Abstract
The radiation-induced reactions of ligands play an important role in the sensitization of metal oxide nanocluster resists. However, the details in the radiation chemistry of ligands for metal oxide nanocluster resists are still unknown. In this study, the radiation-induced reactions of carboxylic acid ligands were investigated using a pulse radiolysis method. The rate constants for the reactions of molecular and ionic forms of tiglic, angelic, o-toluic, and p-toluic acids with hydrated electrons were determined. The rate constants for the reactions of tiglic, angelic, benzoic, o-toluic, and p-toluic acids with dodecane radical cations were also determined. The radical ions of tiglic and angelic acids were more unstable than those of benzoic, o-toluic, and p-toluic acids. The results obtained in this study indicate that the molecular structures of ligands affect their reactivity to cationic and anion species and the stability of their radical cations and anions.
Export citation and abstract BibTeX RIS
1. Introduction
In the development of extreme ultraviolet (EUV) resists, the trade-off relationships between resolution, line width roughness (LWR), and sensitivity are the most serious problem.1–4) For example, LWR is degraded when the sensitivity is improved (the exposure is decreased). In the lithography, the photons from the light source transfer the information written on the photomask to the resist materials and carry the energy required for the solubility change of resist materials in the developer.5,6) The energy for the solubility change can be compensated for through the supply from the external energy source. The postexposure baking of chemically amplified resists is a typical example of the external supply of the energy for the pattern formation. When the information is sufficient, the sensitivity (and resolution) can be improved without degrading LWR, for example, by improving the efficiencies of acid generation and catalytic chain reaction. However, the information is insufficient for meeting the resist requirements for 11 nm half-pitch resolution. The increase of the absorption coefficient of resist materials is essentially required to increase the information without decreasing the sensitivity (increasing the exposure dose).7)
The absorption coefficient of resist films is determined by the product of photoabsorption cross section and density of elements. Therefore, the introduction of elements having a large photoabsorption cross section and/or a high density is an important strategy for the development of high-performance EUV resists.8) With such a background, the metal-containing resists have attracted much attention.9) The metal elements such as Ti,10,11) Zr,10,12–15) Sn,16,17) Sb,18) Hf,11,12,14) and Bi19) have been examined from the viewpoint of applicability to EUV resists. The organic ligands have been used to disperse the metal oxide nanoparticles or nanoclusters. Carboxylic acids have been mostly used as a ligand for the metal-containing resists. Acrylic acid,18) methacrylic acid (MAA),10–13,18,20–22) trans-dimethylacrylic acid,14) isobutyric acid,21) 2-methylbutanoic acid,21) pivalic acid (t-pentanoic acid),19) benzoic acid,11,13,19,20) o-toluic acid,14,15) p-toluic acid,15) bromobenzoic acid,15) 3-(trimethoxysilyl)propyl methacrylic acid,11) and acetic acid18,19) have been used or examined.
The mechanism of solubility change upon exposure has been investigated for the metal oxide nanocluster (or nanoparticle) resists. For the metal oxide nanoparticle resists with acid generators, the ligand exchange with the anions generated from the acid generators was proposed.21) However, the metal oxide resists work well without acid generators.22) The carboxylation has been also suspected as a cause of the solubility change. However, the reasonable amount of carbon dioxide has not been measured as outgas during exposure, although it has been detected. The crosslinking of ligands such as MAA has been also suspected. However, the reasonable amount of the loss of double bonds has not been measured. It is necessary to clarify the radiation chemistry of ligands to discuss the mechanism of solubility change. Upon exposure to the ionizing radiations such as EUV, the organic ligand molecule (L) is ionized and a radical cation (L.+) and a thermalized electron (e−) are generated
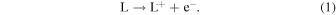
Both a radical cation and a thermalized electron are reactive species. In the subsequent reactions, the radical cation and the thermalized electron typically work as oxidizing and reducing agents, respectively. It is important to understand the reaction of carboxylic acid radical cations and the reactivity of carboxylic acids to thermalized electrons (and the subsequent reaction of their radical anions) for the efficient development of metal oxide nanocluster resists. The radiation chemistry of carboxylic acids has been investigated from the viewpoint of basic science.23–29) However, the details are still unclear. In this study, the radiation-induced reactions of carboxylic acids were investigated for typical carboxylic acids used for metal-containing resists. The chemical systems to elucidate the reaction of carboxylic acid radical cations and the reactivity of carboxylic acids to thermalized electrons by pulse radiolysis were individually designed. The dependence of reactivities on the molecular structures of carboxylic acids was discussed.
