controllability and high sensitivity [16, 17].
Cheng et al in 2015 reported a novel technique for S. aureus detection, in which recognition molecules were combined together. In this study, S. aureus was detected using aptamer-coated magnetic beads (Apt-MB) and vancomycin (Van)-functionalized fluorescent nanoclusters (AuNCs@Van). S. aureus could be discriminated in a mixture of other non-target bacteria, and detected in real-world samples such as milk and human serum. After its synthesis, the structure of AuNCs@Van was confirmed by TEM and XPS. Since nanoprobes should be chemically and photochemically stable, the stability of AuNCs@Van nanoprobes was confirmed by UV illumination and buffer incubation at different pH values. Finally, it was proved that aptamer-coated magnetic beads and antibiotic-capped fluorescent gold nanoclusters could be used to quantify and detect bacteria from complex samples in a dual recognition process [18].
With the development of sophisticated nanomedicine and better aptamer selection methods, aptamer-functionalized NPs have opened a new direction for diagnostic and therapeutic applications. There have been many reports about NP–aptamer conjugates for the delivery of NPs into cancer cells [19].
In 2011, Medley et al applied silica-coated magnetic and fluorophore-doped silica NP–aptamer conjugates to detect exfoliated tumor cells in blood circulation. Due to the low numbers of these cells found in cancer patients, and the fact that they are surrounded by many other cell types and serum proteins, the approach required selective extraction and sensitive detection. They used aptamer-conjugated fluorescent NPs to extract and detect the circulating cancer cells [16].
In 2012, Bamrungsap et al reported the use of aptamers conjugated to magnetic NPs (ACMNPs) for diagnosis of cancer cells [20]. Breast cancer is one of the most deadly cancers and the second most common cause of cancer death in women [21]. Investigations have shown that relatively large number of circulating tumor cells (CTCs) were found in the peripheral blood of women with breast cancer, so the diagnosis of CTCs in blood could be a promising even as a screening method [22]. There are two cancer markers which are commonly over-expressed in breast cancer: HER2 and MUC1. HER2 and MUC1 are over-expressed in 15%–20% and 90% of breast cancer patients, respectively [23]. So, by the use of multi-target HER2 and MUC1 specific probes, breast cancer CTCs could be detected with high accuracy and precision [24].
Recognition of breast cancer cells using (Ru(BPY)3)-doped SiNPs (Dye-SiNPs) is another possibility. In 2015, Jo et al designed a dual aptamer system with MUC1 and HER2 modified-SiNPs for the diagnosis of breast cancer. This newly synthesized probe was characterized successfully. This system was limited to in vitro application because of several difficulties, such as the instability of nucleic acids in blood,the decay of the fluorescence signals in blood and the short half-life of NPs in the circulation system [25].
Heavy metals are agents of concern in environmental contamination. Mercuric ions (Hg2+) are one of the most toxic heavy metals, they are not biodegradable and accumulate in organisms producing toxicity and even death [26]. There are many analytical techniques for measurement of Hg2+ ions [27]. However these methods require expensive and complicated instrumentation, so there is a need to develop new methods with more efficiency to detect Hg2+ ions. An effective approach for the detection of Hg2+ ions may be the use of oligonucleotides. These ions can interact with thymine bases and form thymine–Hg2+–thymine complexes (T–Hg2+–T) [28]. Tanaka et al designed mercury specific aptamer (MSD) functionalized gold NPs (AuNPs) for detection of Hg2+. This process was based on T–Hg2+–T complex formation that suppressed the fluorescence emission of AuNPs. MSDs labeled with fluorescein (FAM) bound to the AuNPs through Au–S bonds to form the AuNPs–MSD probes. This method showed high selectivity for Hg2+ in aqueous solution [29].
Aptamers have been used for antibiotic delivery in a few reports. For example, a nanocarrier for neomycin (an aminoglycoside antibiotic) was prepared using aptamers to immobilize the antibiotic molecules via affinity binding [30].
Mesoporous silica NPs (MSNs) have been shown to be a stimulus-responsive nanocarrier as a drug delivery system [31]. In 2012, an aptamer-gating mechanism was designed to release drugs from MSNs (AS1411). By converting the aptamer sequences to a hairpin structure, a S. aureus specific molecular gate was prepared. The interaction between S. aureus surface antigens and the aptamer disrupted the aptamer structure that prevented the fluorescence emission by self-quenching. Increased fluorescence emission depended on the presence of the target. In this study SA20hp was selected as the molecular gate aptamer because of its high fluorescence response. An antibiotic, vancomycin, was absorbed into the pores of the MSNs and covered with the SA20hp molecular gate. When the NPs were bound to the surface of S. aureus cells through aptamer recognition, the vancomycin antibiotic was released to destroy the bacteria [32].
