Abstract
Carbon black nanoparticles (CB-NPs) have been synthesized from liquid benzene by a solution plasma method at room temperature and atmospheric pressure. The morphological observation by scanning electron microscopy revealed the agglomeration of aggregated fine particles. The synthesized CB-NPs were predominantly amorphous as confirmed by X-ray diffraction. The in vitro cytotoxicity of CB-NPs on the human lung fibroblast (MRC-5) cell line was assessed by the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and systematically compared with those of two types of commercial carbon blacks (i.e., Vulcan XC-72 and Ketjenblack EC-600JD). Cell viabilities were studied at different concentrations of 32.5, 65, 125, and 250 µg/mL. It was found that the CB-NPs derived from solution plasma exhibited a lower cytotoxicity on the MRC-5 cells than the other two comparative carbon blacks. The viability of MRC-5 cells exposed to CB-NPs remained higher than 90% even at a high concentration of 250 µg/mL. This result preliminarily confirmed the biosafety and potential use of CB-NPs in the field of biological applications.
Export citation and abstract BibTeX RIS
1. Introduction
Carbon black nanoparticles (CB-NPs) are nowadays utilized in numerous applications and purposes from scientific to industrial levels, such as in reinforced fillers in polymers,1) pigment in printing ink,2) electrodes in batteries and fuel cells,3–5) and biosensors,6) owing to their unique physical, chemical, and electrical properties. The CB-NPs are a particulate form of carbon that has a nearly spherical shape with less crystallinity than graphite, usually amorphous and/or turbostratic. The mass production of commercial CB-NPs is mostly based on the incomplete combustion or thermal decomposition of gaseous or liquid hydrocarbons under controlled conditions.7–9) Owing to the extensive use of CB-NPs across all areas of applications, human exposure to CB-NPs through respiratory or direct bodily contact could potentially lead to adverse effects on human health.10–14) Therefore, the potential toxicity of CB-NPs on human cells is one of the most important concerns for the further utilization of CB-NPs with the highest level of biological safety.
Over the past decade, researchers have carried out both in vitro and in vivo cytotoxicity assessments of CB-NPs on various human cells.15–24) It has been found that CB-NPs sometimes appeared to be nontoxic or even highly hazardous. These varying results were likely dependent on the synthesis of CB-NPs. Such differences in the synthesis process and conditions could yield CB-NPs with contrasting physicochemical characteristics, such as size, aggregation, surface area, surface chemistry, and sp2/sp3 ratio, thus inducing different inflammatory and/or cytotoxicity effects on human cells.19,22,24) Therefore, the potential cytotoxic risk of CB-NPs on human cells should be evaluated on a case-by-case basis.
Very recently, our group has proposed a solution plasma method as an alternative and facile strategy in the synthesis of CB-NPs with a controllable structure and dopant at room temperature and atmospheric pressure.25–30) Although numerous research efforts have been made in synthesizing the CB-NPs by the solution plasma method, most of them have been focused on the optimization of synthesis conditions to realize desired properties for use as electrocatalysts in fuel cells and batteries26,27) as well as CO2 adsorbents.28) Until now, the cytotoxicity of CB-NPs synthesized by the solution plasma method has not been investigated in detail yet. Therefore, scientific evidence regarding their biosafety and cytotoxicity is greatly needed to confirm whether they have adverse effects on cell viability.
Here, we aim to study the potential toxicity of CB-NPs synthesized by the solution plasma method using an in vitro 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In this study, a human lung fibroblast (MRC-5) cell line was selected as a target model to evaluate cytotoxicity. The concentration dependence on cytotoxicity response was determined by exposing the MRC-5 cells to different concentrations ranging from 32.5 to 250 µg/mL. Moreover, the morphology, particle size, surface area, and crystalline phase were analyzed to elucidate their effects on cytotoxicity. For comparison, two commercially manufactured carbon blacks (i.e., Vulcan XC-72 and Ketjenblack EC600-JD) were also investigated and systematically compared.
2. Experimental methods
2.1. Chemicals
Benzene (C6H6, ≥99.5% purity) was purchased from Qrec Chemical. Ethanol (C2H5OH, ≥99.9% purity) and dimethyl sulfoxide (C2H6OS, ≥99.9% purity) were purchased from RCI LabScan. Vulcan XC-72 (VC) was obtained from Cabot and Ketjenblack EC600-JD (KB) was obtained from Lion Specialty Chemical. Eagle's minimum essential medium (EMEM) containing sodium bicarbonate, nonessential amino acids, l-glutamine, and sodium pyruvate was purchased from Corning. Fetal bovine serum was purchased from Biowest. Penicillin-streptomycin (10,000 U/mL) was purchased from Gibco Life Technologies. All chemicals were of analytical grade and used as received.
