Abstract
Active oxygen species (AOS) generated in the atmosphere can be applied in various industrial processes owing to their extremely strong oxidative ability. Methylene blue (MB) decolors upon exposure to AOS owing to dye degradation; this property can be used to detect the AOS. To detect AOS with higher oxidative ability, it is necessary to stabilize MB by mixing it with sodium alginate. In our previous work, we showed that the OH* concentration in the AOS increased under the high-humidity condition. Herein, the decolorization mechanism of MB-dyed sodium alginate thin films upon exposure to AOS was elucidated under low- and high-humidity conditions; decolorization was observed only under the latter. We analyzed an MB-dyed sodium alginate thin film indicator to elucidate the chemical reactions occurring as well as the decolorization mechanism generated under the high-humidity condition. We found that the decolorization of the film was caused by MB decomposition upon exposure to the AOS generated under the high-humidity condition.
Export citation and abstract BibTeX RIS
1. Introduction
Active oxygen species (AOS) generated in the atmosphere can be applied in various industrial processes owing to their extremely strong oxidative ability. It is well known that atomic oxygen [O(1D)], ozone (O3), and hydroxyl radicals (OH*) are valuable in surface modification. AOS can be generated using ultraviolet (UV) lamps emitting light with 185 and 254 nm wavelengths,1) and it has been proposed that the AOS that are generated in experimental chambers form as follows. The reactions are conducted in the presence of UV light with a 185 nm wavelength, causing a ground-state oxygen molecule (3O2) to decompose into two ground-state oxygen atoms [O(3P)]. Next, O3 is generated when O(3P) binds with another 3O2 molecule. Then, an O(1D) and an excited singlet oxygen molecule (1O2) are generated when O3 is decomposed via irradiation by UV light with a 254 nm wavelength. Finally, OH* radicals are formed when O3 is decomposed and binds with hydrogen atoms. In addition, OH* radicals are generated by the exposure of water molecules (H2O) to UV light at a 185 nm wavelength.2–8) In our previous work, we noted that the OH* concentration increased under a high-humidity condition.2) The use of AOS in sterilization processes has already been reported. Further, we reported a sterilization method in which AOS are generated under UV irradiation.9) AOS sterilization offers various advantages, such as low-temperature and dry processing, no toxicant residual effects, and low operating costs.1) AOS exposure leads to microorganism inactivation.10) In order to establish this technology for use with AOS, determining the presence of AOS and providing an indicator for the sterilization process is necessary; moreover, technology for detecting reactive AOS is highly necessary. However, the measurement methods for AOS in the atmosphere require specialized and expensive equipment, such as an optical laser system11) and a vacuum ultraviolet (UV) light source;12,13) furthermore, electron spin resonance spectroscopy, which is method for detecting radicals with short lifetimes, e.g. O(1D) and OH*,14,15) also requires expensive equipment. In addition, the presence or absence of AOS via such methods cannot be assessed immediately.
We have reported that methylene blue (MB) can be decomposed by O3. MB is a convenient indicator for detecting AOS as it decolors after exposure to AOS owing to degradation of the dye.16) Utilizing this characteristic, we aimed to develop a sensor that can visually determine the presence or absence of AOS via the decolorization of MB. O3 has a lower oxidative ability compared with other AOS. To detect AOS with higher oxidative abilities, it is necessary to stabilize MB by mixing it with sodium alginate, which has a high affinity for MB, thus forming MB/sodium alginate composite films. Sodium alginate is a natural and water-soluble polysaccharide with an excellent film-forming ability. Humidity affects the type of AOS generated: under low-humidity conditions, O(1D) and O3 are generated; under high-humidity conditions, an extremely highly oxidative species, e.g. OH*, is generated.17) Based on these characteristics, the decolorization mechanism of the composite film was investigated. It has been previously observed that decolorization occurs only under high-humidity conditions and is presumably caused by OH*.2) Further, the specific decolorization mechanism of MB-dyed Nafion® films has been reported;16) however, this indicator film was not a uniform thin film. In the present research, we developed a new indicator film using sodium alginate, a water-soluble polymer with a film-forming ability. In addition to the aforementioned decolorization characteristics, the thin film can achieve uniformity and be quantitatively evaluated. We developed the indicator as a thin film that can visually indicate the presence or absence of AOS on the spot. We aimed to analyze the uniform thin film indicator to elucidate the chemical reactions of MB and sodium alginate as well as the decolorization mechanism generated under the high-humidity condition. We cannot employ the MB dye as pristine powder as well as the MB solution in the vacuum process such as surface treatment used in semiconductor industries. To apply for the vacuum process, solid film technology of the MB indicator is required.