Gene therapy is the therapeutic delivery of nucleic acids into cells to remedy faulty genes or provide further biological functions by protein expression. Gene therapy can be carried out via viral and non-viral vectors. On the other hand, gene delivery systems based on non-viral vectors have lower toxicity [33]. Polyethylenimine (PEI) belongs to the class of cationic polymers with good transfection capacity in both in vitro and in vivo conditions [34]. Because of non-specific binding of the pDNA/PEI polyplex to proteoglycans on cell membranes, it is not sufficient for use as a targeted gene delivery system [35].
Kurosaki et al designed and developed pDNA/PEI nanostructures which were coated with a polynucleotide, polyadenylic acid (PolyA). It was shown that that aptamer-coated complexes could be taken up by the cells through aptamer-based molecular recognition [36]. In addition, studies showed that mucin 1 (MUC1) could be used for tumor targeting [37]. Kurosaki et al created pDNA/PEI/MUC1 complexes as tumor targeted drug/gene delivery systems. They were constructed complexes using several different ratios of pDNA:PEI:aptamer. The experiments were carried out using in vivo and in vitro conditions with human A549 lung cancer cells and mouse tumor xenografts. The efficiency of in vivo and in vitro transfection using various weight ratios of the aptamer complexes was investigated. Large doses of MUC1 aptamer significantly decreased transgene expression. It appears that the anionic surface charge may repulse the cellular membrane and this repulsion might be larger than the binding strength of the MUC1 aptamer to the MUC1 antigen. This pDNA/PEI/aptamer hybrid complex might be able to target cells by improved selection of the aptamer to achieve cell-specific gene transfer [38].
Some cancers such as melanoma (skin cancer) are difficult to treat because of resistance to radiotherapy and chemotherapy; BRAF gene mutations in many melanomas also make them difficult to treat [39]. Gene therapy is a possible treatment for melanoma that may have the advantages of high specificity and low toxicity. Gene therapy using siRNA (small interfering RNA) that targets BRAF could be a treatment method for melanoma. A new strategy was suggested by Liyu et al in which nucleolin-targeting liposomes were constructed to deliver siRNA BRAF (anti-BRAF siRNA) for treatment. AS1411 is an aptamer that specifically binds to nucleolin. AS1411 was conjugated to PEGylated liposomes (ASLP) acting as targeting probes. The electrostatic interaction between ASLP and siRNA created an ASLP/SiRNA complex. This system specific showed gene silencing activity in A375 melanoma cells [40].
In 2015, Mohammadi et al designed a new system based on SWCNTs functionalized with piperazine–polyethylenimine and an aptamer for siRNA delivery into breast cancer cells [41]. siRNA can be used for therapeutic purposes [42]. Polyethylenimine (PEI) is a polycation used as an effective non-viral vector with inherent buffering capacity. Branched PEI consists of a mixture of primary, secondary and tertiary amino groups, so it can be protonated and provide buffering capacity. The molecular weight of PEI governs the transfection efficiency of this molecule. As the PEI molecular weight increases, the positive surface charge increases reducing the overall transfection efficiency. Alkylcarboxylation of PEI, improves transfection efficiency. Also, single-walled carbon nanotubes (SWNTs) can be used in siRNA delivery because this molecule can easily cross the plasma membrane [43].
Since SWCNTs have poor solubility, they cannot be used as efficient carriers. By introducing positive charges onto the SWCNT surface, and not only improving their solubility in water, they can also then form strong bonds with DNA and siRNA that possess negative charges. PEI–piperazine-based hybrid molecular nanostructures were coupled to functionalized SWCNTs. Afterwards, the SWCNT–PEI–piperazine NPs (vector) was attached to an EpCAM-specific aptamer. For conjugation of SWCNTs to PEI, the SWCNTs were functionalized by oxidation of surface carbon atoms to COOH groups allowing attachment of of PEI through amide bond formation. Prior studies had shown that conjugation between aptamers and NPs increases the efficiency of delivery. The SWNT–PEI–piperazine/DNA hybrid complex was bound to the EpDT3 aptamer. This vector-aptamer hybrid could enhance the DNA transfection and could be used for breast cancer gene therapy [44, 45].