2.2. Synthesis of CB-NPs by solution plasma method
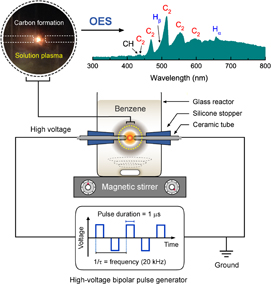
A symmetric pair of 1-mm-diameter tungsten wires (99.9% purity, Nilaco) was employed as the electrodes. The electrodes were shielded with an insulating ceramic tube and placed at the center of a pyrex reactor with a gap distance of 1.0 mm. A bipolar pulsed voltage was applied through the tungsten electrodes using an MPP-HV02 Pekuris bipolar pulse generator (Kurita Seisakusho). The pulse duration and pulse repetition frequency were set at 1 µs and 20 kHz, respectively. Plasma was generated at the gap between electrodes that were immersed in 100 mL of liquid benzene under vigorous stirring and stably kept for 20 min. Figure 1 schematically shows the experimental setup for the synthesis of CB-NPs in this study. Upon plasma generation, benzene molecules were dissociated into small fragments (i.e., C2, CH, and H) as monitored in situ with an Avantes AvaSpec-3648 fiber optic spectrometer system. These dissociated fragments were interacted and recombined with each other, resulting in the rapid formation of carbon particles.30) Such carbon formation was evidenced by the transformation of transparent liquid benzene into muddy black within 1 min. After synthesis, black solid particles were separated by vacuum filtration and then washed with benzene and ethanol, respectively, until the wash solvent became colorless. The resulting products were dried at 80 °C in ambient air for 12 h and subsequently ground with an agate mortar and pestle. The CB-NPs synthesized by the solution plasma method are hereafter denoted as SP.
Fig. 1. Schematic of the experimental setup for the synthesis of carbon nanoparticles by solution plasma and the corresponding optical emission spectrum of the plasma generated in liquid benzene at the initial stage.
Download figure:
Standard image High-resolution image2.3. Characterization
Field-emission scanning electron microscopy (FE-SEM) images were taken using a JEOL JSM-7610F microscope at an acceleration voltage of 5 kV. X-ray diffraction (XRD) patterns were recorded on a Philips X'Pert PRO MPD diffractometer operating at 40 kV and 30 mA with monochromatic Cu Kα radiation (λ = 0.154 nm). The XRD measurement was performed with a step interval of 0.01 and a scanning rate of 0.5 s per step. Nitrogen adsorption was measured on a Micromeritics Gemini 2375 analyzer at liquid nitrogen temperature (−196 °C). Before the measurements, the samples were preheated with a VacPrep 061 degasser at 150 °C for 12 h under vacuum condition. The specific surface area was calculated using the multipoint Brunauer–Emmett–Teller (BET) method in the relative pressure (P/P0) range from 0.05 to 0.30.
2.4. Cytotoxicity test
MRC-5 cells were cultured using a complete medium containing Eagle's Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin prior to culturing in a humidified incubator (37 °C, 5% CO2). The cytotoxicity of SP against MRC-5 cells was evaluated in vitro using the MTT assay. First, MRC-5 cells were seeded into 96-well flat-bottom plates (104 cells/well) and cultured for 24 h. The cultured cells were exposed to SP at different concentrations of 32.5, 65, 125, and 250 µg/mL, followed by incubation for 24 h. After incubation, all supernatants were discarded, and 50 µL of MTT in phosphate-buffered saline (5 mg/mL) was added, followed by incubation at 37 °C for 3 h. The supernatants were completely removed, and then 100 µL of dimethyl sulfoxide was added to dissolve the formazan crystals. Cell viability (or cell death data) in the presence of SP relative to controls was calculated from the absorbance (A) of formazan derived from the MTT in each well at 570 nm with a Varioskan™ flash multimode reader (Thermo Fisher Scientific). All experiments were carried out in duplicate and repeated thrice. The percentage of cell viability was calculated using the following equation:
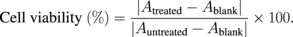
For comparison, two commercially manufactured carbon blacks, i.e., VC and KB, were also evaluated in vitro using identical procedures and concentration range.