2. Experimental methods
Tiglic acid [TiA, (2E)-2-methylbut-2-enoic acid, TCI, purity >98%], angelic acid [AA, (2Z)-2-methylbut-2-enoic acid, TCI, purity >98%], benzoic acid (BA, Sigma-Aldrich, purity >99.5%), o-toluic acid (o-TA, 2-methylbenzoic acid, TCI, purity >98%), and p-toluic acid (p-TA, 4-methylbenzoic acid, Aldrich, purity 98%) were purchased and used for sample preparation. Each molecular structure is shown in Fig. 1. Solvent was appropriately chosen from ultra-pure water (Millipored water), dichloromethane, and dodecane, depending on the reaction system. To control the reaction paths between radiolytic intermediates (cations, trapped- and/or solvated-electrons), a cation scavenger (ethanol) or an electron scavenger (dichloromethane) was added to the sample solutions. Acid-base equilibrium constants (pKa) of TiA, AA, BA, o-TA, and p-TA are 5.0, 4.3, 4.2, 3.9, and 4.4, respectively. Since the prepared solutions (~mM) usually have lower pH than the pKa, the carboxylic acids are present as the molecular form. To investigate reactivity of the anion form, some experiments were carried out under the basic conditions, for which sodium hydroxide (NaOH) was used as pH adjuster. Finally, prior to irradiation experiment, all solutions were purged with Ar gas to avoid the effects of dissolved oxygen.
Fig. 1. Molecular structures of carboxylic acids.
Download figure:
Standard image High-resolution imageFor observation of transient radiolytic species in various time regions from ps to ns, two different electron pulse radiolysis systems were employed at ISIR, Osaka Univ. and Nuclear Professional School, School of Engineering, Univ. of Tokyo. In ns pulse radiolysis system at L-band linac facility in Osaka Univ., ns electron beam [8 ns (FWHM)] with the energy of 27 MeV was used as an irradiation source. The dose was adjusted to ca. 10 Gy/pulse (for evaluation of rate constants) or 70–80 Gy/pulse (for the other experiments). Xe flash lamp was used as the analyzing light which covers the spectral domain between 300 and 1600 nm, and the measurement was performed based on kinetic method. Details of the apparatus for ns pulse radiolysis were described elsewhere.30) The similar ns pulse radiolysis system was also established at S-band linac facility in Univ. of Tokyo,31) and was used in this work. In Univ. of Tokyo, ps pulse radiolysis experiment was also conducted. Ps electron beam [7 ps (FWHM), 22 MeV] generated from a laser-driven photocathode linac (22 MeV S-band, 14.2 Hz operation) was used as the irradiation source. The accelerator is combined with a fs laser [100 fs (FWHM), 782 nm] which is consisting of an Er-doped fiber oscillator (Menlo Systems) and a Ti:Sapphire pre-amplifier (Coherent Inc.). Split into two, one is converted into 3rd harmonics (261 nm) and used for generation of photoelectrons at the photocathode, and the another is used as the probe light after conversion into supercontinuum light. Introducing a multichannel analyzer (PMA20, Hamamatsu Photonics), one shot acquisition of optical spectra ranging from UV to NIR (370–1100 nm) is possible. By using the ps electron beam, the fs laser, and the detection system, 30 ps time-resolved transient absorption measurement based on pulse-probe method was performed. Details of the system are also described elsewhere.32)
3. Results and discussion
The reactivities of organic ligands to the hydrated electrons were investigated using the pulse radiolysis method. Upon exposure to ionizing radiation, a hydrated electron, is generated in aqueous solution through the ionization of water33)
is generated in aqueous solution through the ionization of water33)
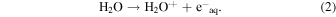
Here, H2O+ is a radical cation of water. H2O+ is immediately decomposed to a hydroxyl radical through the following ion-molecule reaction
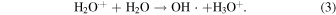
Figure 2 shows the dependences of observed decay rates of hydrated electrons in the presence of carboxylic acids on their concentrations. The representative kinetics of hydrated electrons are shown in the insets. The decay rates were measured under acidic and basic conditions. As discussed in the experimental section, the carboxylic acids used are present as a molecular form and as an anion form under acidic and basic conditions, respectively. Therefore, the decay of hydrated electrons indicates the following reactions
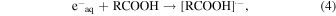
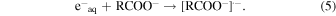
The rate constants for the reaction of hydrated electrons with carboxylic acids were calculated from the slopes of the graphs. The rate constants under acidic and basic conditions are summarized in Table I, together with the reported values for BA,23) MAA,29) and isobutyric acid (IBA).29) The rate constants of TiA, AA, BA, o-TA, p-TA, and MAA were much larger than that of IBA. This is considered to be the effect of C = C double bonds.29) For all the carboxylic acids, the rate constants under acidic condition were larger than those under basic condition. This indicates that the reaction of a molecular form with hydrated electrons is faster than that of an anion form. Under both conditions, the rate constants of TiA and AA with hydrated electrons were smaller than that of MAA. The methyl groups at β positions are considered to affect the reactivity to hydrated electrons. The rate constant of the anion form of AA was significantly smaller than that of TiA. The cis-trans isomerism of methyl group affected not the reactivity of the molecular form but that of the anion form. The rate constants for the reaction of aromatic carboxylic acids with hydrated electrons were approximately the same as those of MAA under the acidic condition, as shown in Table I. Under the basic condition, the rate constant for the reaction of o-TA was significantly smaller than those for BA and p-TA. Similarly to the case of TiA and AA, the methyl group at ortho position affected the reactivity to hydrated electrons under the basic condition.
