Abstract
In a previous report, a self-growing approach was proposed for fabricating complex silver nanostructures, where silver dendrites were grown at silver nanoseeds in silver ion solution owing to plasmonic heating with ultraviolet light. Structures were deformed or destroyed when they were extracted with acetone and dried in air. In this Letter, we discuss the use of supercritical carbon dioxide fluid for the nondestructive extraction of nanostructures. We show the experimental results and discuss the laser power dependence of resultant structures. Another experiment was performed for nanostructure growth inside an agarose gel as a matrix. Silver nanostructures were immobilized without damage in an agarose skeleton network.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Over the last few decades, people have developed a variety of nanotechnologies for fabricating nanodevices with nanomaterials.1) Artificial metallic materials known as metamaterials have, in particular, attracted optical and materials scientists owing to their extraordinary optical properties such as negative refraction and optical cloaking.2–4) They are basically three-dimensional in a large scale. Advanced nanofabrication technology uses either an electron, focused ion, or light beam, while such a beam is not applicable to the fabrication of three-dimensional structures.5–7) Two-photon polymerization or photoreduction can make three-dimensional nanostructures exceeding the diffraction limit.8,9) However, it will take an enormous amount of time to fabricate such three-dimensional nanostructures over a large scale.
In our previous report, we proposed and demonstrated an unconventional nanofabrication method based on metal crystallization.10) Differently from the conventional lithographic or two-photon approach, large-scale three-dimensional structures are self-grown. An ultraviolet (UV) laser beam was used to illuminate silver nanoseeds (nanoparticles) in a solution containing silver ions and ascorbic acid. Localized surface plasmon resonance was hence excited at nanoseeds and then nanocrystals grew owing to the local heating at nanoseeds. The crystal structures looked like nanotrees with nanobranches, being dendrites with fractal geometry. Silver nanodendrites showed broadband extinction between 400 and 900 nm owing to the fractal geometry.11) We used nanodendrites as an efficient substrate for surface-enhanced Raman scattering. The only problem with this fabrication method is that silver nanodendrites bend and lie flat on substrates during the removal of the ion solution and the rinsing of structures with acetone. The nanodendrites are very thin and very fragile. In this Letter, we discuss the use of supercritical fluid for extracting nanostructures without damage.
Supercritical carbon dioxide (CO2) fluid has been used in the industry of semiconductor nanodevices and MEMS for rinsing.12) Supercritical fluid does not have surface tension, which produces capillary force. The viscosity of CO2 supercritical fluid is sufficiently small not to deform or destroy the structures in the rinsing of nanodevices by the fluid. CO2 fluid transits to CO2 gas with the decrease in pressure. As a result, nanodevices are dried and extracted.
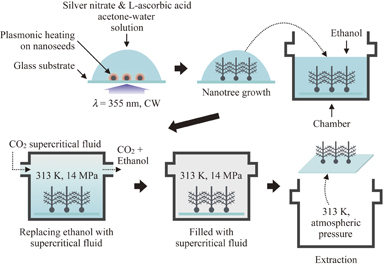
Figure 1 shows the experimental procedure for fabricating and extracting silver nanostructures. Acetone-water (with the ratio  ) solution consisting of silver nitrate (40 mM) and l-ascorbic acid (60 mM) is added dropwise to a glass substrate. Silver nanoseeds with diameters from a few to several nm are prefixed on the substrate. The temperatures of the solution droplets and substrate are maintained at 263 K to inhibit the induction of unnecessary silver nanoparticle reduction in solution. A CW UV laser at λ = 355 nm illuminates silver nanoseeds to excite localized surface plasmon resonance, increasing the local temperature at the nanoseeds. The temperature increase triggers the growth of nanorods from nanoseeds by consuming silver ions in solution through reduction. Owing to the inhomogeneity of the distribution of the silver ion concentration surrounding the surfaces of nanorods, protrusions are grown to produce branches. Branching repeats, forming nanotrees.13,14)
) solution consisting of silver nitrate (40 mM) and l-ascorbic acid (60 mM) is added dropwise to a glass substrate. Silver nanoseeds with diameters from a few to several nm are prefixed on the substrate. The temperatures of the solution droplets and substrate are maintained at 263 K to inhibit the induction of unnecessary silver nanoparticle reduction in solution. A CW UV laser at λ = 355 nm illuminates silver nanoseeds to excite localized surface plasmon resonance, increasing the local temperature at the nanoseeds. The temperature increase triggers the growth of nanorods from nanoseeds by consuming silver ions in solution through reduction. Owing to the inhomogeneity of the distribution of the silver ion concentration surrounding the surfaces of nanorods, protrusions are grown to produce branches. Branching repeats, forming nanotrees.13,14)
Fig. 1. Experimental procedure for growing and extracting silver nanostructures. Silver nanotrees are grown from silver nanoseeds fixed on the substrate in the acetone-water solution of silver nitrate and l-ascorbic acid, with UV laser illumination to induce local heating by localized plasmon resonance at nanoseeds. Nanotrees are grown in the solution, and the solution dissolves in ethanol in a chamber. CO2 supercritical fluid is injected into the chamber, and then ethanol is replaced by the supercritical fluid. CO2 fluid transits to the gas phase, resulting in the drying of the structure without deformation and destruction.