2. Experimental methods
In the present study, we used Active Dry® (Iwasaki Electric CO., Ltd., Tokyo) to generate AOS. Three UV lamps were installed in an experimental chamber with dimensions 24 × 25 × 22 cm3 (height × width × depth): two were 6 W (total 12 W) and one was 4 W. These UV lamps emitted UV radiation at 4 W with 185 and 253.7 nm wavelengths and at 12 W with 253.7 nm wavelength, thereby facilitating the generation of AOS.1,17–19) Although several AOS are known, those with long lifetimes or high oxidization abilities, such as O(1D), O3, OH*, and hydrogen peroxide (H2O2), are of particular interest.3–8,20–22) As humidity is known to affect the type of AOS generated, the chamber temperature was maintained at 30 °C using warm air, which was produced by pumping it through recently boiled water, and the Active Dry® to produce high-humidity conditions (90% or higher humidity). To produce low-humidity conditions (less than 20% humidity), dry air was pumped through a bottle of silica gel (large granules with blue indicator), which was maintained at 30 °C and connected to the Active Dry® (i.e. without passing air over the boiled water).2) The air pumping was ceased when AOS exposure was performed.
To examine the MB-dyed sodium alginate film, we performed thin-layer chromatography (TLC) to assess the affinity between the MB and the sodium alginate. TLC was performed on a glass sheet coated with a thin-layer of silica gel. The developing solvents were chloroform, methanol, and water in a 4:1:0.1 ratio. For TLC analysis, 10 mM MB and 10 mM sodium alginate solutions were used. The molecular weights (Mw) of MB and sodium alginate are 319 and 198 g mol−1, respectively. Three drops of each were placed on the glass sheet: a 10 mM MB solution spot on the left, a 10 mM sodium alginate mixed with 10 mM MB spot on the right, and a mixture of these two spots in the middle. The spots were observed under UV light to examine the behavior of the MB benzene ring in response to the degradation of MB by the UV light. Next, we performed coloration using iodine to examine the behavior of substances that are difficult to observe under UV light. Finally, the sample was heated on a hot plate to apply a color former (anise reagent) to the glass sheet. The behavior of water-soluble polymers can be confirmed via this process.
Decolorization of the MB using a thin film indicator based on MB and sodium alginate indicates that the chemical structure of MB molecules changed; further, the structural change could be confirmed using proton nuclear magnetic resonance (1H-NMR) and carbon-13 NMR (13C-NMR) spectra.23–26) The proton signals of the benzene ring and methyl group of MB were found to be shifted, and the carbon signals of the benzene ring and methyl group of MB disappeared. Both of these effects were caused by the AOS generated under the high-humidity condition. These results suggest that a chemical change occurred in the MB structure. The stabilization of MB and sodium alginate for the AOS was investigated through 1H-NMR, which was performed using a Bruker® AVANCE III 500 MHz Ultra Shield spectrometer. A solution was prepared by mixing 300 μl of 10 mM sodium alginate with 300 μl of 25 mM MB (total volume of 600 μl). In addition, a solution of MB and glucose was prepared by mixing 300 μl of 10 mM glucose with 300 μl of 25 mM MB (total volume of 600 μl). A sodium alginate solution of 600 μl of 10 mM sodium alginate solution was prepared. A glucose solution of 600 μl of 10 mM glucose solution was prepared. Further, an MB solution of 600 μl of 25 mM MB solution was prepared. All the solutions were dissolved in deuterium oxide (D2O).