Fig. 2. (Color online) Dependences of observed decay rates of hydrated electrons in the presence of (a) unsaturated and (b) aromatic carboxylic acids on their concentrations under acidic (low pH) and basic (high pH) conditions. The insets show the kinetics of hydrated electrons obtained in the ns pulse radiolysis of aqueous solutions of (a) 10 mM unsaturated carboxylic acids and (b) 2 mM aromatic carboxylic acids. Kinetics for pure water is also shown. The monitored wavelength was 720 nm.
Download figure:
Standard image High-resolution imageTable I. Rate constants for the reaction of hydrated electrons with carboxylic acids under acidic and basic conditions.
k (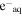 + S) × 10−9 (M−1 s−1) + S) × 10−9 (M−1 s−1) |
||
|---|---|---|
| Carboxylic acid | pH < 4 | pH > 9 |
| Tiglic acid | 8.7 | 0.38 |
| Angelic acid | 6.8 | 0.044 |
| Benzoic acid23) | 16 | 3.2 |
| o-Toluic acid | 15 | 0.29 |
| p-Toluic acid | 18 | 3.8 |
| Methacrylic acid29) | 17 | 3.2 |
| Isobutyric acid29) | 0.88 | 0.0045 |
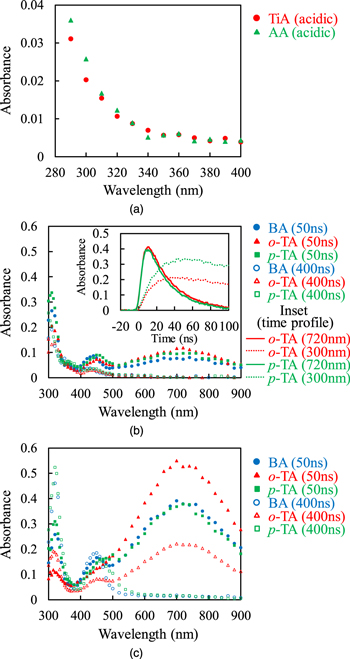
Figure 3(a) shows the transient absorption spectra recorded at 400 ns after irradiation obtained in the ns pulse radiolysis of aqueous solutions of 10 mM unsaturated carboxylic acid under acidic condition. It has been reported that the tails of absorption bands due to the radical anions and α-carbon radicals of MAA are observed at the wavelength of 300 nm.26) The similar tails were observed for TiA and AA under the acidic condition. The tails of absorption bands observed under the acidic condition in Fig. 3(a) are considered to be due to the α-carbon radicals of TiA and AA, similarly to the case of MAA.29) Under the basic condition, the absorptions due to the α-carbon radicals of TiA and AA were extremely low at 400 ns. Because the proton concentration affected the tails observed at the wavelength of 300 nm, in acidic condition, the electron attachment reaction (4) is immediately followed by a protonation reaction to form neutral radical species as
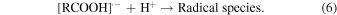
Fig. 3. (Color online) (a) Transient absorption spectra recorded at 400 ns after irradiation obtained in the ns pulse radiolysis of aqueous solutions of 10 mM unsaturated carboxylic acids under acidic condition. (b), (c) Transient absorption spectra recorded at 50 and 400 ns after irradiation obtained in the ns pulse radiolysis of aqueous solutions of 3 mM aromatic carboxylic acids under (b) acidic and (c) basic conditions. The inset in (b) shows the kinetics monitored at the wavelengths of 300 and 720 nm.