Download figure:
Standard image High-resolution imageThe silver nanostructures in solution are put in a chamber filled with ethanol (100 mL). The acetone-water solution with silver nitrate and l-ascorbic acid dissolves in ethanol. Then, CO2 supercritical fluid is injected from the port of the chamber, while the temperature is maintained at 313 K. The pressure and flow rate of the supercritical fluid are kept at 14 MPa and 30 mL/s, respectively (the critical point of CO2 is at 304.1 K and 7.38 MPa from Ref. 15). The ethanol with the acetone-water solution dissolves in CO2 supercritical fluid and exhausts out from the other port of the chamber with CO2 gas. The flow rate of CO2 supercritical fluid is gradually reduced, and CO2 fluid transits to the gas phase. Finally, the nanostructures are extracted from the acetone-water solution without physical damage.
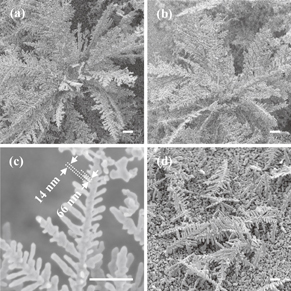
We have carried out an experiment of growing and extracting silver nanostructures. The results are shown in Fig. 2. Figure 2(a) shows a scanning electron microscopy (SEM) image of silver nanodendrites extracted with CO2 supercritical fluid. No structural deformation is observed. The size of this picture is 14 × 14 µm2 (magnification of 4,000×). Figure 2(b) shows the same image as Fig. 2(a) except that the viewing angle is 45° tilted, demonstrating that the tree is three-dimensional. Figure 2(c) shows an enlarged view of part of a nanodendrite with a magnification of 27,000×, where the branches of trees are ∼66 nm thick with a gap of ∼14 nm between adjacent branches. In comparison, a SEM image of nanodendrites extracted with liquid acetone but not with supercritical fluid is shown in Fig. 2(d). Trees lie and overlap each other on the substrate. This is due to the much higher surface tension and viscosity of acetone (23.46 mN/m and 0.324 mPa s at 293 K and ∼0.1 MPa, respectively) than those of CO2 supercritical fluid (0 N/m and 0.08 mPa s at 313 K and 14 MPa, respectively).16–18)
Fig. 2. SEM images of extracted silver nanostructures grown with plasmonic heating at nanoseeds. Panels (a) and (b) show SEM images of silver nanodendrites extracted with CO2 supercritical fluid at different viewing angles of 0 and 45°, respectively, and (c) an enlarged view. Panel (d) shows nanodendrites extracted with acetone for washing (viewing angle of 45°). The scale bar is 1 µm for all samples. The laser power for nanodendrite growth was 20 mW.
Download figure:
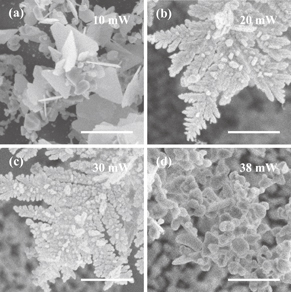
Standard image High-resolution imageThe characteristics of silver nanostructures (either spheres, rods, plates, or cubes, two-dimensional or three-dimensional, and large or small) are determined by the experimental conditions. Here, we discuss the laser power dependence of the structural characteristics (such dependence of other parameters has been discussed in our previous report).10) The UV laser beam with linear polarization is guided to silver nanoseeds on the substrate without any lenses. The diameter of the irradiation spot is 0.98 mm. If the laser power for triggering crystallization at nanoseeds is as low as 10 mW, hexagonal plates are grown as crystals [Fig. 3(a)]. In the case of low-power laser illumination, the reduction rate around nanoseeds is low, such that crystals grow slowly from nanoseeds. In such a slow reaction kinetics, a prismatic shape arises from the seeds containing planar crystallographic defects (e.g., twin planes and stacking faults).19) If the laser power is between 20 and 30 mW, three-dimensional crystals are grown as nanotrees [Figs. 3(b) and 3(c)]. As the laser power increases, the temperature at a nanoseed increases owing to the excitation of localized surface plasmon resonance, resulting in the inhomogeneity of silver ion concentration in solution near the seed. The inhomogeneity triggers crystal growth only at a certain position of the seed surface. As a result, a nanorod is grown. Owing to the inhomogeneity, protrusions are grown on the surface of the nanorod, resulting in three-dimensional branching.13,14) When the power is higher than 35 mW, the reduction occurs not only at the seed but also in the solution owing to the temperature increase. As a result, silver granules are reduced in the solution [Fig. 3(d)].