3. Results and discussion
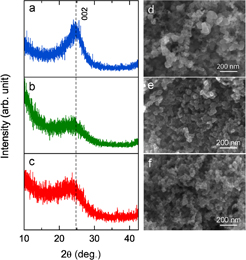
The XRD patterns of all CB-NPs are shown in Figs. 2(a)–2(c). It was observed that the 002 diffraction peak of VC was moderately sharp at about 25°, indicating its intermediate phase between amorphous and graphite, which is known as a turbostratic structure. In contrast, a broader feature at a lower diffraction angle of about 23.5° was evident for KB and SP. The interplanar distances (d002) of VC, KB, and SP calculated from their 002 diffraction peaks using Bragg's law were 0.353, 0.382, and 0.385 nm, respectively. The smaller d002 value of VC close to that of graphite (0.335 nm) indicates its higher crystallinity, while the larger d002 values of KB and SP reflect a dominant disordered structure or amorphous nature in their structure.
Fig. 2. XRD patterns of (a) VC, (b) KB, and SP. FE-SEM images of (d) VC, (e) KB, and (f) SP.
Download figure:
Standard image High-resolution imageThe morphology of all CB-NPs was also investigated by FE-SEM. As illustrated in Figs. 2(d)–2(f), all CB-NPs were composed of aggregated carbon particles with a three-dimensionally interconnected pore structure. The specific surface area determined by the multipoint BET method was found in the following order: KB (∼985 m2/g) > VC (∼252 m2/g) > SP (∼205 m2/g). Other morphological aspects that should be noted here are their particle size and distribution. It was observed that VC had a broad size distribution ranging between 20 and 100 nm with an obvious aggregation. In contrast, both KB and SP revealed almost similar morphologies with a more uniform size distribution of about 20–40 nm. The average particle sizes of KB and SP were estimated to be 33.1 ± 5.9 and 27.3 ± 5.2 nm, respectively.
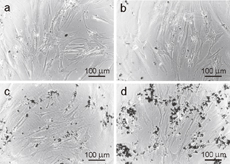
Furthermore, the in vitro cytotoxicities of all CB-NPs against MRC-5 cells were examined using the MTT assay at room temperature after exposure for 24 h. The concentration of carbon was varied at 32.5, 65, 125, and 250 µg/mL for evaluation. The viabilities of MRC-5 cells exposed to VC, KB, and SP are comparatively shown in Fig. 3. The results showed that the number of viable cells exposed to VC, KB, and SP at a low concentration of 32.5 µg/mL had no significant difference with the control group (close to 100%). With increasing concentration, the cytotoxicity effect became obvious for VC and KB, as reflected by the decrease in cell viability. At the concentration of 250 µg/mL, the cell viability decreased to 79.5 and 72.1% for VC and KB, respectively. In contrast, in the case of SP, the cell viability remained above 90% throughout the concentration range investigated, indicating its slight cytotoxicity to the MRC-5 cells. Typically, the surface of carbon materials can induce the formation of reactive oxygen species (ROS), which is a major cause of the oxidative damage of various biological materials and eventually cell death.18,20) The different cytotoxicity effects of CB-NPs were further analyzed by associating their characterization results and cytotoxicity responses toward MRC-5 cells. Although the particle sizes of KB and SP were found to be almost the same, the surface area of KB was much larger than that of SP. A larger surface area of KB could cause a greater reactivity to exposed cells near carbon particles. This could promote more oxidation and harmful effects, leading to the high cytotoxicity of KB. Next, considering the effect of particle size, it has been reported that CB-NP size significantly affects cellular uptake and cytotoxicity.19,22) Sahu et al. showed a comparative cytotoxicity study of nano- and microsized carbon blacks on the human monocyte (THP-1) cell line. They found that nanosized carbon black (∼50 nm) exhibited a higher cytotoxicity to THP-1 cells than microsized carbon black (∼500 nm).22) Kong et al. investigated the cytotoxicity of CB-NPs with different sizes (i.e., 14, 51, and 95 nm) to the mouse macrophage (RAW264.7) cells. The result demonstrated that the cellular uptake of CB-NPs increased as their size increased. This in turn led to the higher cytotoxicity induced by a higher oxidative stress and more ROS formation.19) By associating with our results indicating that the sizes of VC, KB, and SP were smaller than 100 nm, the larger particle size and broader distribution of VC may be the reasons why VC had a higher toxicity than SP. In addition, another possible factor that may cause the higher cytotoxicity of VC is its higher degree of graphitization or sp2/sp3 ratio.24) However, the factors and mechanisms for inducing cell death upon exposure to CB-NPs are still unclear at the present, and further in-depth investigation and analysis are still required to arrive at a definite conclusion. The morphology of untreated and treated MRC-5 cells with SP at the concentrations of 32.5 and 250 µg/mL was further observed and imaged using an inverted microscope, as shown in Fig. 4. The morphology of all treated MRC-5 cells remained in normal. The results obtained from the microscopic images are in good agreement with the result of the cytotoxicity test discussed above.