6.2. Virus-based approaches
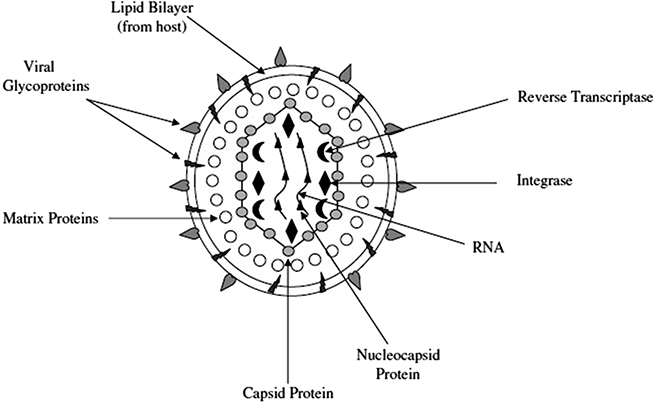
A retrovirus is an enveloped virus with a genome consisting of ssRNA (figure 6.2) [46]. However retroviruses have disadvantages, such as possible cytotoxicity and carcinogenesis.
Figure 6.2. The physical structure of retrovirus NPs. Reprinted from [46], with permission from Elsevier.
Download figure:
Standard image High-resolution imageVirus-based NPs (VNPs) have some of the advantages of native viruses, without the drawbacks of being infectious agents. VNPs are considered to be an important member of the class of nanosystems commonly known as 'bio-inspired'. These particles exhibit controllable self-assembly properties, and are non-infectious in mammals, as well as having good biocompatibility and biodegradability. Viruses have a wide-range of different sizes and shapes, which can allow them to evade the host immune response. The hydrophobicity and surface charge of viral particles are also critical factors for immune escape. The positive charge on the particles can lead to long circulation times in comparison to negatively charged particles [47, 48].
VNPs exhibit novel chemical properties, and can be genetically engineered in order to carry imaging agents and drugs for diagnostic and therapeutic applications. They may be a particularly suitable nanotechnology for oral drug delivery. VNPs for mammalian use are generally derived from plant viruses, and other virus-like particles (VLPs). Many VNPs are produced by spontaneous assembly of virus structural proteins, which then form non-infectious protein cage-like structures [49, 50].
Various type of virus structural proteins are able to form VLPs in heterologous expression systems, such as mammalian cells, plants, Escherichia coli and yeasts. The proteinaceous shell is an important part of VLPs, which has been naturally formed by evolution in order to contain the nucleic acids required for viral replication. Moreover, the inner cavity of VLPs can serve to incorporate other rigid NPs, such as AuNPs as a nanocell concept [51, 52].
VLPs can be an ideal targeted delivery vehicle for drugs, proteins, peptides, siRNAs and RNA aptamers. Moreover, they can act as scaffolds to deliver epitopes in subunit vaccines. Protein engineering via DNA design is a promising approach to create new functionalities at internal, external and inter-subunits of VLPs [53].
The multi-dentate nature of the capsid proteins provides high potential for surface modification that can allow incorporation of multiple ligands and cargoes, such as contrast agents, targeting ligands and therapeutic drugs into a single nano-formulation [54].
Viral capsid proteins (VCP) can self-assemble into a new class of nanomaterial derived from viruses. They form self-assembled hollow nanostructures that are commonly used as nano-capsules for gene therapy, drug delivery and vaccines against infectious diseases. Virus capsid proteins are mono-disperse (all identical), and can be produced by cell culture on a large scale. Advanced structures of these materials can be produced by genetic, chemical or biological engineering techniques. High biocompatibility and facile functionalization makes them an ideal candidate for wide applications. Various sized protein caged can be internalized by living cells with high efficiency due to the natural ability of viruses to cross the cell membrane [51, 55].
Cowpea mosaic virus (CPMV) is used as a model capsid protein derived from a plant virus, that is able to be conjugated with polymers via lysine residues. The cages produced from CPMV have been used for drug delivery systems and bio-imaging. They have an icosahedral shape with a high tendency to bind to vimentin receptors. These receptors are over-expressed on the surface of many endothelial and cancer tissues [56].
Various compounds can be loaded into CPMV nanocages, such as cytotoxic drugs. CPMV-based NPs have good stability in the stomach and intestine after oral delivery. However, the low pH in fasting gastric secretions may lead to denaturation and digestion of these NPs [53].
Moreover, the cowpea chloritic mottle virus (CCMV) is another model capsid protein that can be functionalized with PEG–PLA diblock copolymers and PEG polymers. CCMV can be coupled with photosensitizers leading to improvement in targeted delivery. For example, they can be targeted to bacteria in order to carry out antimicrobial photodynamic inactivation [57].
Studies have shown that capsids can be modified by PEGylated polymers to increase the thermal stability of the tobacco mosaic virus in hydrophobic solvents. In addition, functionalization of CPMV with PEG can increase the density of polymer grafting [57].
Another instance is cucumber mosaic virus (CMV) that can encapsulate various drugs such as doxorubicin inside the capsid via non-covalent attachment to the encapsulated RNA genome, leading to enhanced targeting of the drug in cancer tissue and reduced cardiotoxicity.