Download figure:
Standard image High-resolution imageThe transient absorption spectra obtained in the ns pulse radiolysis of aqueous solutions of 3 mM aromatic carboxylic acid under the acidic condition are shown in Fig. 3(b). The absorption band with the peak wavelength of 720 nm is due to the hydrated electrons.33) The absorption band with the peak wavelength of 320 nm observed for BA aqueous solution has been reported to be due to BA radical anions, which are generated through the reaction with the hydrated electrons.23) The absorption band with the peak wavelength of 450 nm is its subband.23) Similar absorption bands were observed for o-TA and p-TA. These absorption bands are considered to be due to the radical anions of o-TA and p-TA, because their absorption intensities increased with the decrease of the absorption intensities of hydrated electrons, as shown in Fig. 3(b). Under the basic condition, the similar absorption bands were observed as shown in Fig. 3(c). Unlike the cases of MAA, TiA, and AA, the radical anions of BA, o-TA, and p-TA existed stably in the observed time range under both acidic and basic conditions.
The reactivities of organic ligands to the radical cations were investigated using dodecane as a solvent. Upon exposure to ionizing radiation, a dodecane radical cation is generated through the ionization of dodecane (solvent)34,35)
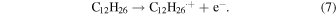
Here, C12H26.+ is a radical cation of dodecane. In absence of any scavengers, the radical cation and the electron are known to recombine quickly in picosesecond time region (geminate ion recombination) to form an excited state.34,35) By adding the dichloromethane, an electron scavenger, to the sample solution, C12H26.+ can be enhanced because e− is eliminated through the following reaction (8) and the geminate ion recombination is also inhibited
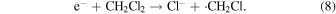
The insets of Figs. 4(a) and 4(b) show the representative kinetics of dodecane radical cations obtained in the ps pulse radiolysis of TiA solution and BA solution in dodecane with 1 M dichloromethane, respectively. By adding the carboxylic acids to dodecane solution, the decay of dodecane radical cations became fast, which indicates the hole transfer from dodecane radical cation to carboxylic acids
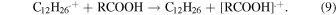
The dependences of observed decay rates of dodecane radical cations in the presence of carboxylic acids on their concentrations are shown in Fig. 4. The rate constants for the reaction of dodecane radical cations with carboxylic acids were calculated from the slopes of the graphs, as summarized in Table II, together with the reported values for MAA29) and IBA.29) Although the reactions of TiA and AA with hydrated electrons became slow due to methyl groups at β positions, the reactions of TiA and AA with dodecane radical cations became fast, compared with that of MAA. The introduction of methyl group to β positions is considered to increase the reactivity to cationic species and decrease the reactivity to anionic species. The rate constants of aromatic carboxylic acids with dodecane radical rations were comparable to those of TiA and AA and larger than that of MAA.
Fig. 4. (Color online) Dependences of observed decay rates of dodecane radical cations in the presence of (a) unsaturated and (b) aromatic carboxylic acids on their concentrations. The insets show the representative kinetics of dodecane radical cations obtained in the ps pulse radiolysis of (a) tiglic acid solution and (b) benzoic acid solution in dodecane. The numerical values next to M represent the solute concentrations. The monitored wavelength was 820 nm.
Download figure:
Standard image High-resolution imageTable II. Rate constants for the reaction of dodecane radical cations with carboxylic acids.
| Carboxylic acid | k (C12H26.+ + S) × 10−9 (M−1 s−1) |
|---|---|
| Tiglic acid | 7.86 |
| Angelic acid | 7.59 |
| Benzoic acid | 7.09 |
| o-Toluic acid | 9.22 |
| p-Toluic acid | 6.44 |
| Methacrylic acid29) | 2.58 |
| Isobutyric acid29) | 3.46 |
The nanosecond pulse radiolysis of carboxylic acid solutions in dichloromethane was carried out to investigate the radical cation of carboxylic acids. Upon exposure to an ionizing radiation, a thermalized electron, e−, is generated through the ionization of dichloromethane (solvent)
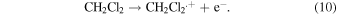
Here, CH2Cl2.+ is a radical cation of dichloromethane. Since e− immediately disappears in ps time scale with the following dissociative attachment to the solvent molecule,36,37) the radical cation survives and is available for reactions of our interests
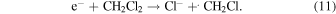
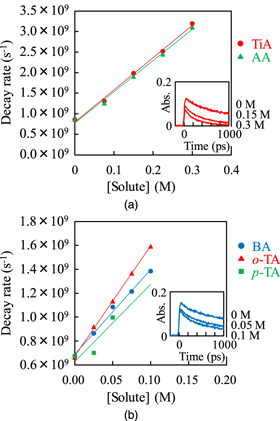