The AOS generated under the high-humidity condition induced the decolorization of the MB-dyed sodium alginate film; this phenomenon was investigated by comparing the 13C-NMR spectra before and after exposure of the solution of MB and sodium alginate to the AOS generated under the high-humidity condition. Under high-humidity conditions, the mixture after the AOS exposure was prepared by mixing 300 μl of 10 mM sodium alginate with 300 μl of 25 mM MB (total volume = 600 μl). This solution was spread on a glass substrate and dried to form a thin film. Three glass substrates were placed in a sterilization bag comprising plastic film and a nonwoven fabric (Johnson and Johnson K.K.) to eliminate the effects of UV light on the decolorization reaction. UV light could not penetrate the sterilization bag; however, AOS could permeate the bag, as has been reported previously.1) The samples were subjected to NMR analyzes. The thin film was subjected to AOS exposure under high-humidity conditions until it completely decolorized after 7 h. Thereafter, the thin film-coated glass substrate was placed in 15 ml D2O; the thin film dissolved using an ultrasonic cleaner. To increase the concentration of the solution mixture for 1H-NMR and 13C-NMR analyzes, the resulting solution was heated and evaporated to a volume of 10 ml.
To examine the stabilization of MB dye in the MB/sodium alginate composite films, we analyzed chemical bonds between MB dye and sodium alginate using a microplate reader. Sodium alginate is a polysaccharide; therefore, we used glucose, which is a monosaccharide with a Mw of 180 g mol−1, to compare the stabilities of MB and sodium alginate. Microplate reader analyzes were performed using a Corona Grating Microplate reader® SH-9000 series with 0.05 mM sodium alginate, 0.05 mM glucose, and 0.05 mM MB aqueous solutions. A mixture of these solutions mixture was measured in a 1:1 ratio of MB/sodium alginate and MB/glucose. In each hole of a 96-well plate, 200 μl of these solutions was dropped for microplate reader analyzes. The measurement was performed at an absorbance/wavelength of 450–800 nm.
3. Results and discussion
3.1. Results
Figure 1 shows the molecular structures of the MB dye, sodium alginate, and glucose. MB is a redox dye used as a colorimetric oxygen indicator.27,28) MB has been used in the investigation of the photochemical stability of biopolymers.29) Among the various dyes, MB is known to be difficult to degrade by conventional waste water treatment methods owing to its stability.30) Sodium alginate (C6H7O6Na) is a linear polysaccharide derivative of alginic acid. Alginate is linear binary copolymers comprised of homopolymeric regions of β-D-mannuronic acids (M) and α-L-guluronic acids (G), residues connected via (1,4)-glycoside linkages in regions of M-, G-, and MG-sequential block structures as shown in Fig. 1(b).31,32) Sodium alginate is a cell-wall component of marine brown algae and contains approximately 30%–60% alginic acid. The conversion of alginic acid to sodium alginate permits the solubility in water, which assists its extraction.33) Furthermore, sodium alginate contains OH and COONa, which are considered to interact with MB at N+/S+. Glucose is a carbohydrate compound of comprising six carbon atoms and an aldehyde group; it is a simple sugar (monosaccharide).34)
Fig. 1. (Color online) Molecular structures of (a) MB, (b) sodium alginate, and (c) glucose. A, B and C indicate the positions of protons in the 1H-NMR spectra. G and M correspond to α-L-guluronic (G) acids and β-D-mannuronic (M). A, B, and D correspond to the proton positions shown in Fig. 5. A, B, C, D, E, and F correspond to the carbon positions shown in Fig. 7.