Potato virus X (PVX) is another promising candidate for drug delivery, derived from self-assembly of identical coat proteins to produce a highly homogeneous and flexible filamentous shaped nanostructure. The filamentous shape of these NPs can enhance tissue penetration and tumor targeting compared to their spherical counterparts [58]. They can be designed with controlled size and shape, enabling a large-scale production process. They are particularly suitable when a narrow size distribution is needed. Finally, PVX is biocompatible, biodegradable and is a non-infectious agent in humans [59]. PVX can also be used as an immunotherapeutic agent for in situ vaccine monotherapy. A study showed that co-administration of the VLP-based in situ vaccine combined with drug chemotherapy could improve therapeutic efficacy [60].
Tobacco mosaic virus (TMV) is another kind of plant virus-based NP (VNP) that can form a nanotube structure. TMV forms proteinaceous NPs with biocompatibility, biodegradability and lacking any pathogenicity for humans [61]. Physical modification of TMV can lead to transformation from its natural rod-shape to a spherical form by heating. When injected into the bloodstream, TMV moved towards the vascular walls which led to effective interaction with the diseased vessels such as sites of thrombosis. Conjugation of TMV with streptokinase (STK) was used as a carrier for the delivery of thrombolytic enzymes. TMV nanotubes and TMV-derived disk-shaped NPs have both been conjugated with DOX via strong covalent bonds [62]. TMV contains single stranded RNA surrounded by 2130 identical coat protein molecules that assemble into a helical structure that forms a macro one-dimensional rod-like structure. The three-dimensional structure of the coat protein cage makes it suitable for chemical conjugation, and allows protection of the incorporated drug from adverse environmental effects. A tumor-targeting ligand is often added to improve the tumor-specific performance and to reduce the side effects of TMV [63, 64].
Furthermore, modification with a tumor-homing cyclic peptide containing the Arg-Gly-Asp motif (cRGD) can improve the cellular uptake via the αVβ3 integrin-mediated internalization mechanism. For example, attachment of cRGD to the tyrosine139 residue on the TMV exterior surface led to enhanced tumor targeting for chemotherapeutic drugs. TMV could also be an appropriate carrier for the delivery of photosensitizers, in particular for porphyrin-based photosensitizers [65].
Glybera, a viral vector extracted from adeno-associated virus (AAV) was used in gene therapy to treat lipoprotein lipase deficiency. However, the production of these viral vectors is expensive, although it may be considered low cost in comparison to the cost of traditional treatment, such as enzyme replacement therapy [66].
Encapsulation of synthetic cargoes such as inorganic NPs, small molecule drugs, synthetic RNA sequences, metal ions, and organic fluorophores into VNPs has attracted attention for various goals [67].
VNPs can be used to produce mineralized inorganic NPs with controllable sizes. In this process, metal ions can be trapped within the inner cavity of VNPs using electrostatic interaction, or by the preparation of tiny seeds to allow mineralization to occur. The pores on the VNP surface display a pH-dependent gating mechanism [68].
Simian virus 40 (SV40) forms VNPs with a cage-like structure derived by self-assembly of the main capsid protein of SV40. They have a high potential for internalization of foreign nanomaterials, such as quantum dots and AuNPs, and can be engineered to produce hybrid forms with different functionalities [69].
Several types of VLPs are immunogenic for B cells, and induce strong and prolonged IgG responses, even without the use of any adjuvants. Several antigens can be co-administered with VLPs for example, HBsAg and HBcAg antigens can be produced in VLPs by a genetic engineering method in Pichia pastoris and E. coli for preventive vaccination [70].
6.3. Bacterial-based approaches
Another interesting strategy in pathogen-based carriers is the use of bacterial-based NCs. Bacteria belong to the class of prokaryotic unicellular organisms without any nucleus or other intracellular membrane-surrounded organelles. Many bacterial species (particularly anaerobic species) but also including Salmonella spp and Escherichia coli have the ability to actively target particular tissues and organs. Biologically designed variants of anaerobic bacterial species expressing specified proteins can display improved uptake by mammalian cells, and have been utilized to deliver therapeutic cargos. Moreover, subsidiary components of bacterial cells, such as the S-layer, bacterial ghosts and other constituent components, have been investigated as promising carriers for drugs and nucleic acids. These types of components are considered as biomimetic structures with controlled targeting capability or, from another perspective, have multi-task targeting ability with the addition of specific ligands [71–73].