Fig. 3. Comparison of structural characteristics of nanostructures with different laser powers for triggering crystallization. Panels (a)–(d) are SEM images of the structures grown with laser powers of 10, 20, 30, and 38 mW, respectively. The scale bar is 1 µm for all samples.
Download figure:
Standard image High-resolution imageAnother experiment that we have performed involves the growth of silver nanostructures inside an agarose gel and the removal of the solution without the destruction or deformation of structures in the skeleton of agarose. In this experiment, agarose powder is dissolved in pure water, and the agarose gel is immersed in the acetone-water solution consisting of silver nitrate and l-ascorbic acid. This agarose gel containing silver ions is poured into the chamber, into which CO2 supercritical fluid is injected. The experimental conditions and parameters for extraction with CO2 supercritical fluid are the same as those explained above with Fig. 1. The results are shown in Fig. 4. Figures 4(a) and 4(b) show the SEM images of a silver nanotree grown in an agarose gel observed at viewing angles of 0 and 45°, respectively. Three-dimensional nanotrees are observed to be surrounded and immobilized with a fibrous agarose network. The fibrous agarose network is formed as a result of drying the agarose gel with the supercritical fluid. Figure 4(c) shows the agarose network without dendrite drying in the supercritical fluid (upper part) and air (lower part). In the air, agarose fibers aggregate during drying, breaking silver nanostructures [lower part in Fig. 4(c)]. Figure 4(d) shows silver nanostructures in agarose dried in air. It is observed that nanodendrites are not self-standing and lie and overlap each other.
Fig. 4. Experimental results of silver nanostructures grown in agarose gel. Panels (a) and (b) show SEM images of a nanotree grown with the UV laser and dried with CO2 supercritical fluid. The observation angles are 0° for (a) and 45° for (b). The two SEM images in (c) show the agarose network without nanodendrite drying in the supercritical fluid (upper part) and air (lower part). In the air, agarose fibers aggregate during drying. Panel (d) shows silver nanostructures in an agarose gel dried in air. The scale bar corresponds to 1 µm for all samples.
Download figure:
Standard image High-resolution imageIn summary, we demonstrated the nondestructive extraction of three-dimensional silver nanostructures grown in UV light from nanoseeds with CO2 supercritical fluid for washing and drying. We analyzed the laser power dependence of structural characteristics for nondeformed silver nanostructures. For the practical use of the developed method, we grew the nanostructures in a transparent matrix. We used an agarose gel and successfully grew silver nanodendrites in it, and extracted the structures by supercritical fluid drying.
One of the future aims is the increase in the area of nanostructure growth with plasmonic heating. The in-plane area can be expanded as the UV illumination area enlarges. To increase the height, stacking layers of nanostructures is effective. With the present method, nanostructures rise until the silver ions in solution are exhausted. However, lateral growth also occurs, leading to collision with adjacent trees. To prevent such collision, nanoseeds should be sparsely dispersed on the substrate. Photopolymerizable resin is a possible matrix in which nanostructures can grow. There was a report on two-photon metal reduction in a gold-ion-doped polyvinyl alcohol film, although this did not involve the self-growing of nanostructures.20) Three-dimensional polymer nano/microstructures fabricated with two-photon polymerization or multibeam interference can also be used as the skeleton network to immobilize silver nanostructures.21,22) Metals other than silver can be used as materials of nanostructures grown with the developed method, while their operating frequency range is different from that of silver. Gold and copper have been used for growing nanotrees with electrochemical deposition and chemical de-alloying, respectively.23,24) In our previous study, we used the grown nanodendrites as the substrate for a surface-enhanced Raman scattering experiment for the detection of molecules. The factor of enhancement was ∼5 × 106.10) Zhao has reported that nanodendrites show a negative refraction index as metamaterials.25) Another possible application of these large-scale materials with nanostructures would be as a perfect absorber in a wide spectral range between UV and IR as reported in a previous paper, with a wide viewing angle range due to the fractal geometry and three-dimensionality of their nanostructures.
Acknowledgments
The authors would like to thank N. Nishimura for his contribution to the optical setup, experiment, and parameter optimization, and Professors J. Takahara and T. Sekitani for advice regarding the growth and extraction of three-dimensional nanostructures.