Fig. 3. Viabilities of MRC-5 cells exposed to VC, KB, and SP at different carbon concentrations determined by the MTT assay. The vertical error bar represents the standard deviation of three replicates.
Download figure:
Standard image High-resolution imageFig. 4. Microscopy images (magnification: ×20) of MRC-5 cells exposed to SP at different concentrations for 24 h: (a) 32.5, (b) 65, (c) 125, and (d) 250 µg/mL.
Download figure:
Standard image High-resolution image4. Conclusions
CB-NPs were synthesized from liquid benzene by a simple solution plasma method. The synthesis could be achieved at room temperature and atmospheric pressure. The in vitro cytotoxicity of SP was assessed by measuring the viability of MRC-5 cells using the MTT assay. The cytotoxicity test was also performed on two commercial CB-NPs, i.e., VC and KB, for comparison. As the concentration increased, the cell viability remained almost constant for SP (∼90%), whereas a significant decrease was observed for VC and KB. This result indicates that the cytotoxicity of SP was relatively lower than those of VC and KB. The low cytotoxicity of SP may possibly be attributed to its moderate specific surface area and uniform size distribution. Although SP showed an acceptable biosafety level for MRC-5 cells in this study, results of evaluating other cell lines are still questionable; thus, further investigation should be carried out. Our result presented here provides the initial and important information on the cytotoxicity assessment of CB-NPs derived from solution plasma, which will pave the way to future research on biological applications with minimal side effects.
Acknowledgments
The authors wish to thank Dr. Sewan Theeramunkong of the Faculty of Pharmacy, Thammasat University for her technical assistance and the Drug Discovery and Development Center at Thammasat University for providing all the facilities that were used for the cytotoxicity tests. This work was partially supported by JST/CREST (Grant No. GJPMJCR12L1).
Biographies

Gasidit Panomsuwan received the D.Eng. degree from the Graduate School of Engineering, Nagoya University in 2013, under the supervision of Prof. Nagahiro Saito. He was a postdoctoral researcher under the guidance of Prof. Takahiro Ishizaki at Shibaura Institute and Technology during 2013–2015, where he worked on the synthesis of heteroatom-doped carbons by solution plasma for use as electrocatalysts in fuel cells and metal-air batteries. He has been a lecturer at the Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Thailand, since 2016. His current research interests are carbon-based materials, dielectric materials, energy storage, and photocatalysis.

Chayanaphat Chokradjaroen is a Ph.D. student at the faculty of polymer science at the Petroleum and Petrochemical College, Chulalongkorn University, Thailand. She holds the bachelor's degree in chemical engineering from the Sirindhorn International Institute of Technology, Thammasat University, Thailand. For her further study, she continued and completed her master's degree at Kanazawa University, Japan. Currently, her research focuses on the degradation and the modification of chitin and chitosan, and the evaluation of biological activities of the obtained products.

Ratana Rujiravanit is an associate professor of the Polymer Science Program at the Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, Thailand. She received her master's and doctorate degrees from Hokkaido University, Japan. After that she earned post-doctoral fellowship at Japan Advanced Institute of Science and Technology (JAIST). Ratana's main research interest is the development of biopolymer-derived functionalized materials. Her published research works have been cited more than 2,000 times with the h-index of 27. One of her publication in Carbohydrate Polymers became one of the Top Ten Cited Articles in the year 2008–2010. In addition, her invention on bio-cellulose composite wound dressing received the Gold Medal Award from the 45th International Exhibition of Inventions, Geneva, Switzerland.

Tomonaga Ueno received the B.E. and M.E. from Nagoya University, Japan in 2006 and 2008, respectively. He received the D.Eng. Degrees from The University of Tokyo, Japan in 2011. He became an assistant professor at the Graduate School of Engineering, Nagoya University in 2011. His research focuses on light-weight materials, polymer degradation, self-organization, and plasma chemistry.

Nagahiro Saito is the research director for "Deepening of the Precise Reaction Field in Solution Plasma and Development of Advanced Carbon Catalyst" in JST/CREST research area; Establish of Molecular Technology towards the Creation of New Functions by Research Supervisor, Hisashi Yamamoto, Ph.D. The author is venturing into the novel and new organic reactions through solution plasma.