The bacterial surface-layer (S-layer) acts as a specific envelope on the surface of the bacterial cell in most bacterial species, both Gram-negative and Gram-positive. S-layers are composed of specific proteins which form an interconnected network of protein molecules. S-layers can be applied in drug delivery systems due to their structural regularity, self-assembly ability and specific physicochemical properties. S-layer-based NCs have several promising applications for vaccine delivery, and in particular as a targeting platform for drug carriers. Furthermore, they easily form supramolecular structures because of their tunable molecular properties. To be more precise, their surface functional groups, morphology and surface patterning makes them a biostructure that has great potential in biotechnology applications [74–76].
A research group investigated the S-layer from Lactobacillus kefiri, and attached the S-layer to specific liposomes by tuning the lipid–protein ratios and their zeta potential. After coating of the liposome by the proteins (S-layer), the zeta potential became positive, which is suitable for oral administration of drug carriers. In addition, S-layers have been utilized as NCs for therapeutic delivery in cancer, antibacterial applications and allergies [77, 78].
The bacterial ghost (BG) of Gram-negative bacteria is an empty non-denatured envelope that does not have any cytoplasmic content. However BGs preserve the original cellular morphology as well as retaining the chemical structures present on the surface of the cell. Their ability to be loaded with large quantities of cargo makes them a good choice for therapeutic delivery systems. Recently, BGs have been considered to be a promising non-living bio-inspired delivery vehicle. The main advantages of utilizing them as NCs is that there is no need for any denaturing environment to destroy any possible infectivity. For instance, Pantoea cypripedii BGs have been applied as a promising pesticide delivery system including the lipophilic compounds inside the shell. The system showed a significant resistance against water, and retained good activity against some agricultural pathogens. BGs are intact bacterial cell envelopes from which most of the contents have been removed. The procedure is carried out by generation of a specific tunnel within the cell membrane mediated by protein E. In the case of E. coli, the protein E (the tunnel forming species) is encoded by bacteriophage PhiX174. By inducing protein E, a transmembrane tunnel is created allowing the contents to leak out through the outer membrane, leaving the residual envelope intact. In order to trigger the tunnel forming process, a single lysis gene of the phage is sufficient. Recent studies have shown that first the cytoplasmic material is released from the cell, followed by the external medium filling up the inner space of the rigid BGs. Moreover, the same PhiX174 E gene in plasmid vectors can be used to allow BGs to be generated from other bacterial species. These plasmids can be introduced into bacteria using electroporation. BGs are a promising and novel NC for delivery of drugs as well as antigens [79–83].
Another bacterium-based vehicle is based on magnetotactic bacteria that have evolved to take up specific orientations along the direction of the Earth's magnetic field. This phenomenon originated from cells with a specific kind of intracellular organelle, called magnetosomes, which contain a number of nanometer-sized magnetic iron crystals. Magnetosomes can be isolated and utilized as bio-inspired magnetic NPs, and as NCs for drug delivery systems. Magnetospirillum gryphiswaldense magnetosomes were investigated as NCs for DOX delivery to hepatic cancer. DOX was conjugated to the magnetosome surface but did not increase cytotoxicity toward the cancer tissue. However, the morality rate of the mice whose tumor was treated with magnetosome conjugated DOX was about 60% lower than that with free DOX, showing that magnetosomes improved the overall treatment outcome. In another study, M. magneticum AMB-1 magnetosomes were investigated as an anti-tumor targeted drug delivery system for treatment of acute leukemia, and the result was same as in the previous study [84–87].
Outer membrane vesicles (OMVs) function as communication vehicles between individual bacterial cells and between them and other living systems in the environment. OMVs are usually produced by Gram-negative bacteria and are released by a 'pinching-off' mechanism. In spite of the fact that Gram-positive bacteria do not have any outer membrane, some species can still generate OMVs from peptide-based cell walls. The OMVs that are utilized for drug delivery systems have nanosizes between 10 to 300 nm in diameter and a spherical morphology made from lipid-bilayer membrane vesicles. They have been used for therapeutic delivery especially against cancer tissues. They are generated through natural bacterial growth and contain a trace number of inner membrane proteins. Gram-negative OMVs require a detoxification procedure for the purpose of removing any contaminating lipopolysaccharide (LPS). LPS is a strong Toll-like receptor 4 activator that can overstimulate the host innate immune system. OMVs can protect the cargo from extracellular degradation and from different environmental conditions [88–93].