Figure 5 shows the transient absorption spectra recorded immediately after irradiation and kinetics at the wavelength of 440 nm (insets) obtained in the ns pulse radiolysis of dichloromethane containing carboxylic acids with and without the additive of 1 M ethanol. Upon the irradiation by an electron pulse, a characterless broad absorption was observed for each unsaturated carboxylic acid solution without ethanol, as shown in Figs. 5(a) and 5(b). On the other hand, characteristic absorption bands were observed for each aromatic carboxylic acid solution without ethanol, as shown in Figs. 5(c)–5(e). Ethanol was added to the sample solution to identify the intermediates which have an absorption band in the observed wavelength range. By adding 1 M ethanol, the absorption intensity decreased in the wavelength range shorter than 650 nm for unsaturated carboxylic acids and in the whole observed wavelength range for aromatic carboxylic acids, as shown in Fig. 5. For the broad absorption observed in the unsaturated carboxylic acid solution without ethanol, the kinetics observed at 440 nm with and without ethanol is shown in the insets of Figs. 5(a) and 5(b). For the absorption bands in the visible wavelength region observed in the aromatic carboxylic acid solutions without ethanol, the kinetics observed at 450 nm with and without ethanol are shown in the insets of Figs. 5(c)–5(e). Ethanol being a radical cation scavenger, the difference observed between samples with and without ethanol will be due to related species of the radical cation (CH2Cl2.+), which are possibly a product of reaction (12) and/or the other products originating from CH2Cl2.+, similarly to the case of MAA29)
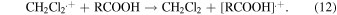
The lifetimes of the intermediates of unsaturated carboxylic acids were significantly shorter than those of aromatic carboxylic acids. This difference is considered to be related to the stability of the radical cations. The radical cations of carboxylic acids are decomposed to neutral radicals through deprotonation.
Fig. 5. (Color online) Transient absorption spectra recorded immediately after irradiation and kinetics (insets) obtained in the ns pulse radiolysis of dichloromethane containing (a) 0.1 M tiglic acid, (b) 0.1 M angelic acid, (c) 0.1 M benzoic acid, (d) 0.1 M o-toluic acid, and (e) 0.1 M p-toluic acid, with and without the additive of 1 M ethanol. The monitored wavelengths for the kinetics were (a), (b) 440 nm and (c)–(e) 450 nm.
Download figure:
Standard image High-resolution imageAs thus far described, the radical ions are considered to be generated through both the reactions starting from ligand radical cations and thermalized electrons. These radical ions are considered to be basically decomposed to neutral radicals, although their lifetimes depend on their molecular structures and their surroundings. The neutral radicals of organic ligands are considered to result in the radical recombination to the other radicals, the radical addition to the unsaturated ligands, and/or decarboxylation.38) The radical recombination and radical addition lead to the insolubilization of resist films though the crosslinking with the ligands of neighboring nanoclusters (or nanoparticles). The decarboxylation also leads to the insolubilization of resist films through the loss of function as a ligand. As described in the introduction, the major mechanism of insolubilization has not been determined. The results obtained in this study confirmed that the decarboxylation and crosslinking are possible from the viewpoint of radiation-induced reactions of ligands. Our results also do not deny the ligand exchange. Therefore, in the real current metal oxide nanocluster (or nanoparticle) resists, plural reaction paths are considered to contribute to the solubility change of the resist film. In the future design, we should closely investigate all paths and may need to simplify the reaction path (eliminate some paths and enhance the other paths) to improve the total performance of metal oxide nanocluster (or nanoparticle) resists.
4. Conclusions
The radiation-induced reactions of carboxylic acid ligands were investigated using a pulse radiolysis method. The rate constants for the reactions of molecular and ionic forms of TiA, AA, o-TA, and p-TA with hydrated electrons were determined. The rate constants for the reactions of TiA, AA, BA, o-TA, and p-TA with dodecane radical cations were also determined. The radical ions of TiA and AA were more unstable than those of BA, o-TA, and p-TA. The results obtained in this study indicate that the molecular structures of ligands affect their reactivity to cationic and anion species and the stability of their radical cations and anions.
Acknowledgments
This research was partly supported by a Grant-in-Aid for Scientific Research, 15H04243. We thank Mr. K. Furukawa and Mr. Y. Okada in the Research Laboratory for Quantum Beam Science, Institute of Scientific and Industrial Research, Osaka University for the technical assistance of electron linear accelerator. Finally, part of this work was carried out as a collaborative research project at Nuclear Professional School, School of Engineering, The University of Tokyo, and we thank Prof. M. Uesaka, Mr. T. Ueda, and Dr. E. Hashimoto for providing support in the experiment.