Download figure:
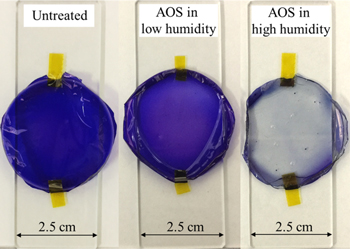
Standard image High-resolution imageFigure 2 shows the uniform thin films of MB-dyed sodium alginate, indicating the decolorization of the films deposited on glass substrates after AOS exposure for 1 h. In this experiment, the MB-dyed sodium alginate thin films were prepared by dissolving 700 μl of 20 g l−1 sodium alginate and 400 μl of 0.6 g l−1 MB in 1,300 μl of water (H2O) in a Ø 40 mm × 13.5 mm petri dish, which was placed in an incubator (40 °C) for 15 h. The thickness of the thin film was 7.6 ± 0.02 μm at its middle and edge. It is evident that the MB-dyed sodium alginate film lost coloration after the AOS exposure under high-humidity conditions (>90%), as shown in Fig. 3. However, the film was hardly decolorized by AOS exposure under low-humidity conditions. It is well known that under high-humidity conditions the AOS generated under UV light are hydroperoxide (HO2) and OH*, of which OH* is of particular interest owing to its stability and oxidative ability in the atmosphere.2) The major reactive species produced during photocatalytic reactions are the generated superoxide anion and OH*.35) Moreover, the generated amount of OH* depends on humidity1) and it is considered that the decolorization of the film under high-humidity conditions was caused by the OH* species.2)
Fig. 2. (Color online) Decolorization of MB-dyed sodium alginate thin films set on glass substrates owing to AOS exposure for 1 h under low- and high-humidity conditions.
Download figure:
Standard image High-resolution imageFig. 3. (Color online) Transmittance of decolorization of MB-dyed sodium alginate thin films owing to AOS exposure for 1 h under low- and high-humidity conditions and untreated conditions.
Download figure:
Standard image High-resolution imageFigure 3 shows the decolorization transmittance spectra of the MB-dyed sodium alginate thin films owing to AOS exposure for 1 h under low- and high-humidity and untreated conditions. The decolorization signal of the thin film after the AOS exposure for 1 h under high-humidity at a wavelength of 600–700 nm was dramatically increased compared with that of the untreated signal and AOS exposure for 1 h under low-humidity conditions.
Figure 4 shows the TLC patterns of MB and mixtures of MB and sodium alginate. In the TLC experiment, the mixing ratio of sodium alginate and MB was 1:1 (Mw), and the changes in distance of the spots are barely observable. The retention factor, Rf (Rf = a/b), is defined as the distance from the origin to the spot center (a) divided by the distance from the origin to the mobile phase front (b). However, there was a slight difference between the left side Rf = 0.30 and the right side Rf = 0.28, for which repeatability was confirmed. It is considered that a weak affinity exists between MB and sodium alginate.
Fig. 4. (Color online) TLC analysis of the affinity between MB and sodium alginate. Rf: retention factor, a: distance moved from the origin point to the spot point, b: represents distance moved by solvent from the origin point.
Download figure:
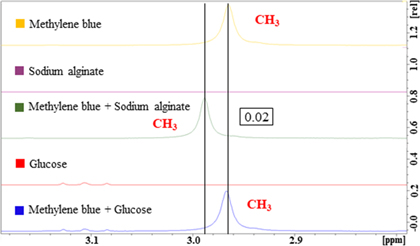
Standard image High-resolution imageThe 1H-NMR spectra of MB, sodium alginate, the mixture of MB and sodium alginate, glucose, and the mixture of MB and glucose are shown in Figs. 5 and 6. The characteristic peaks corresponding to the benzene ring of MB in the mixture of MB and sodium alginate can be observed at approximately 6.66, 6.88, and 7.14 ppm, whereas those of the mixture of MB and glucose can be observed at approximately 6.62, 6.85, and 7.10 ppm (Fig. 5). The methyl group (CH3) of MB in the mixture of MB and sodium alginate can be observed at approximately 2.99 ppm, whereas that of the mixture of MB and glucose can be observed at approximately 2.98 ppm (Fig. 6). These are assigned to the protons in the functional groups. Although the shapes of the peaks corresponding to the benzene ring and CH3 of MB in the mixture of MB and sodium alginate are the same as those of pristine MB, there are some peak shifts relative to the pristine signals (0.05, 0.03, 0.04, and 0.02 ppm). As such, the MB peak shifted because sodium alginate and MB exhibit an intermolecular interaction with one another. However, the positions and shapes of the peaks corresponding to the benzene ring and CH3 of MB in the mixture of MB and glucose are the same as those of pristine MB, i.e. glucose and MB do not interact. These results suggest that sodium alginate and MB ionically bond with one another at the –COO− (carboxylate salt) of sodium alginate and the N+ (nitrogen) or S+ (sulfur) position of MB.