References
- [1]Stoltenburg R, Reinemann C and Strehlitz B 2007 SELEX—a revolutionary method to generate high-affinity nucleic acid ligands Biomol. Eng. 24 381–403
- [2]Dollins C M, Nair S and Sullenger B A 2008 Aptamers in immunotherapy Hum. Gene Ther. 19 443–50
- [3]Proske D et al 2005 Aptamers—basic research, drug development, and clinical applications Appl. Microbiol. Biotechnol. 69 367–74
- [4]Han K, Liang Z and Zhou N 2010 Design strategies for aptamer-based biosensors Sensors 10 4541–57
- [5]Mascini M 2008 Aptamers and their applications Anal. Bioanal. Chem. 390 987–88
- [6]Ireson C R and Kelland L R 2006 Discovery and development of anticancer aptamers Mol. Cancer Ther. 5 2957–62
- [7]Song K-M, Lee S and Ban C 2012 Aptamers and their biological applications Sensors 12 612–31
- [8]Nitsche A et al 2007 One-step selection of Vaccinia virus-binding DNA aptamers by MonoLEX BMC Biotechnol. 7 48
- [9]Vivekananda J and Kiel J L 2003 Methods and compositions for aptamers against anthrax Google Patents
- [10]Duan N et al 2012 Selection and identification of a DNA aptamer targeted to Vibrio parahemolyticus J. Agric. Food Chem. 60 4034–38
- [11]Charoenphol P and Bermudez H 2014 Aptamer-targeted DNA nanostructures for therapeutic delivery Mol. Pharm. 11 1721–25
- [12]Charoenphol P and Bermudez H 2014 Design and application of multifunctional DNA nanocarriers for therapeutic delivery Acta Biomater. 10 1683–91
- [13]Sun T-M et al 2011 Simultaneous delivery of siRNA and paclitaxel via a 'two-in-one' micelleplex promotes synergistic tumor suppression ACS Nano 5 1483–94
- [14]Luzzago C et al 2014 Clonal diversity, virulence-associated genes and antimicrobial resistance profile of Staphylococcus aureus isolates from nasal cavities and soft tissue infections in wild ruminants in Italian Alps Vet. Microbiol. 170 157–61
- [15]Wu S et al 2014 Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels Anal. Chem. 86 3100–07
- [16]Medley C D et al 2011 Aptamer-conjugated nanoparticles for cancer cell detection Anal. Chem. 83 727–34
- [17]Chan P-H and Chen Y-C 2012 Human serum albumin stabilized gold nanoclusters as selective luminescent probes for Staphylococcus aureus and methicillin-resistant Staphylococcus aureus Anal. Chem. 84 8952–56
- [18]Cheng D et al 2015 Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters Anal. Chem. 88 820–25
- [19]Farokhzad O C et al 2004 Nanoparticle-aptamer bioconjugates Cancer Res. 64 7668–72
- [20]Bamrungsap S et al 2012 Pattern recognition of cancer cells using aptamer-conjugated magnetic nanoparticles ACS Nano 6 3974–81
- [21]Siegel R, Naishadham D and Jemal A 2013 Cancer statistics, 2013 CA: Cancer J. Clin. 63 11–30
- [22]Bidard F-C et al 2013 Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials Cancer Metastasis Rev. 32 179–88
- [23]Rakha E A et al 2005 Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer Mod. Pathol. 18 1295–304
- [24]Howes P D, Chandrawati R and Stevens M M 2014 Colloidal nanoparticles as advanced biological sensors Science 346 1247390
- [25]Jo H, Her J and Ban C 2015 Dual aptamer-functionalized silica nanoparticles for the highly sensitive detection of breast cancer Biosens. Bioelectron. 71 129–36
- [26]Clarkson T W, Magos L and Myers G J 2003 The toxicology of mercury—current exposures and clinical manifestations N. Engl. J. Med. 2003 1731–37
- [27]Bernaus A et al 2006 Microprobe techniques for speciation analysis and geochemical characterization of mine environments: the mercury district of Almadén in Spain Environ. Sci. Technol. 40 4090–95
- [28]Tanaka Y et al 2007 15N− 15N J-coupling across HgII: direct observation of HgII-mediated T−T base pairs in a DNA duplex J. Am. Chem. Soc. 129 244–45
- [29]Tan D et al 2013 Aptamer functionalized gold nanoparticles based fluorescent probe for the detection of mercury (II) ion in aqueous solution Talanta 113 26–30
- [30]Sundaram P, Wower J and Byrne M E 2012 A nanoscale drug delivery carrier using nucleic acid aptamers for extended release of therapeutic Nanomed.: Nanotechnol. Biol. Med. 8 1143–51
- [31]Zhu C-L et al 2014 Cell microenvironment stimuli-responsive controlled-release delivery systems based on mesoporous silica nanoparticles J. Food Drug Anal. 22 18–28
- [32]Kavruk M et al 2015 Antibiotic loaded nanocapsules functionalized with aptamer gates for targeted destruction of pathogens Chem. Commun. 51 8492–95
- [33]Elsabahy M, Nazarali A and Foldvari M 2011 Non-viral nucleic acid delivery: key challenges and future directions Curr. Drug Deliv. 8 235–44
- [34]Hatakeyama H, Akita H and Harashima H 2013 The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors Biol. Pharm. Bull. 36 892–99
- [35]Wiethoff C M and Middaugh C R 2003 Barriers to nonviral gene delivery J. Pharm. Sci. 92 203–17
- [36]Sadeqzadeh E et al 2011 Combined MUC1-specific nanobody-tagged PEG-polyethylenimine polyplex targeting and transcriptional targeting of tBid transgene for directed killing of MUC1 over-expressing tumour cells J. Control. Release 156 85–91
- [37]Kim C J et al 2002 Immunotherapy for melanoma Cancer Control 9 22–30
- [38]Li L et al 2014 Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas Biomaterials 35 3840–50
- [39]Kichler A et al 2001 Polyethylenimine‐mediated gene delivery: a mechanistic study J. Gene Med. 3 135–44
- [40]Zintchenko A et al 2008 Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity Bioconjug. Chem. 19 1448–55
- [41]Nimesh S et al 2007 Influence of acyl chain length on transfection mediated by acylated PEI nanoparticles Int. J. Pharm. 337 265–74
- [42]Demeneix B and Behr J P 2005 Polyethylenimine (PEI) Adv. Genet. 53 215–30
- [43]Rahn J J et al 2001 The importance of MUC1 cellular localization in patients with breast carcinoma Cancer 91 1973–82
- [44]Shahidi‐Hamedani N et al 2013 Targeted gene delivery with noncovalent electrostatic conjugates of sgc‐8c aptamer and polyethylenimine J. Gene Med. 15 261–69
- [45]Kurosaki T et al 2012 Self-assemble gene delivery system for molecular targeting using nucleic acid aptamer Gene 491 205–9
- [46]Zhang X and Godbey W 2006 Viral vectors for gene delivery in tissue engineering Adv. Drug Deliv. Rev. 58 515–34
- [47]Parodi A et al 2017 Bio-inspired engineering of cell-and virus-like nanoparticles for drug delivery Biomaterials 147 155–68
- [48]Li N et al 2017 Bio-inspired virus imprinted polymer for prevention of viral infections Acta Biomater. 51 175–83
- [49]Raeeszadeh-Sarmazdeh M et al 2016 Protein nanoparticles as multifunctional biocatalysts and health assessment sensors Curr. Opin. Chem. Eng. 13 109–18
- [50]Llauró A et al 2016 Tuning viral capsid nanoparticle stability with symmetrical morphogenesis ACS Nano 10 8465–73
- [51]Ye Z et al 2018 Background-free imaging of viral capsid proteins-coated anisotropic nanoparticle on living cell membrane with dark-field optical microscopy Anal. Chem. 16 1177–85
- [52]Alkubaisi N A and Aref N M 2017 Dispersed gold nanoparticles potentially ruin gold barley yellow dwarf virus and eliminate virus infectivity hazards Appl. Nanosci. 7 31–40
- [53]Berardi A et al 2018 Stability of plant virus-based nanocarriers in gastrointestinal fluids Nanoscale 10 1667–79
- [54]Guerrero Y et al 2017 Optical characteristics and tumor imaging capabilities of near infrared dyes in free and nano-encapsulated formulations comprised of viral capsids ACS Appl. Mater. Interfaces 9 19601–11
- [55]Béthune J and Wieland F T 2018 Assembly of COPI and COPII vesicular coat proteins on membranes Annu. Rev. Biophys. 20 63–83
- [56]Chatterji A et al 2004 New addresses on an addressable virus nanoblock: uniquely reactive Lys residues on cowpea mosaic virus Chem. Biol. 11 855–63
- [57]Dutt M et al 2017 Self-assembly of virus capsids decorated with block copolymers: a simulation study J. Mater. Res. 32 143–52
- [58]Zeng Q et al 2013 Cucumber mosaic virus as drug delivery vehicle for doxorubicin Biomaterials 34 4632–42
- [59]Le D H et al 2017 Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy Nanoscale 9 2348–57
- [60]Lee K L et al 2017 Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response Nano Lett. 17 4019–28
- [61]Pirie N 2009 Tobacco mosaic virus Adv. Enzymol. Relat. Areas Mol. Biol. 5 1
- [62]Pitek A S et al 2017 Elongated plant virus-based nanoparticles for enhanced delivery of thrombolytic therapies Mol. Pharm. 14 3815–23
- [63]Ishikawa M 2017 Studies on the mechanism of tobacco mosaic virus RNA replication J. Gen. Plant Pathol. 83 410–13
- [64]Saunders K and Lomonossoff G P 2017 In planta synthesis of designer-length tobacco mosaic virus-based nano-rods that can be used to fabricate nano-wires Front. Plant Sci. 8 1335