Fig. 5. (Color online) 1H-NMR spectra of MB, sodium alginate, a mixture of MB and sodium alginate, glucose, and a mixture of MB and glucose. A, B, and D correspond to the proton positions shown in Fig. 1(a).
Download figure:
Standard image High-resolution imageFig. 6. (Color online) 1H-NMR spectra of MB, sodium alginate, a mixture of MB and sodium alginate, glucose, and a mixture of MB and glucose. CH3 corresponds to the proton positions shown in Fig. 1(a).
Download figure:
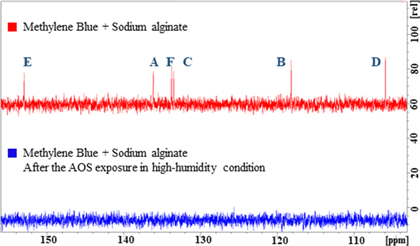
Standard image High-resolution imageFigure 7 shows the 13C-NMR spectra of the mixtures of MB and sodium alginate before and after the AOS exposure under high-humidity conditions. The results suggest that the six peaks between 105 and 153 ppm (A, B, C, D, E, and F) corresponding to the benzene ring of MB disappeared owing to the AOS exposure under high-humidity conditions. Based on quantum chemical calculations, the benzene ring is known to have decomposed.36–39) Therefore, the decolorization of the MB-dyed sodium alginate film was achieved via AOS exposure under high-humidity conditions. This result indicates that MB was decomposed by the AOS generated under the high-humidity condition, as reported previously.16) Additionally, the MB degradation reaction owing to AOS has been previously reported.40)
Fig. 7. (Color online) 13C-NMR spectra of mixtures of MB and sodium alginate before and after AOS exposure under high-humidity conditions. A, B, C, D, E, and F correspond to the carbon positions shown in Fig. 1(a).
Download figure:
Standard image High-resolution imageFigure 8(a) shows the absorbance from the microplate reader spectra of MB, sodium alginate, and the mixture of MB and sodium alginate (1:1 ratio). Figure 8(b) shows the absorbance of the microplate reader spectra for MB, glucose, and the mixture of MB and glucose (1:1 ratio). A peak at 577 nm in the mixture of MB and sodium alginate spectrum shifted to the peak at 620 nm in the MB spectrum as shown in Fig. 8(a). Moreover, the absorbances of the peaks between the microplate reader spectra of (i) 577 and 660 nm of the mixture of MB and sodium alginate spectrum and (ii) 620 and 670 nm of the MB spectrum [Fig. 8(a)] also show differences. These results indicate that an intermolecular force (interaction between molecules) occurred, suggesting that sodium alginate and MB undergo an intermolecular interaction with one another at the –COO− (carboxylate salt) of sodium alginate and the N+ (nitrogen) or S+ (sulfur) position of MB. However, the peaks shift at approximately 613 nm in the microplate reader spectra of the mixture of MB and glucose; the MB spectrum could not be confirmed in Fig. 8(b). Moreover, the absorbances of all the peaks around 613 and 664 nm in the microplate reader spectra of the mixture of MB and glucose as well as MB [Fig. 8(b)] are the same. These results indicate that there is no interaction between glucose and MB.
Fig. 8. (Color online) Absorbance of microplate reader spectra of (a) MB, SA is sodium alginate, and MB + SA is a mixture of MB and sodium alginate (1:1 ratio); (b) MB, G is glucose, and MB + G is a mixture of MB and glucose (1:1 ratio).
Download figure:
Standard image High-resolution imageThe TLC and 1H-NMR results suggest that sodium alginate and MB exhibit an intermolecular interaction with one another. Therefore, sodium alginate is considered to be suitable for stabilizing MB.