- [65]Wang Z et al 2017 Programming self-assembly of tobacco mosaic virus coat proteins at Pickering emulsion interfaces for nanorod-constructed capsules ACS Appl. Mater. Interfaces 9 27383–89
- [66]Kurreck J 2017 Rethinking delivery: viral vectors and RNA interference J. RNA Genom. 13 545–7
- [67]Zhang W et al 2017 Encapsulation of inorganic nanomaterials inside virus-based nanoparticles for bioimaging Nanotheranostics 1 358
- [68]Du G et al 2017 Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles J. Control. Release 266 109–18
- [69]Zhang W and Li F 2018 Virus‐based nanoparticles of simian virus 40 in the field of nanobiotechnology Biotechnol. J. 13 e1700619
- [70]Lopez M et al 2017 Characterization of the size distribution and aggregation of virus-like nanoparticles used as active ingredients of the HeberNasvac therapeutic vaccine against chronic hepatitis B Adv. Nat. Sci.: Nanosci. Nanotechnol. 8 025009
- [71]Abed N and Couvreur P 2014 Nanocarriers for antibiotics: a promising solution to treat intracellular bacterial infections Int. J. Antimicrob. Agents 43 485–96
- [72]Zhao Q et al 2014 Smart nanocarrier: self-assembly of bacteria-like vesicles with photoswitchable cilia ACS Nano 8 11341–49
- [73]Farjadian F et al 2018 Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol. Adv. 36 968–85
- [74]Sára M 2001 Conserved anchoring mechanisms between crystalline cell surface S-layer proteins and secondary cell wall polymers in Gram-positive bacteria? Trends Microbiol. 9 47–9
- [75]Sahoo S K and Labhasetwar V 2003 Nanotech approaches to drug delivery and imaging Drug Discov. Today 8 1112–20
- [76]Sleytr U B and Sára M 1997 Bacterial and archaeal S-layer proteins: structure–function relationships and their biotechnological applications Trends Biotechnol. 15 20–6
- [77]Hollmann A et al 2007 Characterization of liposomes coated with S-layer proteins from lactobacilli Biochim. Biophys. Acta Biomembr. 1768 393–400
- [78]Ristl R et al 2010 The S-layer glycome—adding to the sugar coat of bacteria Int. J. Microbiol. 2011 127870
- [79]Paukner S, Kohl G and Lubitz W 2004 Bacterial ghosts as novel advanced drug delivery systems: antiproliferative activity of loaded doxorubicin in human Caco-2 cells J. Control. Release 94 63–74
- [80]Kudela P, Koller V J and Lubitz W 2010 Bacterial ghosts (BGs)—advanced antigen and drug delivery system Vaccine 28 5760–67
- [81]Yoo J-W et al 2011 Bio-inspired, bioengineered and biomimetic drug delivery carriers Nat. Rev. Drug Disc. 10 521
- [82]Paukner S et al 2003 Sealed bacterial ghosts—novel targeting vehicles for advanced drug delivery of water-soluble substances J. Drug Target. 11 151–61
- [83]Tabrizi C A et al 2004 Bacterial ghosts–biological particles as delivery systems for antigens, nucleic acids and drugs Curr. Opin. Biotechnol. 15 530–37
- [84]Matsunaga T and Kamiya S 1987 Use of magnetic particles isolated from magnetotactic bacteria for enzyme immobilization Appl. Microbiol. Biotechnol. 26 328–32
- [85]De Lanauze D et al 2014 Three-dimensional remote aggregation and steering of magnetotactic bacteria microrobots for drug delivery applications Int. J. Robot. Res. 33 359–74
- [86]Mura S, Nicolas J and Couvreur P 2013 Stimuli-responsive nanocarriers for drug delivery Nat. Mater. 12 991
- [87]Felfoul O et al 2016 Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions Nat. Nanotechnol. 11 941
- [88]Gujrati V et al 2014 Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy ACS Nano 8 1525–37
- [89]Rösler A, Vandermeulen G W and Klok H-A 2012 Advanced drug delivery devices via self-assembly of amphiphilic block copolymers Adv. Drug Deliv. Rev. 64 270–9
- [90]van Dommelen S M et al 2012 Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery J. Control. Release 161 635–44
- [91]Berleman J and Auer M 2013 The role of bacterial outer membrane vesicles for intra‐ and interspecies delivery Environ. Microbiol. 15 347–54
- [92]Schwechheimer C and Kuehn M J 2015 Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions Nat. Rev. Microbiol. 13 605
- [93]McBroom A J and Kuehn M J 2007 Release of outer membrane vesicles by Gram‐negative bacteria is a novel envelope stress response Mol. Microbiol. 63 545–58
Footnotes
- 1
These authors contributed equally to this work.