3.2. Discussion
The mechanism of the decolorization of MB through AOS exposure is assumed to involve the destruction of the MB complex. In the present study, decolorization of the thin film indicator was observed upon its exposure to AOS under high-humidity conditions. Moreover, the MB-dyed sodium alginate film selectively reacted with the AOS generated under the high-humidity conditions. Ono et al. have reported on the effect of humidity on the densities of AOS in a cylindrical reaction chamber equipped with UV lamps.41) Humid air was introduced, and the temperature (T) was maintained at 25 °C. The effect of humidity on the densities of various radicals was examined. The relative humidities (RH) under the low- and high-humidity conditions in the present study were 20% and 90% RH, respectively. In their work on O3 density in a chamber, Ono et al. installed UV lamps with a radiation powers of 1.9 and 12.2 W at 185 and 254 nm, respectively.41) These radiation powers are approximately the same as those of the UV lamps used in the present study. As already discussed, the O3 densities generated under the low- and high-humidity conditions were predicted to be approximately 120 and 40 ppm, respectively.2) However, Ono et al. controlled the O3 density between 100 and 1000 ppm and predicted the O3 density to be 275 and 104 ppm in the low- and high-humidity conditions, respectively. The chamber volume in the present study is 13 200 cm3, which is larger than that used in Ono's study (1508 cm3), and the difference in the O3 densities between One's study and the present study is attributable to this difference in chamber size. However, when we evaluate AOS concentrations on the basis of previously reported simulation results,41) the O3 density ratios—the O3 density under the low- and high-humidity conditions—in Ono's study and in this study are 3.0 and 2.6, respectively.
The results show that the concentration of the O3, excited singlet oxygen [O2(1aΔg)], hydroperoxyl (HO2), OH*, and oxygen atoms (O) are of the order of 6.88 × 1015 cm−3, 4 × 1013 cm−3, 2.4 × 1012 cm−3, 2 × 1011 cm−3, and 3.4 × 1010 cm−3, which represent 98.58%, 0.57%, 0.034%, 0.0029%, and 0.0005% of the total AOS density, respectively, under the low-humidity condition. These densities are of the order of 2.6 × 1015 cm−3, 3.1 × 1013 cm−3, 2.2 × 1012 cm−3, 6 × 1011 cm−3, and 1.2 × 1010 cm−3, which represent 97.57%, 1.16%, 0.083%, 0.023%, and 0.0005% of the total AOS density, respectively under the high-humidity condition.41) These results indicate that the O3 concentration is highest among these radicals under both the low- and high-humidity conditions. OH* and HO2 are produced under the high-humidity condition; however, the reaction rate constants for HO2 and OH* are 102–103 l mol−1 s−1 and 108–109 l−1 mol−1 s−1, respectively.17) We considered that the OH* plays an important role in the oxidation reaction under both humidity conditions.
We have already discussed the model of OH* production by O3 and water inside the nonwoven fabric.42) In addition, an OH* formation reaction by O3 and water has already been reported. The autolysis reaction of the O3 under water is as follows:
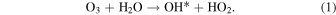
The O3 under water is easily decomposed. The decomposition reaction starts with the production of H2O2 via the reaction of O3 and hydroxide ion (OH−), which is a dissociation component of water:
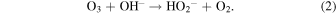
HO2− is a dissociation component of H2O2, which decomposes O3 and generates products such as OH radicals. Therefore, O3 is decomposed into oxygen molecules and OH* radicals are generated in the process. However, the OH* radicals at the surface and/or in the indicator react quickly and disappear; thus, they do not accumulate.
Thus, we deduced that the decolorization of the MB-dyed sodium alginate thin film due to the AOS generated under the high-humidity condition is related to the decomposition of MB by OH*.
4. Conclusions
In conclusion, we have succeeded in preparing uniform MB-dyed sodium alginate thin films and investigating their decolorization characteristics upon their exposure to the AOS generated under the high-humidity condition. Results of TLC, 1H-NMR, 13C-NMR, and microplate reader analyzes suggest that MB and sodium alginate share an ionic bond, as evidenced by the shifts in the peaks corresponding to the benzene ring of MB in the mixture of MB and sodium alginate. Moreover, these peaks disappeared after the AOS exposure under high-humidity conditions, indicating that the decolorization of the film is caused by the decomposition of MB upon exposure to the AOS generated under the high-humidity condition.
Acknowledgments
This work was partially supported by Grant-in-Aids for matching planner program from the Japan Science and Technology Agency, (MP27115663427), and Scientific Research C from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17K06823).